互营乙酸氧化菌研究进展
2016-12-14郑珍珍麻婷婷
吴 伟, 郑珍珍, 麻婷婷, 承 磊, 张 辉
(1. 中国石化集团胜利油田分公司油气开发管理中心, 山东 东营 257097; 2. 农业部沼气科学研究所 农业部农村可再生能源开发利用重点实验室, 四川 成都 610041)
互营乙酸氧化菌研究进展
吴 伟1, 郑珍珍2, 麻婷婷2, 承 磊2, 张 辉2
(1. 中国石化集团胜利油田分公司油气开发管理中心, 山东 东营 257097; 2. 农业部沼气科学研究所 农业部农村可再生能源开发利用重点实验室, 四川 成都 610041)
乙酸是沼气发酵过程中的重要中间代谢产物,可以通过乙酸裂解途径和互营乙酸氧化产甲烷途径代谢产生甲烷。文章主要综述了互营乙酸氧化菌的研究历史和最新进展,讨论了影响互营乙酸氧化产甲烷代谢的环境因素,并展望了互营乙酸氧化菌的研究趋势。
沼气发酵; 互营乙酸氧化; 产甲烷途径; 环境胁迫
沼气发酵是在厌氧条件下,由多种不同类型的细菌和古菌微生物,通过互营代谢等协同作用,将复杂生物质,如秸秆、畜禽粪便、石油烃等转化为CH4和CO2的微生物学过程(见图1)。沼气发酵过程不仅发生在沼气池等人工环境中,也普遍存在于油藏等地下缺氧环境中[1-2]。参与沼气发酵的厌氧微生物是地下油藏的主要功能微生物,在油藏生物地球化学循环和提高石油采收率中起着重要的作用[3-4]。在沼气发酵过程中,乙酸是重要的中间代谢产物。目前已知乙酸转化为甲烷可通过两种代谢途径来实施,一是乙酸裂解型产甲烷途径,乙酸营养型产甲烷烷古菌(AM)直接降解乙酸产生CH4和CO2,其中乙酸的甲基部分转化为CH4,羧基端转化为CO2。迄今已发现2个属的产甲烷古菌(Methanothrix和Methanosarcina)可以通过该途径降解乙酸产生甲烷[5]。另一种是互营乙酸氧化产甲烷途径(SAO-HM)[6],乙酸先分解为H2和CO2,再通过氢营养型产甲烷古菌(HM)转化产生CH4,这需要互营乙酸氧化菌(SAOB)和氢营养型产甲烷古菌(HM)通过互营代谢作用来完成。本文主要介绍SAOB的研究历史和国内外研究进展,以期读者对沼气发酵过程有一个全面的了解。
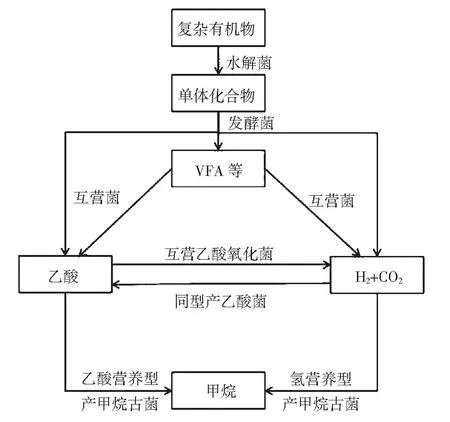
图1 沼气发酵过程的一般代谢途径
1 国内外研究现状
1.1 互营乙酸氧化菌的研究历史
1.2 互营乙酸氧化菌的热力学特征
