ZnCo2O4纳米颗粒组装的毛线团状的微球用于锂离子电池负极材料
2016-12-20王瑛林宁
王瑛 林宁
ZnCo2O4纳米颗粒组装的毛线团状的微球用于锂离子电池负极材料
王瑛*,1林宁2
(1山东玉皇新能源技术有限公司,菏泽274000)
(2中国科学技术大学化学与材料科学学院,合肥230026)
通过液相共沉淀法获得Zn和Co的前驱,经过600℃煅烧处理获得ZnCo2O4纳米颗粒组装的毛线团状的微球。电化学测试表明,在0.5 A·g-1的电流密度下循环200次可逆比容量保持为965 mAh·g-1;在0.8 A·g-1的电流密度下循环350次可逆比容量保持为882 mAh·g-1。倍率性能测试表明在2 A·g-1的电流密度时可逆比容量为736 mAh·g-1。
ZnCo2O4;纳米颗粒;线团状微球;锂离子电池
0 Introduction
Recently,spineltype ZnCo2O4hasbeen considered as an attractive anode material because both Zn and Co are electrochemical active for lithium storage[1-2], thus resulting in higher theoretical capacity(975 mAh·g-1)than pure Co3O4(890 mAh·g-1)[3-6].To improve the Li-ion storage performance,several efforts have been made to fabricate nanostructured ZnCo2O4materials.Sharma et al.prepared porous ZnCo2O4nanotubes which showed a reversible capacity of 900 mAh·g-1at60 mA·g-1over 60 cycles[3].The ZnCo2O4nanorods prepared by hydrothermal method at 180℃delivered a reversible capacity of 767 mAh·g-1at 0.2 mA·cm-2over 60 cycles[5].Qiu et al.fabricated porous ZnCo2O4nanoflakes which exhibited a capacity of 750mAh·g-1at 80 mA·g-1over 50 cycles[7].Du et al.synthesized ZnCo2O4nanowires via sacrificial templates, delivering a reversible capacity of 957 mAh·g-1at 100 mA·g-1over 20 cycles[8].
Mesoporous micro/nano-structures as an important family of functional materials have attracted considerable attention in recent years.This unique structure could take advantage of both micro and nano components,such as better permeability,large surface area,high tap density,and mechanical integrity[2-3,11]. Bai et al.prepared ZnCo2O43D hierarchical twin microspheres that delivers a specific capacity of 550 mAh·g-1at 5 A·g-1for 2 000 cycles[12].Wang etal. prepared mesoporous ZnCo2O4microspheres which exhibited initialspecific capacity of 1 332 mAh·g-1at a current density of 100 mA·g-1,and maintained at 721 mAh·g-1after 80 discharge/charge cycles[13].
Herein,the hierarchical clew-like ZnCo2O4microspheres composed of uniform nanoparticles were synthesized by a two-step method.First,a typical coprecipitation reaction is carried out to fabricate the sub-carbonate precursor.In this process,the Zn2+and Co2+are co-precipitated by bicarbonate ion to form a large amount of nanoparticles which would subsequently assemble as microspheres.Second,postannealing treatment at 600℃is able to decompose and oxidize the precursor.As an anode material for rechargeable lithium-ion batteries,the as-prepared ZnCo2O4microspheres exhibit the reversible capacity of 965 mAh·g-1at 500 mA·g-1after 200 cycles,882 mAh·g-1at800 mA·g-1after 350 cycles,and good rate -capability with a capacity of736 mAh·g-1at2.0 A·g-1.
1 Experimental
1.1Preparation of clew-like microspheres of ZnCo2O4
All of chemical reagents were of analytical grade and were used without any further purification.In a typical experiment,ZnCo2O4microspheres were synthesized through two steps.Firstly,0.294 5 g of Zn(NO3)2·6H20,0.584 97 g of Co(NO3)2·6H20,and 3.964 g of (NH4)2SO4were dissolved in 210 mL deionized water, marked as solution A.2.372 g of NH4HCO3was dissolved in 210 mL deionized water,marked as solution B.0.2 g of tartaric acid was dissolved in 21 mL absolute ethanol,marked as solution C.Then,the solution B and C were injected into solution A.After stirring vigorously atroom temperature for 30 minutes, the mixed solution was sealed in glass beaker,which was heated at 60℃for 9 hours.Subsequently,the pink precipitate was collected by centrifugation,washed by deionized water and absolute ethanol several times, and followed by vacuum-drying at60℃overnight.
