Caspase-1 inhibition attenuates activation of BV2 microglia induced by LPS-treated RAW264.7 macrophages
2016-12-13YangPanBoShenQinGaoJunZhuJingdeDongLiZhangYingdongZhang
Yang Pan, Bo Shen, Qin Gao, Jun Zhu, Jingde Dong, Li Zhang,✉, Yingdong Zhang,✉
1Department of Neurology, Nanjing First Hospital affiliated to Nanjing Medical University, Nanjing, Jiangsu 210006, China;
2Department of Geriatrics, Nanjing Brain Hospital affiliated to Nanjing Medical University, Nanjing, Jiangsu 210029, China.
Caspase-1 inhibition attenuates activation of BV2 microglia induced by LPS-treated RAW264.7 macrophages
Yang Pan1,2, Bo Shen2, Qin Gao1, Jun Zhu2, Jingde Dong2, Li Zhang2,✉, Yingdong Zhang1,✉
1Department of Neurology, Nanjing First Hospital affiliated to Nanjing Medical University, Nanjing, Jiangsu 210006, China;
2Department of Geriatrics, Nanjing Brain Hospital affiliated to Nanjing Medical University, Nanjing, Jiangsu 210029, China.
Neuroinflammation has been recognized as a factor in the pathogenesis of neurodegenerative diseases. Emerging evidence suggests that peripheral inflammation, besides neuroinflammation, functions as a modulator of disease progression and neuropathology in several neurodegenerative diseases. However, detailed correlations among peripheral inflammation, neuroinflammation and neurodegeneration remain unknown. In the present study, we prepared a peripheral inflammation model with lipopolysaccharides (LPS)-stimulated RAW264.7 macrophages to explore its activation on BV2 microglia. We found that LPS induced the production of IL-1β, IL-6 and TNF-α in the culture medium of RAW264.7 macrophages. We further showed that LPS plus ATP activated inflammasome, evidenced by the upregulation of caspase-1 and IL-1β, which was suppressed by ZYVAD, a caspase-1 inhibitor. Furthermore, the conditioned medium obtained from LPS-treated RAW264.7 macrophages activated BV2 microglia, stimulating the release of IL-1β, IL-6 and TNF-α from BV2 cells. ZYVAD pretreatment markedly suppressed BV2 microglia activation induced by RAW264.7 cells conditioned medium. Taken together, our study indicates that macrophage-mediated peripheral inflammation subsequently evokes neuroinflammation and may aggravate neural damage. Inflammasome and caspase-1 may be potential targets for modulating systemic inflammatory responses in neurodegenerative diseases.
peripheral inflammation, neuroinflammation, neurodegenerative diseases, NLRP3 inflammasome, caspase-1
Introduction
The World Health Organization (WHO) predicts that over 70 million people will be living with neurodegenerative diseases in 2030[1]. Neurodegenerative diseases are a collection of interconnected disorders such as Alzheimer's disease (AD), Parkinson's disease (PD), genetic disorders, and a few others. The explosion of neurodegenerative diseases has become a global health concern and has created a huge burdenon economies and society. Disability from such diseases is primarily caused by death and dysfunction of neurons[2]. Although neurodegenerative diseases have been intensively investigated in the last two decades, their precise etiology is still unclear. So far researchers have focused on chronic activation of neuroinflammation, including those mediated by microglia and resident CNS macrophages which are traditionally recognized to increase the risk of neurodegeneration[3].
Currently, a cluster of peripheral inflammation factors are recognized to increase the risk of neurodegeneration. Holmes et al. showed that an increased rate of cognitive decline in AD patients correlates with high level of peripheral proinflammatory cytokines[4]. Similarly, respiratory infections have been linked to a more frequent cause of death in PD patients than in the general population[5,6]. These facts indicated a direct link between systemic cytokine levels and neuropathology[7]. Increased serum peripheral cytokine levels due to systemic inflammation have been associated with AD and PD, suggesting a link between systemic inflammation and neurodegeneration[8]. However, the detailed mechanism underlying the association of peripheral inflammation and neurodegeneration remains unknown. Peripheral inflammation has functioned as a regulator of neuropathology in several neurodegenerative diseases, which has emerged as critical target in new therapeutic method[9].