吉布斯自由能(△G)决定了一个反应是否可以自发进行,乙酸是氧化态最高的有机物之一,其进一步发酵产生H2和CO2的反应,在标准状况下的吉普斯自由能(△G0’)为+104.6 kJ·mol-1(见表1反应2),难以自发进行。HM可以利用H2和CO2产生CH4,其△G0’为 -135.6 kJ·mol-1。当这两个反应耦联,SAOB“发酵”乙酸产生的H2,可以快速的被HM转化为CH4,整个反应体系中保持着极低的氢分压。SAOB-HM途径的总反应式是1 mol乙酸产生1 mol CH4和1 mol CO2,△G0’为-31.0 kJ·mol-1(见表1反应3),这样SAOB产生H2和CO2的反应就可以源源不断地进行。整个反应与AM直接利用乙酸产CH4的△G0’一样(见表1反应1),但是这些能量需要维持SAOB和HM这2种微生物的生命代谢活动,其能量劣势导致了SAOB的代谢速率低、生长非常缓慢[29]。
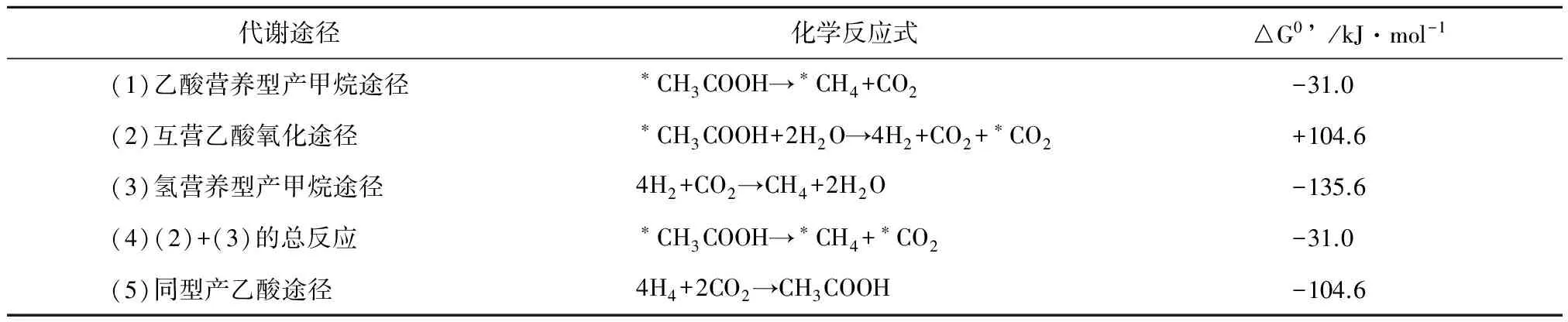
表1 乙酸降解产甲烷的代谢反应及其热力学特征[6]
注:*代表乙酸的甲基碳的流向,标准吉布斯自由能数据参考文献[30]。
1.3 互营乙酸氧化菌的微生物多样性研究
目前已分离的SAOB都是严格厌氧菌,除AOR丢失外,共有5个被鉴定为新种,它们都具有与产甲烷古菌共培养,进行互营乙酸氧化产甲烷代谢的功能(见表2)。根据文献报道,其中有5个菌是嗜热菌,最适生长温度都在55℃ ~65℃,只有ClostridiumultunenseBST是中温菌,最适生长温度为37℃[10]。AOR,ThermacetogeniumphaeumPBT和ThermotogalettingaeTMOT不仅可以互营氧化乙酸,还具有进行同型产乙酸功能[9,11-12](见表2)。多个SAOB可以利用甜菜碱、半胱氨酸、丙酮酸、葡萄糖等有机物生长,具有不同的代谢功能。SAOB生长缓慢,分离周期长,基于分离培养的传统方法难以全面认识SAOB。而基于未培养的微生物分子生态学方法和同位素示踪技术的应用,科学家发现SAOB广泛分布在人工和自然环境中。Sun[21]等发现在13个连续流搅拌(CSTR)沼气工程中,其中有10个反应器中存在各种类型的SAOB。Lee[31]等还发现厌氧消化反应器中SpirochaetesCluster II可能代表一类新的SAOB。Ito等通过RNA-SIP和MAR-FISH等证实Synergistes group 4中也含有SAOB[32]。在高氨[33-35]、高温[19-36]、高浓度挥发性脂肪酸(VFA)[37]和低水力停留时间(HRT)[38]等环境条件下,互营乙酸氧化产甲烷代谢通常是有机质降解产甲烷的主要途径。