Secondly,the as-prepared pink precursor was annealed at 600℃in muffle furnace for 2 hours with a ramp rate of 2℃·min-1under air atmosphere. Contrast experiment was carried out following the similar procedure without adding tartaric acid.
1.2Characterization
The final product in this work was characterized by X-ray diffraction(XRD)on Philips X′Pert Super diffractometer with Cu Kαradiation(λ=0.154 178 nm), the working voltage and current is 40 kV and 40 mA, respectively.The thermal performance of as-prepared precursor Zn-Co co-precipitation was studied by thermogravimetric analysis(TGA)on Micrometrics ASAO 2020M at a heating rate of 10℃·min-1in air. The scanning electron microscope(SEM)images were taken by using a JEOL-JSM-6700F field-emitting(FE) scanning electron microscope with an accelerating voltage of 5 kV.The high-resolution transmission electron microscope(HRTEM)was taken on a JEOL-2010 transmission electron microscope at an accelerating voltage of 200 kV.Brunauer-Emmett-Teller (BET)surface area and Barrett-Joyne-Halenda(BJH) pore distribution plots were calculated on basis of the N2absorption-desorption isotherms that were measured on Micromeritics ASAP 2020 accelerated surface area and porosimetry system.Surface analysis by X-ray photoelectron spectra(XPS)was performed on VGESCA-LABMKIIX-ray photoelectronic spectrometer.
1.3Electrochemical measurements
Half-cell tests were conducted using two electrode coin cells(CR2016)with pure Li metalfoil as counter electrode.For preparing working electrode, a slurry mixture of as-prepared active material,superP,and PVDF ata weight ratio of 80:10:10,was coated on copper foil(99.9%).The active material density of each electrode was determined to be about 1.5 mg· cm-2.A solution of1 mol·L-1LiPF6in ethylene carbonate (EC)and diethyl carbonate(DEC)(1:1,V/V)was served as electrolyte.The cells were assembled in an argon-filled glove box.Galvanostatic charge/discharge measurements were carried out on a LAMD-CT2001A instrument with a fixed voltage range of 0.005~3.0 V (vs Li+/Li).Cyclic voltammetry(CV)was performed on electrochemistry workstation(CHI 660D),with a scanning rate of 0.1 mV·s-1at room temperature. Electrochemical impedance spectroscopy(EIS)was measured with an electrochemical station(CHI 660D) by applying an AC voltage of 5 mV in the frequency ranging from 100 kHz to 0.04 Hz.
2 Results and discussion
Fig.1a shows the thermogravimetric analysis (TGA)curves of the co-precipitated precursor.The first weight loss of 27.69%before 183℃mainly corresponds to the evaporation of absorbed moisture and the loss of lattice water of the precipitation.As the temperature further increases,the 26.9%weight loss between 183 and 325℃isassigned to the complete decomposition of co-precipitated metal(cobalt and zinc)carbonates.At 600℃,there is no significant weight change,and high temperature treatment would improve the crystallinity ofthe product.
The crystalline structure and phase information of the product were verified by powder X-ray diffraction(XRD)patterns,as shown in Fig.1b.Eight main peaks at 18.97°,31.22°,36.79°,38.49°,44.74°, 55.57°,59.36°and 61.36°are attributed to the diffraction peaks of(111),(220),(311),(222),(400),(422), (511),(440)and(533)planes of cubic spinel ZnCo2O4(a=0.809 64 nm,space group Fd3m,PDF card No.23-1390).The Zn2+and Co3+ions occupy the tetrahedral sites and the octahedral sites in this crystal structure, respectively.Based on the full width at half maximum (FWHM)of the main diffraction peaks,the primary particle size of obtained sample was calculated to be about32 nm using Scherrer′s equation:D=Κλ/(B cosθ), whereΚis Scherrerconstant,B is FWHM,λisradiation wavelength,θis diffraction angle.