Some studies showed systemic inflammation induced by obesity and type 2 diabetes (T2D) has a close relationship with neuroinflammation, and an important systemic inflammatory mediator is the I kappa B kinase (IKK) β/ nuclear factor kappa B (NF-κB) pathway, which controls cell survival and apoptosis[10]. NF-κB regulates various inflammatory mediators and plays an important role in inflammatory responses[11-12]. The most inflammatory reactions are mediated by proinflammatory cytokines, which are regulated in part by NF-κB. In non-stimulated cells, NF-κB is located in the cytosol[13-15]. NF-κB is free and enters the nucleus and binds to the promoter region of genes such as interleukin (IL)-1β, tumor necrosis factor-alpha (TNF-α), IL-6, and IL-18, which are associated with the development of neurodegenerative diseases[16-18]. In recent decades, inflammasome has been recognized to be a component of the inflammatory process and its aberrant activation is shown to be upregulated in LPS-induced systemic inflammation[19]. The upregulation of mature caspase-1 is the premise of the activation of inflammasome. Activated caspase-1 is required for IL-1β and IL-18 release and plays a key role in inflammation. Caspase-1 is an endogenous cysteine protease synthesized as inactive pro-caspase-1 and activated by dimerization and autoproteolysis within multiprotein complexes including the ASC and NLRP3 inflammasome[20-21]. NLRP3, as the highest expression of inflammasome in macrophages and microglia and recognized as a component of the inflammatory process and its aberrant activation, is pathogenic in metabolic diseases and neurodegenarative diseases[22]. It is one of the best studied inflammasome, so we focused on the NLRP3 inflammasome in our study. Among these responses, inflammasome has been shown to have important functions in systemic inflammation, yet the precise mechanism of NLRP3 inflammasome action in neuroinflammation is not clear.
In the current study, we prepared a peripheral inflammation model with lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages to explore its activation on BV2 microglia. Our study indicates that macrophage-mediated peripheral inflammation can evoke neuroinflammation and subsequently aggravate neural damage. Inflammasome and caspase-1 may be potential targets for modulating systemic inflammatory responses in neurodegenerative diseases.
Materials and methods
Reagents
LPS (Escherichia coli O55:B5) was purchased from Beyotime Institute of Biotechnology, Nanjing, China. Dulbecco's modified eagle medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco BRL Co., Ltd., Grand Island, NY, USA. Mouse IL-1β, IL-6, and TNF-α enzyme-linked immunosorbent assay (ELISA) kits were purchased from Biolegend, San Diego, CA, USA. Primary antibodies (against T-IKKβ, P-IKKβ, P65, NLRP3, caspase-1), internal control (GAPDH, Lamin B) and horseradish peroxidaseconjugated anti-rabbit antibodies were all purchased from Cell Signaling Technology, Beverly, MA, USA.
Cell culture
RAW264.7 macrophages and BV2 micro glia were obtained from Shanghai Cell Bank of Chinese Academy of Sciences. Cells were all cultured in DMEM (with 10% FBS, 100 U/mL penicillin and 100 U/mL streptomycin), which were grown in a 37°C humidified incubator with 5% CO2.
Cell treatment
Murine RAW264.7 macrophages were treated with LPS (200 ng/mL) for 1 hour followed by ATP (5 mmol/L) incubation for 30 minutes, and after removal of the medium, washed the cells 2-3 times with sterile PBS. Subsequently, the RAW264.7 cells were treated with the fresh medium for 24 hours. We collected the RAW264.7 cell culture supernatants to induce the activation of BV2 cells for another 24 hours. RAW264.7cells and BV2 cells were pretreated with ZYVAD (10 μmol/L) for 1 hour before LPS or conditioned medium incubation, respectively.
ELISA
After incubation with ZYVAD for 1 hour, the RAW 264.7 cells were subsequently induced with LPS (100 ng/mL) and/or ATP for 24 hours[9]. We obtained the RAW264.7 cell culture supernatants in order to (i) perform cytokine analysis and (ii) induce the activation of BV2 microglia. After incubation by RAW264.7 conditioned media obtained from LPS+ATP-stimulated cells, we get the BV2 cell supernatants for further cytokine analysis. The expression of IL-6, IL-1β, and TNF-α in supernatants were examined with their respective ELISA kits according to the manufacturer's instructions. Then, the microplate spectrophotometer was used to test the absorbance of each well at 450 nm.
Preparation of cytosolic and nuclear fractions
RAW264.7 macrophages and BV2 microglia were homogenized in PER-Mammalian Protein Extraction Buffer (1: 20, W:V) (Pierce Biotechnology, Rockford, IL, USA) containing freshly added protease inhibitor cocktail I (EMD Biosciences, San Diego, CA, USA) and 1 mmol/L phenylmethanesulfonyl fluoride (PMSF). The cytosolic fraction of the cells was prepared by centrifugation at 15,000 g for 10 minutes at 4°C. Nuclear and cytoplasmic extracts of cells were prepared using NE-PER nuclear and cytoplasmic extraction reagents (Pierce Biotechnology), respectively.