在高温水稻土中,Thermacetogenium和Thermoanaerobacteriaceae是乙酸互营氧化代谢产甲烷过程中的关键细菌类群[22-23]。在高温油藏中也发现了参与互营乙酸氧化产甲烷的细菌类群。Nazina等发现中国大港油田,Thermoanaerobacteriales、Thermotogales、Nitrospirales和Planctomycetales是参与互营乙酸氧化产甲烷的主要细菌类群[28],Mayumi发现Thermacetogenium和Methanothermobacter是日本Yabase 油藏互营乙酸氧化产甲烷的主要细菌和古菌类群[27]。Gieg[26]等推测SAO-HM途径是原油降解过程中的主要产甲烷途径。Conrad小组报道在以色列Kinneret湖底沉积物中(15℃ ~30℃),互营乙酸氧化产甲烷也是乙酸代谢的主要途径[24],但是它们后来的同位素示踪实验却发现Methanothrix是代谢乙酸产甲烷的主要功能菌[39]。这些研究表明在很多独特的生态系统中,还存在大量未分离培养的SAOB,它们在碳素生物地球化学循环中起着重要的生理生态学功能。
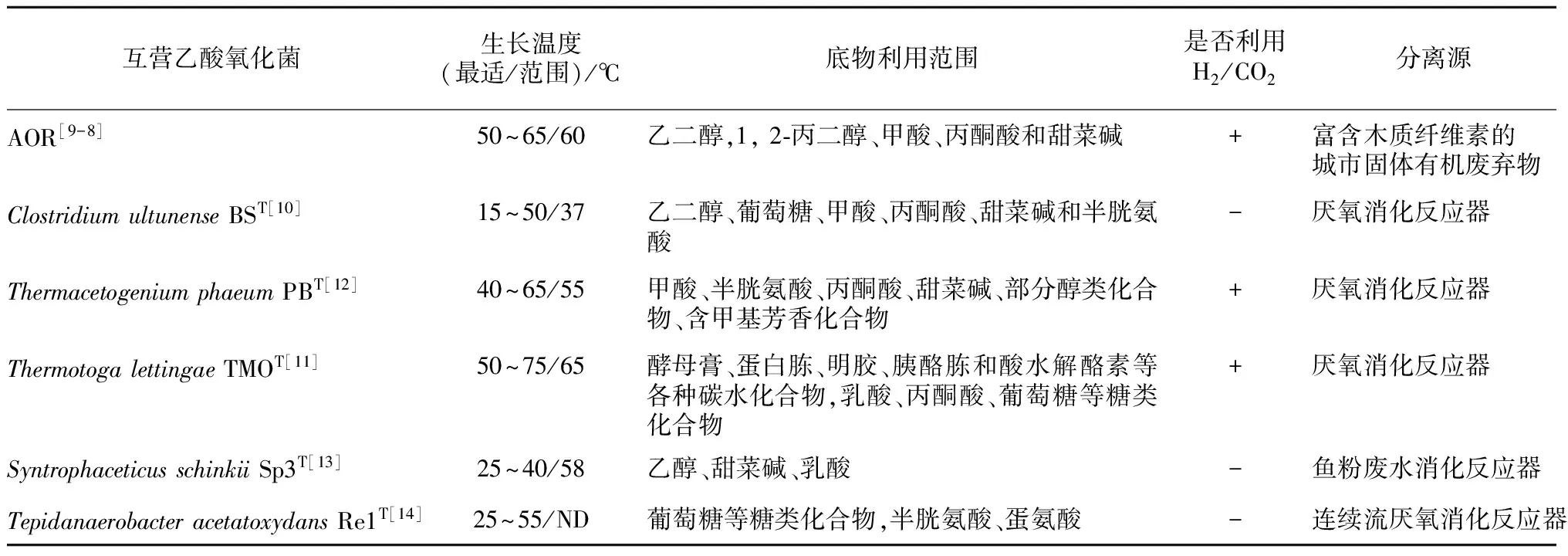
表2 互营乙酸氧化菌的纯培养物
注:ND为未测出。
1.4 影响互营乙酸氧化产甲烷代谢的环境因子

1.5 互营乙酸氧化菌的生物强化作用

2 研究展望
综上所述,通过培养和未培养方法,发现SAOB广泛参与了沼气发酵过程,并起着重要的生理生态学功能,但是迄今为止,还不清楚SAOB响应和调控乙酸代谢的分子机理。氨,温度,pH值,HRT和VFA等环境因子的改变,会影响SAOB的丰度和群落组成,但是,对SAOB应答环境胁迫的分子机制研究还非常有限,这些有待更深入去探讨。
[1] Magot M, Ollivier B, Patel B K C. Microbiology of petroleum reservoirs[J]. Antonie Leeuwenho, 2000, 77(2): 103-116.
[2] 承 磊, 仇天雷, 邓 宇, 张 辉. 油藏厌氧微生物研究进展[J]. 应用与环境生物学报, 2006, 12(5): 740-744.
[3] Mbadinga S M, Wang L Y, Zhou L, Liu J F, Gu J D, Mu B Z. Microbial communities involved in anaerobic degradation of alkanes[J]. Int Biodeterior Biodegrad, 2011, 65(1): 1-13.