The surface electronic state and the composition of the obtained ZnCo2O4were analyzed by X-ray photoelectron spectra(XPS).The binding energies for the C1s peak(284.6 eV)are served as correction in the XPS analysis.Fig.2a exhibited a typical XPS spectrum which is consisted of Zn,Co,O,and C elements.No other impure peaks were observed.Fig. 2b presented the high-resolution Zn2p spectrum,in which two strong peak at 1 020.5 and 1 043.9 eV were ascribed to Zn2p1/2and Zn2p3/2orbits of Zn(Ⅱ), respectively.Fig.2c showed the deconvoluted Co2p spectrum,where two main peaks are centered at794.9 eV for Co2p3/2and 780 eV for 2p1/2,indicating the Co(Ⅱ)oxidation state formed in this composition.Fig.2d displays the major O1s peak located at 529.95 and 531.5 eV,which suggests the oxygen species of the Co-O and Zn-O in ZnCo2O4.
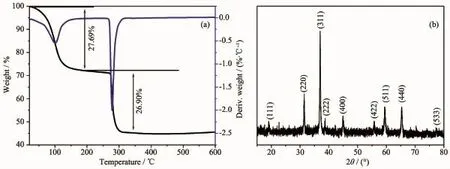
Fig.1(a)Thermogravimetric analysis(TGA)and deriv.weight curves of the precursor heated in air; (b)XRD pattern of the as-synthesized ZnCo2O4
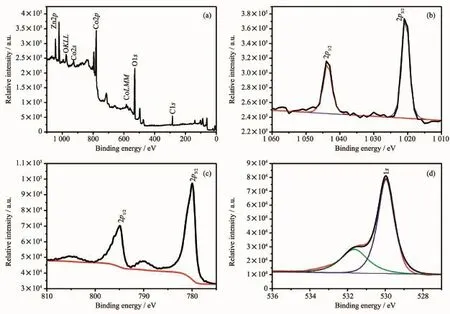
Fig.2 XPS spectra:survey spectrum(a),Zn2p(b),Co2p(c)and O1s(d)for ZnCo2O4sample
The structure and morphology of the synthesized ZnCo2O4was investigated by scanning electron microscope(SEM)and transmission electron microscope (TEM)images.As shown in Fig.3a,the obtained sample exhibits clew-like microspheres with an average diameter of 2~3μm.Fig.3b shows the higher magnification image of the chapped microspheres.It was clearly observed that those hierarchical microspheres are composed of interconnected uniform nanoparticles with a mean size of~30 nm.Obviously, these nanoparticles would allow for better release of stress withoutthe mechanicalfracture during cycling[3]. Fig.S1 shows the sample prepared from contrast experiment without using tartaric acid,which is consisted of disordered nanoparticles.So,it was reasonable to believe that the tartaric acid played an important role in constructing the clew-like ZnCo2O4microspheres.Fig.3d showed the HRTEM image.The measured d-spacing of lattice fringe was 0.461 nm, corresponding to the(111)plane of cubic spinel ZnCo2O4crystals.
Fig.3c shows the N2adsorption-desorption isotherms at 77 K,and the corresponding pore size distribution calculated by Barrett-Joyne-Halenda(BJH) method from the desorption branch.The BET specific surface area of the ZnCo2O4microspheres is 8 m2·g-1, associated with a pore volume of 0.055 cm3·g-1.The pore size is ranging from 10 to 110 nm,with an average size of 46.7 nm.The porous hierarchical structure could provide a channel for electrolyte to contact with active materials completely,making sure of the effective lithium ion/electron diffusion between the solid/liquid interfaces.