Western blotting analysis
Firstly, a total of 1 × 106RAW264.7 macrophages per well were grown in 12-well plates and pre-treated with ZYVAD for 1 hour, then incubated with LPS (1 μg/mL) and/or ATP for 24 hours, or untreated (control). Secondly, a total of 1 × 106BV2 microglia per well were grown in 12-well plates and incubated in RAW264.7 conditioned media obtained from LPS+ATP-stimulated cells for 24 hours, or untreated (control). Then, the RAW264.7 and BV2 cells were obtained on ice, washed once with ice-cold PBS, and lysed by Lysis buffer with phos phatase and protease inhibitors obtained from Sangon Biotech of China. 30 minutes after the lysis, cellular extracts were centrifuged in a refrigerated centrifuge (5418 R, Eppendorf, Germany) at 4°C for 10 minutes. Then, a BCA pro tein assay kit (Biomiga, USA) was employed to record the amount of collected total proteins. 10% SDS-PAGE was used to separate proteins. These proteins were electro-transferred to PVDF membranes (Millipore, USA), which were blocked with 5% (W/V) dried skimmed milk for 1 hour. To probe corresponding target proteins, these proteins were incubated with anti-GAPDH, anti-P65, anti-NLRP3, anti-caspase-1, anti-IKKβ, and anti-phospho-IKKβ. Peroxidase-conjugated secondary antibodies were used to detect bound antibodies, of which the amount was assessed by enhanced chemilumi nescence (ECL). Relative expression of target proteins were qualified via the optical density of electrophoresis bands with GAPDH as an internal control.
Immunofluorescent staining
The cells grown in two-well chamber slides were fixed and permeabilized as previously described[32]. The cells were incubated with rabbit polyclonal anti-P65 antibody diluted 1:100 in 1% BSA for 30 minutes. The cells were incubated with rhodamine isothiocyanate-conjugated goat anti-rabbit immunoglobulin G antibody diluted 1:100 in 1% BSA for 30 minutes. After mounting with 50% glycerol, the slides were analyzed with a fluorescence light microscope.
Statistical analysis
Data are all expressed as the mean (SD) of 3 independent experiments. Least significant difference (LSD) tests were used to determine the statistical difference between two groups. P-values less than 0.05 were considered representing significant differences between means.
Results
Effects of ZYVAD on proinflammatory cytokines in activated RAW264.7 cells
Proinflammatory cytokines are essential mediators in regulating host responses to inflammation. To further determine the anti-inflammatory effect of ZYVAD on proinflammatory cytokines in RAW264.7 cells stimulated by LPS and ATP, we investigated the productions of TNF-α, IL-1β and IL-6 from activated RAW264.7 cells treating with LPS plus ATP. As expected, ELISA revealed that the levels of proinflammatory cytokines increased significantly upon LPS and ATP treatment (Fig. 1). Furthermore, these culture supernatants were pre-treated with ZYVAD for 1 hour. The results indicated that ZYVAD inhibited the expression of proinflammatory cytokines IL-1β, IL-6 and TNF-α in activated RAW264.7 cells (Fig. 1).
Effects of ZYVAD on NF-kB signaling in activated RAW264.7 cells
The NF-κB signaling pathway functions as a modulator in regulating the level of proinflammatory cytokines in activated RAW264.7 cells. Western blotting assaysshowed that dual stimulation with LPS and ATP had a synergistic effect on NF-κB signaling (Fig. 2). To further explain the mechanisms underlying the inhibition of NF-κB signaling pathway overexpression in RAW264.7 cells by ZYVAD, we used Western blotting to examine the phosphorylation of IKKβ and P65 and total IKKβ. The levels of phospho-IKKβ and P65 were significantly enhanced after cells were challenged with LPS and ATP for 24 hours. Treatment with ZYVAD apparently inhibited the levels of phospho-IKKβ and P65 (Fig. 2). However, ZYVAD had no obvious effect on the levels of total IKKβ and P65.