[4] Xiong S, Li X, Chen J, Zhao L, Zhang H, Zhang X. Crude oil degradation by bacterial consortia under four different redox and temperature conditions[J]. Appl Microbiol Biotechnol, 2015, 99(3): 1451-1461.
[5] Oren A. The Family Methanosarcinaceae // Oren A The Prokaryotes[M]. Springer Berlin Heidelberg, 2014: 259-281.
[6] Hattori S. Syntrophic Acetate-Oxidizing Microbes in Methanogenic Environments[J]. Microbes and Environments,2008, 23(2): 118-127.
[7] Barker H A. On the biochemistry of the methane fermentation[J]. Arch Microbiol, 1936, 7(404-419).
[8] Zinder S H, Koch M. Non-aceticlastic methanogenesis from acetate: acetate oxidation by a thermophilic syntrophic coculture[J]. Arch Microbiol, 1984, 138(3): 263-272.
[9] Lee M J, Zinder S H. Isolation and characterization of a thermophilic bacterium which oxidizes acetate in syntrophic association with a methanogen and which grows acetogenically on H2-CO2[J]. Appl Environ Microbiol, 1988, 54(1): 124-129.
[10] Schnürer A, Schink B, Svensson B H. Clostridium ultunense sp. nov, a mesophilic bacterium oxidizing acetate in syntrophic association with a hydrogenotrophic methanogenic bacterium[J]. Int J Syst Bacteriol, 1996, 46(4): 1145-1152.
[11] Balk M, Weijma J, Stams A J M. Thermotoga lettingae sp. nov., a novel thermophilic, methanol-degrading bacterium isolated from a thermophilic anaerobic reactor[J]. Int J Syst Evol Microbiol, 2002, 52(4): 1361-1368.
[12] Hattori S, Kamagata Y, Hanada S, Shoun H. Thermacetogenium phaeum gen. nov., sp. nov., a strictly anaerobic, thermophilic, syntrophic acetate-oxidizing bacterium[J]. Int J Syst Evol Microbiol, 2000, 50(4): 1601-1609.
[13] Westerholm M, Roos S, Schnürer A. Syntrophaceticus schinkii gen. nov, sp. nov, an anaerobic, syntrophic acetate-oxidizing bacterium isolated from a mesophilic anaerobic filter[J]. FEMS Microbiol Lett, 2010, 309(1): 100-104.
[14] Westerholm M, Roos S, Schnürer A. Tepidanaerobacter acetatoxydans sp. nov., an anaerobic, syntrophic acetate-oxidizing bacterium isolated from two ammonium-enriched mesophilic methanogenic processes[J]. Syst Appl Microbiol, 2011, 34(4): 260-266.
[15] Hattori S, Galushko A S, Kamagata Y, Schink B. Operation of the CO Dehydrogenase/Acetyl Coenzyme A Pathway in both Acetate Oxidation and Acetate Formation by the Syntrophically Acetate-Oxidizing Bacterium Thermacetogenium phaeum[J]. J Bacteriol, 2005, 187(10): 3471-3476.
[16] Manzoor S, Bongcam-Rudloff E, Schnürer A, Müller B. First Genome Sequence of a Syntrophic Acetate-Oxidizing Bacterium, Tepidanaerobacter acetatoxydans Strain Re1[J]. Genome Announcements, 2013, 1(1).
[17] Schnürer A, Svensson B H, Schink B. Enzyme activities in and energetics of acetate metabolism by the mesophilic syntrophically acetate-oxidizing anaerobe Clostridium ultunense[J]. FEMS Microbiol Lett, 1997, 154(2): 331-336.
[18] Hao L P, Lü F, He P J, Li L, Shao L M. Predominant contribution of syntrophic acetate oxidation to thermophilic methane formation at high acetate concentrations[J]. Environ Sci Technol, 2010.
[19] Krakat N, Westphal A, Schmidt S, Scherer P. Anaerobic digestion of renewable biomass: thermophilic temperature governs methanogen population dynamics[J]. Appl Environ Microbiol, 2010, 76(6): 1842-1850.
[20] Schnürer A, Zellner G, Svensson BH. Mesophilic syntrophic acetate oxidation during methane formation in biogas reactors[J]. FEMS Microbiol Ecol, 1999, 29(3): 249-261.
[21] Sun L, Müller B, Westerholm M, Schnürer A. Syntrophic acetate oxidation in industrial CSTR biogas digesters[J]. J Biotechnol, 2014, 171: 39-44.