The electrochemical properties of the as-synthesized ZnCo2O4microspheres were first investigated by cyclic voltammogram(CV),as shown in Fig.4.In the first cycle,the sharp cathodic peak at 0.86 V is assigned to the reduction of ZnCo2O4by Li to Zn0and Co0[3].Two broad oxidation peaks located at 1.8 V and 2.2 V in the first anodic scan are attributed to the oxidation of Zn to Zn2+and Co to Co3+,respectively.In the subsequent cycles,the reduction peaks are gradually shifted to 1.0 V and became much broader, which implies the irreversible electrochemicalreaction during the first discharge process[13,15].From second cycle forward,the CV curves are overlapped verywell,indicating the good reversible electrochemical reactions.Base on the above analysis,the lithium-ion insertion/extraction reaction with ZnCo2O4microspheres could be determined as the following equations 1~5[3]:
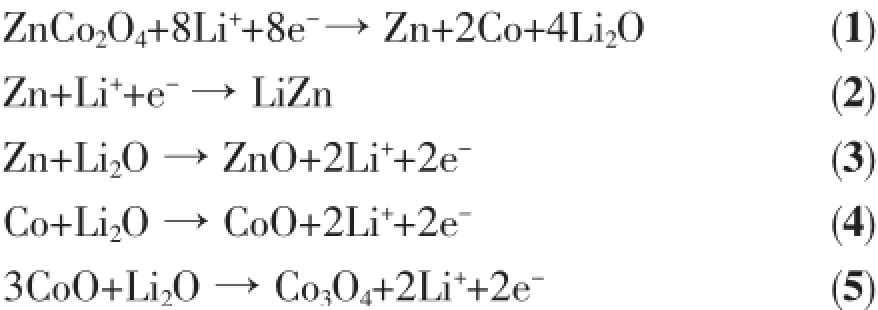
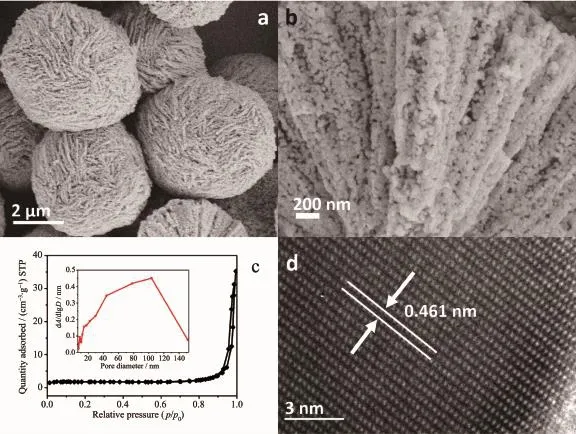
Fig.3(a)SEM image of the obtained ZnCo2O4microspheres;(b)SEM image of the chapped surface of ZnCo2O4microspheres;(c)Typical nitrogen adsorption isotherms and corresponding BJH pore size distribution; (d)HRTEM image of the as-synthesized ZnCo2O4sample
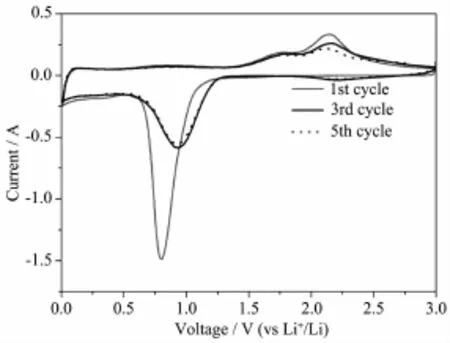
Fig.4 Cyclic voltammograms(1st,3rd and 5th cycles)of ZnCo2O4microspheres electrode
Fig.5a shows the discharge and charge profiles of ZnCo2O4microspheres ata currentdensity of0.2 A·g-1. In the first discharge curve,there was a clear potential plateau located at near 1 V(vs Li+/Li),and the overall specific capacity was as high as 1 260 mAh·g-1,the initial charge capacity is 832 mAh·g-1. The large irreversible capacity is caused by the irreversible equation 1 and the formation of unstable solid electrolyte interphase(SEI).From the second cycle onward,the long potential plateau was replaced by a sloping discharge curves,which is similar to previous reports[13-14].Noteworthy,the charge/discharge curves are overlapped well for the second and third cycles.