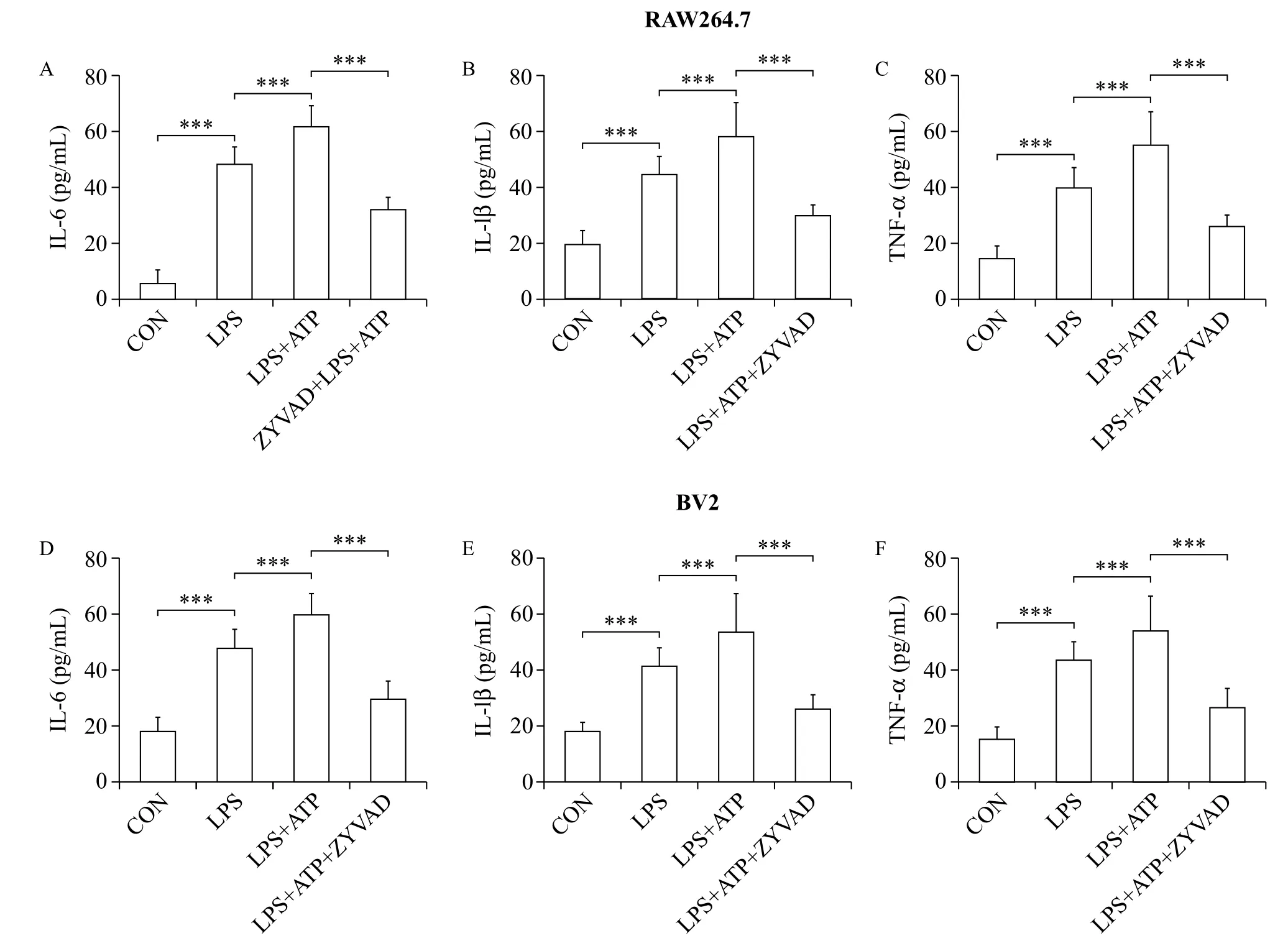
Fig. 1 ZYVAD suppresses proinflammatory cytokines IL-1β, IL-6, and TNF-α in RAW264.7 cells treated with LPS+ATP and BV2 microglia induced by activated peripheral RAW264.7 macrophages. A, D: ZYVAD reduced IL-1β overproduction in LPS-stimulated RAW264.7 macrophages and BV2 microglia induced by activated peripheral RAW264.7 macrophages. B, E: ZYVAD reduced IL-6 overproduction in LPS-stimulated RAW264.7 macrophages and BV2 microglia induced by activated peripheral RAW264.7 macrophages. C, F: ZYVAD reduced TNF-α overproduction in LPS-stimulated RAW264.7 macrophages and BV2 microglia induced by activated peripheral RAW264.7 macrophages. Data are all presented as the mean (SD). LSD tests were used to determine the statistical difference between two groups. The P-values show the statistical differences between each group and the corresponding positive control (without ZYVAD and with LPS and/or ATP treated). All P-values are less than 0.001. CON: control; LPS: lipopolysaccharides.
Effects of ZYVAD on the NLRP3 inflammasome in activated RAW264.7 cells
Similarly, to elucidate whether the inhibition of NLRP3 inflammasome was related with ZYVAD, we further explored the level of NLRP3 and caspase-1 in RAW264.7 cells (Fig. 2). Western blot analysis showed that treatment with ZYVAD and then co-treatment with LPS and ATP further induced the inhibition of NLRP3 and caspase-1 (Fig. 2).
Activation of BV2 microglia by conditioned medium from activated RAW264.7 macrophages
Overt activation of RAW264.7 macrophages may induce the activation of BV2 microglia and we examined this possibility by adding the culture supernatants obtained from RAW264.7 macrophages culture to BV2 microglia (Fig. 3). Although addition of RAW264.7 conditioned culture media itself did not induce significant activation of BV2 microglia, RAW264.7 conditioned media obtained from LPS+ATP-stimulated cells induced significantupregulation of proinflammatory cytokines, the NF-κB signaling pathway and NLRP3 inflammasome in BV2 cells (Fig. 1 and Fig. 3). The expression of proinflammatory cytokines IL-1β, IL-6 and TNF-α in the BV2 microglia treated with RAW264.7 conditioned media obtained from LPS+ATP-stimulated cells was higher than that in those stimulated with LPS alone, as shown by ELISA (Fig. 1). At the same time, RAW264.7 conditioned media obtained from dual stimulation with LPS and ATP had a synergistic effect on the expression of the NF-κB signaling pathway by Western blot analysis (Fig. 3). Consequently, we explored the expression of NLRP3 inflammasome in BV2 cells (Fig. 3) by western blot analysis. The results showed that RAW264.7 conditioned media co-treated with LPS and ATP further induced the overexpression of NLRP3 and caspase-1 compared with treatment with LPS alone (Fig. 3). Indirect immunofluorescence and confocal microscopy indicated nuclear translocation of NF-κB, while P65 mostly translocated into the nucleus treated with LPS and ATP (Fig. 4). These results suggest that synergistic activation of RAW264.7 macrophages by LPS and ATP in an inflammation-challenged condition may adversely affect BV2 microglia.