[22] Liu F, Conrad R. Thermoanaerobacteriaceae oxidize acetate in methanogenic rice field soil at 50℃[J]. Environ Microbiol, 2010, 12(8): 2341-2354.
[23] Rui J, Qiu Q, Lu Y. Syntrophic acetate oxidation under thermophilic methanogenic condition in Chinese paddy field soil[J]. FEMS Microbiol Ecol, 2011, 77(2): 264-273.
[24] Nusslein B, Chin K J, Eckert W, Conrad R. Evidence for anaerobic syntrophic acetate oxidation during methane production in the profundal sediment of subtropical Lake Kinneret (Israel) [J]. Environ Microbiol, 2001, 3(7): 460-470.
[25] Cheng L, Dai L, Li X, Zhang H, Lu Y. Isolation and characterization of Methanothermobacter crinale sp. nov, a novel hydrogenotrophic methanogen from Shengli Oilfields[J]. Appl Environ Microbiol, 2011, 77(15): 5212-5219.
[26] Gieg L M, Davidova I A, Duncan K E, Suflita J M. Methanogenesis, sulfate reduction and crude oil biodegradation in hot Alaskan oilfields[J]. Environ Microbiol, 2010, 12(11): 3074-3086.
[27] Mayumi D, Mochimaru H, Yoshioka H, Sakata S, Maeda H, Miyagawa Y, Ikarashi M, Takeuchi M, Kamagata Y. Evidence for syntrophic acetate oxidation coupled to hydrogenotrophic methanogenesis in the high-temperature petroleum reservoir of Yabase oil field (Japan) [J]. Environ Microbiol, 2011, 13(8): 1995-2006.
[28] Nazina T N, Shestakova N M, Grigor’yan A A, Mikhailova E M, Tourova T P, Poltaraus A B, Cingxian F, Fangtian N, Belyaev S S. Phylogenetic diversity and activity of anaerobic microorganisms of high-temperature horizons of the Dagang oil field (P. R. China) [J]. Microbiology, 2006, 75(1): 55-65.
[29] Jackson B E, McInerney M J. Anaerobic microbial metabolism can proceed close to thermodynamic limits[J]. Nature, 2002, 415(6870): 454-456.
[30] Thauer R K, Jungermann K, Decker K. Energy conservation in chemotrophic anaerobic bacteria[J]. Microbiol Mol Biol Rev, 1977, 41(1): 100-180.
[31] Lee S H, Park J H, Kim S H, Yu B J, Yoon J J, Park H D. Evidence of syntrophic acetate oxidation by Spirochaetes during anaerobic methane production[J]. Bioresour Technol, 2015, 190: 543-549.
[32] Ito T, Yoshiguchi K, Ariesyady H D, Okabe S. Identification of a novel acetate-utilizing bacterium belonging to Synergistes group 4 in anaerobic digester sludge[J]. ISME J, 2011, 5(12): 1844-1856.
[33] Schnürer A, Nordberg A. Ammonia, a selective agent for methane production by syntrophic acetate oxidation at mesophilic temperature[J]. Water Sci Technol, 2008, 57: 735-740.
[34] Wang H, Fotidis IA, Angelidaki I. Ammonia effect on hydrogenotrophic methanogens and syntrophic acetate oxidizing bacteria (DOI: 10.1093/femsec/fiv130)[J]. FEMS Microbiol Ecol, 2015.
[35] Werner J J, Garcia M L, Perkins S D, Yarasheski K E, Smith S R, Muegge B D, Stadermann F J, DeRito C M, Floss C, Madsen E L, Gordon J I, Angenent L T. Microbial Community Dynamics and Stability during an Ammonia-Induced Shift to Syntrophic Acetate Oxidation[J]. Appl Environ Microbiol, 2014, 80(11): 3375-3383.
[36] Tang Y Q, Matsui T, Morimura S, Wu X L, Kida K. Effect of temperature on microbial community of a glucose-degrading methanogenic consortium under hyperthermophilic chemostat cultivation[J]. J Biosci Bioeng, 2008, 106(2): 180-187.
[37] Hao L P, Lü F, He P J, Li L, Shao L M. Predominant contribution of syntrophic acetate oxidation to thermophilic methane formation at high acetate concentrations[J]. Environ Sci Technol, 2011, 45(2): 508-513.