Fig.5b exhibits the cycling behavior of the ZnCo2O4microspheres ata currentdensity of0.5 A·g-1and the corresponding coulombic efficiency.At a current density of 0.5 A·g-1,the electrode delivers a reversible capacity of 965 mAh·g-1over 200 cycles, which was further higher than that of graphite(372 mAh·g-1).The coulombic efficiency increases from68%for the first cycle up to 90%for the second cycle,and then maintains at about 98%for the subsequentcycles.
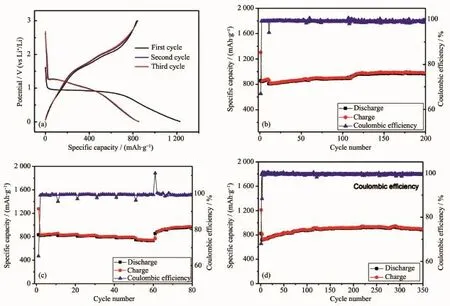
Fig.5(a)Typical charge-discharge curves for selected cycles of ZnCo2O4microspheres at 0.2 A·g-1;Cycling performances at different current densities:(b)0.5 A·g-1,(d)0.8 A·g-1;(c)Rate performance of the ZnCo2O4microspheres
Fig.5c shows the rate performance at a current density ranging from 0.2 to 2.0 A·g-1.The ZnCo2O4microspheres deliver the reversible capacity of 846, 823,810,800,786,and 736 mAh·g-1at the current density of 0.2,0.4,0.6,0.8,1.0 and 2.0 A·g-1, respectively.As the current density is returned back to 0.2 A·g-1,the specific capacity of 980 mAh·g-1is retained,indicating fine rate performance.
At a higher current density of 0.8 A·g-1,the ZnCo2O4microspheres shows a reversible capacity of 882 mAh·g-1after 350 cycles,as shown in Fig.5d. The slight capacity increase was common for several mental oxides anode materials,which could be explained by the activation process of inner metal oxides electrode during cycling and the reversible growth of polymeric gel-like films caused by kinetically activated electrolyte degradation[3-4,6,14].The columbic efficiency is able to reach above 98%after two cycles,which was important for practical application.
Electrochemical impedance spectroscopy(EIS)of the ZnCo2O4microspheres electrode was measured before cycling and after 10 cycles.Fig.6 shows the Nyquist plots.A semicircle in the high-frequency range was assigned to the charge transfer impedance in the electrode/electrolyte interface,and an inclined line in the low-frequency range was corresponding to the Li-ion diffusion process[5].Remarkably,the surface layer resistance was increased significantly after 10 cycles,which was mainly caused by the formation of SEI film[16].Fig.7 displays SEM image of the cycled ZnCo2O4microspheres electrode.It was clearly to find that the diameter of these microspheres increased up to over 4μm,but still maintained their initial structure integrity,which was significant to improve the cycling stability ofthe electrode.
The improved electrochemical performance such as high specific capacity,good cycling stability and rate capability ofthe as-synthesized clew-like ZnCo2O4microspheres should be ascribed to the multiadvantageous structure of the ZnCo2O4.First,primaryZnCo2O4nanoparticles could effectively release the strain induced by volume changes,thus preventing the active materials from cracking.Second,the clew-like hierarchical microspheres have stable mechanical skeleton,which prevent the active materials pulverization during lithium-ion insertion/extraction process.Whats more,a large amount of nano-sized pores throughout the microspheres would not only offer diffusion channels for fast electric and ionic diffusion,but also provide void space to buffer volume variation.