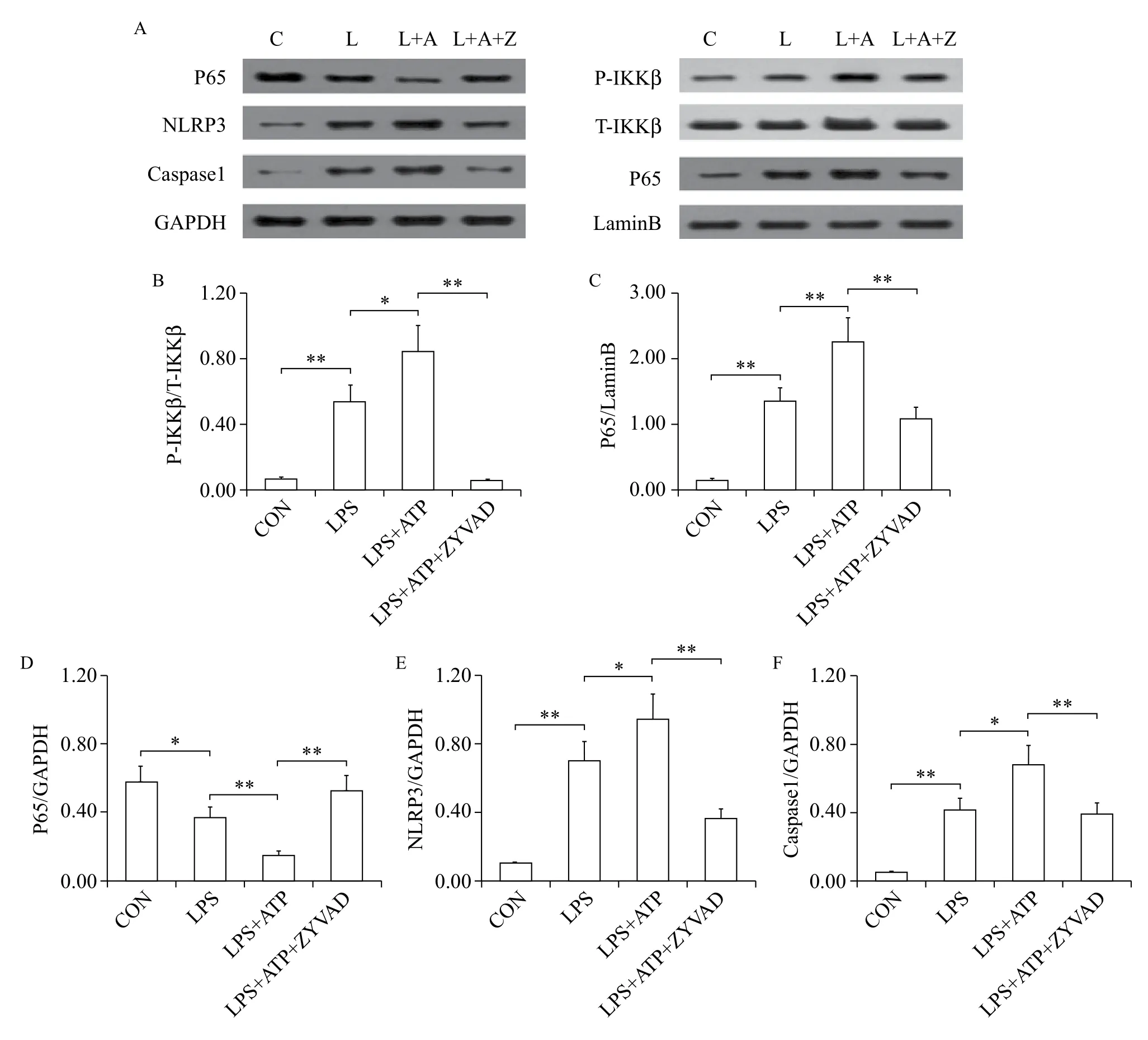
Fig. 2 ZYVAD suppresses NF-κB signaling and the NLRP3 inflammasome in BV2 microglia induced by activated peripheral RAW264.7 macrophages. A-D: ZYVAD suppresses NF-κB signaling activation in BV2 microglia induced by activated peripheral RAW264.7 macrophages. A, E, F: ZYVAD induces the overexpression of NLRP3, caspase-1 in BV2 microglia induced by activated peripheral RAW264.7 macrophages. Data are all presented as the mean (SD). LSD tests were used to determine the statistical difference between two groups. * means P < 0.05 and ** means P < 0.01.