[38] Shigematsu T, Tang Y, Kobayashi T, Kawaguchi H, Morimura S, Kida K. Effect of Dilution Rate on Metabolic Pathway Shift between Aceticlastic and Nonaceticlastic Methanogenesis in Chemostat Cultivation[J]. Appl Environ Microbiol, 2004, 70(7): 4048-4052.
[39] Schwarz J I K, Lueders T, Eckert W, Conrad R. Identification of acetate-utilizing Bacteria and Archaea in methanogenic profundal sediments of Lake Kinneret (Israel) by stable isotope probing of rRNA[J]. Environ Microbiol, 2007, 9(1): 223-237.
[40] Petersen S, Ahring B K. Acetate oxidation in a thermophilic anaerobic sewage-sludge digestor: the importance of non-aceticlastic methanogenesis from acetate. FEMS Microbiol[J]. Lett, 1991, 86(2): 149-158.
[41] Hao L P, Lü F, Li L, Shao L M, He P J. Shift of pathways during initiation of thermophilic methanogenesis at different initial pH[J]. Bioresour Technol, 2012, 126: 418-424.
[42] Shigematsu T, Tang Y, Kawaguchi H, Ninomiya K, Kijima J, Kobayashi T, Morimura S, Kida K. Effect of dilution rate on structure of a mesophilic acetate-degrading methanogenic community during continuous cultivation[J]. J Biosci Bioeng, 2003, 96(6): 547-558.
[43] de Baere L A, Devocht M, Van Assche P, Verstraete W. Influence of high NaCl and NH4Cl salt levels on methanogenic associations[J]. Water Res, 1984, 18(5): 543-548.
[44] Chen Y, Cheng J J, Creamer K S. Inhibition of anaerobic digestion process: A review[J]. Bioresour Technol, 2008, 99(10): 4044-4064.
[45] Jarrell K F, Saulnier M, Ley A. Inhibition of methanogenesis in pure cultures by ammonia, fatty acids, and heavy metals, and protection against heavy metal toxicity by sewage sludge[J]. Can J Microbiol, 1987, 33(6): 551-554.
[46] Westerholm M, Levén L, Schnürer A. Bioaugmentation of syntrophic acetate-oxidising culture in biogas reactors exposed to increasing levels of ammonia[J]. Appl Environ Microbiol, 2012, 78(21): 7619-7625.
[47] Fotidis I A, Karakashev D, Angelidaki I. Bioaugmentation with an acetate-oxidising consortium as a tool to tackle ammonia inhibition of anaerobic digestion. Bioresour[J]. Technol, 2013, 146: 57-62.
[48] Fotidis I A, Wang H, Fiedel N R, Luo G, Karakashev DB, Angelidaki I. Bioaugmentation as a Solution To Increase Methane Production from an Ammonia-Rich Substrate[J]. Environ Sci Technol, 2014, 48(13): 7669-7676.
[49] Cavaleiro A J, Sousa D Z, Alves M M. Methane production from oleate: Assessing the bioaugmentation potential of Syntrophomonas zehnderi[J]. Water Res, 2010, 44(17): 4940-4947.
Recent Advances on Syntrophic Acetate Oxidation Bacteria /
WU Wei1, ZHENG Zhen-zhen2, MA Ting-ting2, CHENG Lei2, ZHANG Hui2/
(1. Center for oil and gas development of Shengli Oifield Company, China Petroleum & Chemical Corporation, Dongying 257091,China; 2. Biogas Institute of Ministry of Agriculture, Key Laboratory of Development and Application of Rural Renewable Energy of Ministry of Agriculture, Chengdu 610041, China )
Acetate is an important intermediate during biogas fermentation, which could be converted into methane through acetoclastic methanogenesis and syntrophic acetate oxidation coupled with hydrogenotrophic methanogenesis. This paper mainly reviewed the history and progress of research on syntrophic acetate oxidation bacteria, and discussed the environmental factors affecting the pathway of syntrophic acetate oxidation coupled hydrogenotrophic methanogenesis. And the further study was prospected.
Biogas fermentation; syntrophic acetate oxidation; methanogenic pathway; environmental stress
2015-12-20
项目来源: 国家高技术研究发展计划(2013aa064401); 中国农业科学院基本科研业务费 (2013ZL001); 微生物资源前期开发国家重点实验室项目(SKLMR-20150605)
吴 伟(1968- ),男,四川简阳人,高级工程师,主要研究方向为提高石油采收率技术,E-mail: wuwei656.slyt@sinopec
张 辉,E-mail: zhanghuits@aliyun.com
S216.4;X172
A
1000-1166(2016)02-0003-06