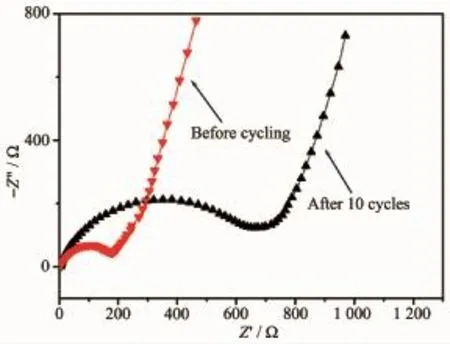
Fig.6 EIS of ZnCo2O4microspheres electrode before and after cycling

Fig.7 SEM image of ZnCo2O4microspheres electrode after 200 cycles
3 Conclusions
In summary,hierarchical clew-like ZnCo2O4microspheres composed of interconnected uniform nanoparticles was synthesized through a simple method,which is easily to scale up.As an anode for rechargeable lithium-ion batteries,the obtained clewlike ZnCo2O4microspheres deliver reversible capacity of 965 mAh·g-1at a currentdensity of 0.5 A·g-1after 200 cycles.At a higher current density of 0.8 A·g-1, the ZnCo2O4microspheres still deliver a reversible capacity of 882 mAh·g-1even over 350 cycles.The superior electrochemical performance such as high specific capacity,good cycling stability and rate capability is attributed to the unique architecture. This study paves a way to explore the promising anode material for next-generation rechargeable lithium-ion batteries.
Supporting information is available athttp://www.wjhxxb.cn
References:
[1]Park J,Moon W G,Kim G P,et al.Electrochim.Acta,2013, 105:110-114
[2]Zhang X X,Xie Q S,Yue G H,et al.Electrochim.Acta, 2013,111:746-75
[3]Sharma Y,Sharma N,Subba Rao G V,et al.Adv.Funct. Mater.,2007,17(15):2855-2861
[4]Liu B,Zhang J,Wang X F,et al.Nano Lett.,2012,12(6): 3005-3011
[5]Liu H W,Wang J.Electrochim.Acta,2013,92:371-375
[6]Luo W,Hu X L,Sun Y M,et al.J.Mater.Chem.,2012,22 (18):8916-8921
[7]Qiu Y C,Xu G L,Yan K Y,et al.J.Mater.Chem.,2011,21 (17):6346-6353
[8]Du N,Xu Y F,Zhang H,et al.Inorg.Chem.,2011,50(8): 3320-3324
[9]Chou S L,Wang J Z,Liu H K,et al.J.Phys.Chem.C, 2011,115(32):16220-16227
[10]Zhong K F,Zhang B,Luo S H,et al.J.Power Sources, 2011,196(16):6802-6808
[11]Yang J,Zhou X Y,Zou Y L,et al.Electrochim.Acta,2011, 56(24):8576-8581
[12]Bai J,Li X G,Liu G Z,et al.Adv.Funct.Mater.,2014,24 (20):3012-3020
[13]Li J F,Wang J Z,Wexler D,et al.J.Mater.Chem.A,2013, 1(48):15292-15299
[14]Liu B,Liu B Y,Wang Q F,et al.ACS Appl.Mater.Inter., 2013,5(20):10011-10017
[15]Reddy M V,Kenrick K Y H,Wei T Y,etal.J.Electrochem. Soc.,2011,158(12):A1423-A1430
[16]Chen C H,Liu J,Amine K.J.Power Sources,2001,96(2): 321-328
Clew-like Microspheres Composed of Uniform ZnCo2O4Nanoparticles as Anode Material for Lithium-Ion Batteries
WANG Ying*,1LIN Ning2
(1Shandong Yuhuang New Energy Technology Co.,Ltd.,Heze,Shandong 274000,China)
(2Department of chemistry,University of Science and Technology of China,Hefei 230026,China)
Clew-like microshperes,composed of ZnCo2O4nanoparticles,are synthesized by heat treatment of coprecipitated Zn and Co contained precursor at 600℃.As anode for Li ion battery,the obtained ZnCo2O4microspheres deliver a reversible capacity of 965 mAh·g-1at 0.5 A·g-1after 200 cycles,and the capacity of the ZnCo2O4microspheres still remains 882 mAh·g-1at 0.8 A·g-1over 350 cycles.The rate capability shows that a reversible capacity of736 mAh·g-1is maintained athigh currentdensity of2.0 A·g-1.
ZnCo2O4;nanoparticles;clew-like microspheres;lithium-ion batteries
TM912
A
1001-4861(2016)12-2191-07
10.11862/CJIC.2016.259
2016-06-22。收修改稿日期:2016-09-28。
*通信联系人。E-mail:yhwangying@163.com