ZYVAD significantly inhibits production of proinflammatory cytokines in activated BV2 cells
To further evaluate the anti-inflammatory effect of ZYVAD on BV2 microglia activation by adding the culture supernatants obtained from activated RAW264.7macrophage culture, we investigated the productions of proinflammatory cytokines TNF-α, IL-1β and IL-6 in BV2 cells. As expected, the levels of proinflammatory cytokines increased obviously with LPS and ATP (Fig. 1). Whereas these supernatants were pre-treatment with ZYVAD, the results showed that ZYVAD inhibited the syn thesis and expression of proinflammatory cytokines IL-1β, IL-6 and TNF-α in BV2 cells stimulated RAW264.7 conditioned media obtained from dual stimulation with LPS and ATP (Fig. 1).

Fig. 3 ZYVAD suppresses NF-κB signaling pathway, NLRP3 inflammasome in RAW264.7 cells treated with LPS+ATP. A-D: ZYVAD supressed NF-κB signaling pathway activation in LPS-stimulated RAW264.7 macrophages. A, E, F: ZYVAD induced the overexpression of NLRP3, caspase-1 in LPS-stimulated RAW264.7 macrophages. Data are all presented as the mean (SD). LSD tests were used to determine the statistical difference between two groups. *, ** and *** indicate P < 0.05, P < 0.01 and P < 0.001, respectively.
ZYVAD significantly inhibits NF-kB signaling in activated BV2 cells
To further explain the mechanisms underlying inhibition of NF-κB signaling in activated BV2 cells by ZYVAD, we used Western blotting assays to examine the phosphorylation of phospho-IKKβ and P65 and the production of corresponding total IKKβ. The levels of phospho-IKKβ and P65 were significantly enhanced after cells were challenged with RAW264.7 conditioned media obtained from dual stimulation with LPS and ATP. Treatment with ZYVAD apparently inhibited the overexpression of phospho-IKKβ and P65 (Fig. 3). Therefore, data from confocal microscopy indicated that the nuclear translocation of NF-κB was significantly blocked in BV2 cells treated with ZYVAD (Fig. 4), while P65 mostly translocated into the nucleus with RAW264.7 conditioned media obtained from dual stimulation with LPS and ATP.
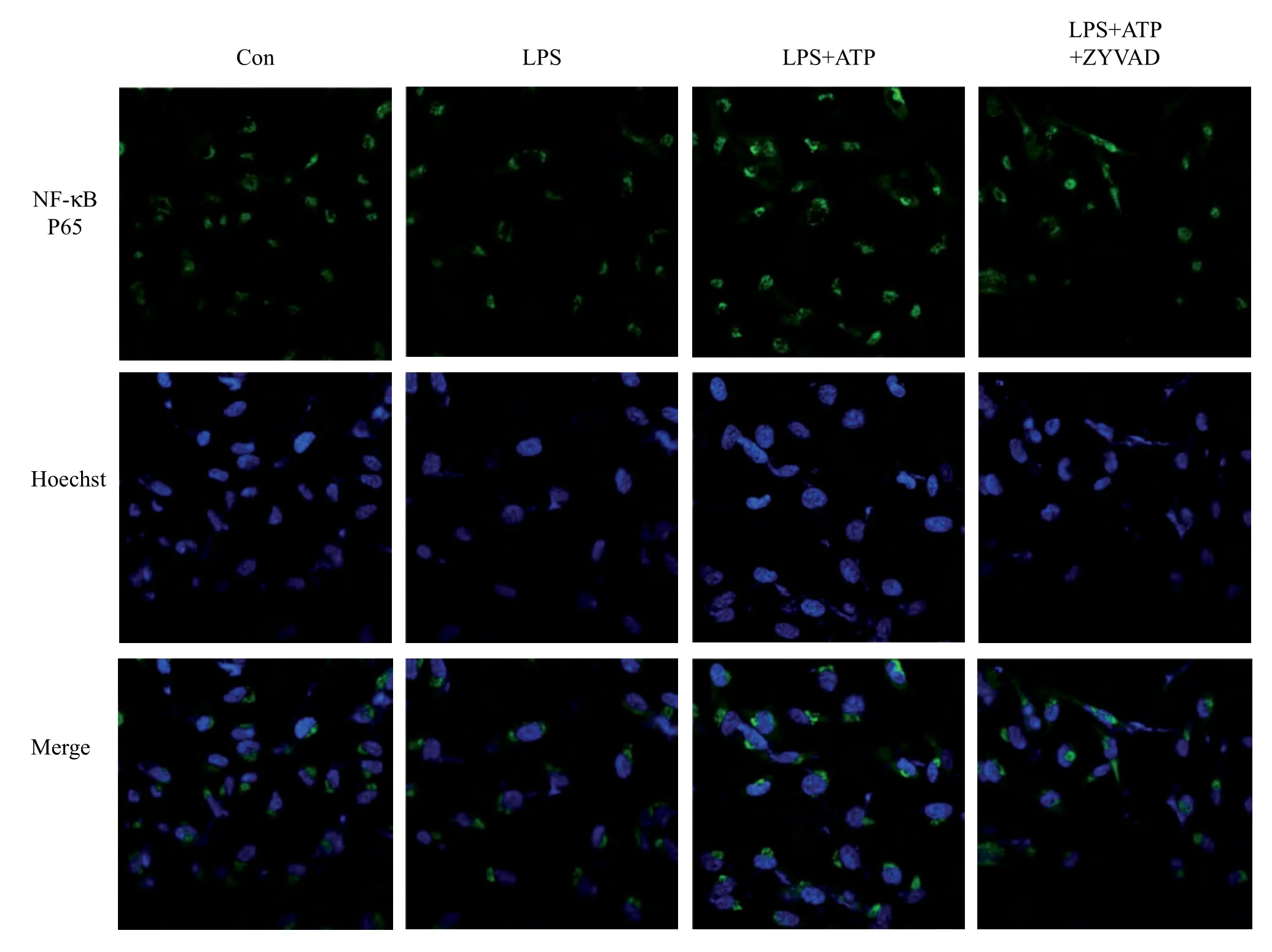
Fig. 4 Immunofluorescence and laser scanning confocal microscopy examining the effects of ZYVAD on the localization of NF-κB in BV2 cells. Con: control; LPS: BV2 cells were pretreated with RAW264.7 conditioned media obtained from pre-treatment with LPS for 24h; LPS+ATP: BV2 cells were pretreated with RAW264.7 conditioned media obtained from pre-treatment with LPS plus ATP; LPS+ATP+ZYVAD: BV2 cells were pretreated with RAW264.7 conditioned media obtained from pre-treatment with ZYVAD followed by dual stimulation with LPS and ATP. NF-κB p65, Hoechst and merged images show the translocation of p65 into the nucleus. Images were acquired using confocal laser scanning microscopy (× 400).
ZYVAD significantly inhibits the NLRP3 inflammasome in activated BV2 cells
To elucidate whether inhibition of NLRP3 inflammasome was related with ZYVAD, we further explored the level of NLRP3 and caspase-1 in BV2 cells. Western blot analysis showed that treatment with ZYVAD and then stimulated RAW264.7 conditioned media obtained from co-treatment with LPS and ATP further induced the inhibition of NLRP3 and caspase-1 (Fig. 3).
Discussion
In recent decades, neuroinflammation has been implicated in the pathogenesis of neurodegenerative diseases[23]. Emerging evidence suggests that peripheral inflammation, apart from neuroinflammation, functions as a modulator of disease progression and neuropathology in several neurodegenerative diseases[24-25]. However, correlations among peripheral inflammation, neuroinflammation and neurodegeneration remain unknown. Proinflammatory mediators can re-activate or further stimulate previously activated central microglia, which are the main inflammatory cells of the CNS and are important in response to CNS damage[26]. The activated micro glia produc es high levels of inflammatory cytokines mediated through the NF-κB signaling pathway[27]. The blood brain barrier restricts access of larger mole cules into the brain[28]. Nonetheless, there are a number of potential mechanisms whereby peripheral proinflammatory cells can modulate neuronal function. The inflammatory mediators produced in the periphery can injury the blood brain barrier, and then the signal can spread into the brain with immune cells[29]. These results indicated the blood brain barrier may be compromised in neurodegenerative diseases[30], which caused the exchange of proinflammatory cells and markers between the brain and periphery.
In the present study, we prepared a peripheral inflammation cell model with LPS-stimulated RAW264.7 macrophages to explore its activation onBV2 microglia[31]. We found that LPS induced the production of IL-1β, IL-6 and TNF-α in the culture medium of RAW264.7 macrophages. We further showed that LPS plus ATP activated the NLRP3 inflammasome, evidenced by the upregulation of caspase-1 and IL-1β. Furthermore, the conditioned medium obtained from LPS-treated RAW264.7 macrophages activated BV2 microglia, stimulating the release of IL-1β, IL-6 and TNF-α from BV2 cells. The expression of proinflammatory cytokines IL-1β, IL-6 and TNF-α, and the NF-κB signaling pathway and inflammasome was higher than the control group, as shown by ELISA, and then the subcellular localization of NF-κB in BV2 cells determined activation of central BV2 cells. Indeed, the medium obtained from BV2 cells still contained the cytokines produced by the macrophages. Given the ELISA results, we speculated that macrophagemediated peripheral inflammation may induce BV2 cell activation and cytokine production, but we could not exclude the possibility that elevated cytokines in the BV2 medium were from the macrophage conditioned medium. Therefore, we further detected intracellular pathways, including phospho-IKKb expression and p65 nuclear translocation in BV2 cells by Western blotting and immunocytochemistry. These results confirmed our hypothesis that macrophage-mediated peripheral inflammation can evoke BV2 cell activation and neuroinflammation.
Early detection of microglial activation and antiinflammatory therapy may delay or halt disease progression before irreversible damage and clinical symptoms occur. As we know, ZYVAD is an irreversible caspase 1 inhibitor which was associated with significant inhibition of IL-1β. In the present study, we also investigated whether macrophage-mediated peripheral inflammation could evoke neuroinflammation and subsequently aggravate neural damage. ZYVAD administration in RAW264.7 cells for 24 h markedly reduced the proinflammatory cytokines, the NF-κB signaling pathway and inflammasome of RAW264.7 cells. Moreover, ZYVAD pretreatment markedly suppressed BV2 microglia activation induced by RAW264.7 macrophage conditioned medium. Furthermore, the results suggested that inflammasome and caspase-1 may be potential targets for modulating systemic inflammatory responses in neurodegenerative diseases.
In conclusion, this study demonstrates for the first time that peripheral inflammation, besides neuroinflammation, functions as a modulator of disease progression and neuropathology in several neurodegenerative diseases. Consequently, the conditioned medium obtained from LPS-treated RAW264.7 macrophages activated BV2 microglia, which could be suppressed by ZYVAD. Our study indicates that macrophagemediated peripheral inflammation subsequently evokes neuroinflammation and may aggravate neural damage. Inflammasome and caspase-1 may be potential targets for modulating systemic inflammatory responses in neurodegenerative diseases.
References
[1] World Health Organization. Dementia a public health priority. World Health Organization 2012. http://site. ebrary.com/lib/ucmerced/Doc?id=10718026 [accessed 28 September 2015].
[2] Träger U, Tabrizi SJ. Peripheral inflammation in neurodegeneration[J]. J Mol Med, 2013,91:673-681.
[3] Ajami B, Bennett JL, Krieger C, et al. Local self- renewal can sustain CNS microglia maintenance and function throughout adult life[J]. Nat Neurosci, 2007,10:1538-1543.
[4] Holmes C, Cunningham C, Zotova E, et al. Systemic inflammation and disease progression in Alzheimer disease[J]. Neurology, 2009,73:768-774.
农民工返乡创业集群能够驱动组织发展成长。农民工返乡创业集群根植于地方的人际关系网络,能够推动如农民专业合作社、农民股份合作社、相关产业服务协会等组织成立,从而有助于培育新型农业经营主体,把分散的小农经济、零散的乡村工业和低端的服务业向有组织的、适度规模的二三产融合发展过渡,提高农民之间的互动互助,打破农产品和市场之间的隔阂,推进家庭联产承包条件下小农生产、加工和服务的组织化、市场化、社会化,以实现乡村振兴战略。
[5] Harris MA, Tsui JK, Marion SA, et al. Association of Parkinson’s disease with infections and occupational exposure to possible vectors[J]. Mov Disord, 2012,27:1111-1117.
[6] D’Amelio M, Ragonese P, Morgante L, et al. Longterm survival of Parkinson’s disease: a population-based study[J]. J Neurol, 2006,253:33-37.
[7] Peng XX, Zhang SH, Wang XL, et al. Panax Notoginseng flower saponins (PNFS) inhibit LPS-stimulated NO overproduction and iNOS gene overexpression via the suppression of TLR4-mediated MAPK/NF-kappa B signaling pathways in RAW264.7 macrophages[J]. Chinese Medicine, 2015,10:15.
[8] Inoue M, Kamada H, Abe Y, et al. P3 (APP3), a novelmember of the TNF/TNFR2 signaling complex, induces phosphorylation of JNK[J]. Journal of Cell Science, 2015, 128(4):656-669.
[9] Xie Q, Shen WW, Zhong J, et al. Lipopolysaccharide/ adenosine triphosphate induces IL1β and IL-18 secretion through the NLRP3 inflammasome in RAW264.7 murine macrophage cells[J]. Int J Mol Med, 2014,34:341-349.
[10] Wang L, Zhai YQ, Xu LL, et al. Metabolic inflammation exacerbates dopaminergic neuronal degeneration in response to acute MPTP challenge in type 2 diabetes mice[J]. Exp Neurol, 2014,251:22-29.
[11] Dai JN, Zong Y, Zhong LM, et al. Gastrodin inhibits expression of inducible NO synthase, cyclooxygenase-2 and proinflammatory cytokines in cultured LPS-stimulated microglia via MAPK pathways[J]. PLoS One, 2011,6:e21891.
[13] Li Q, Verma IM. NF-κB regulation in the immune system[J]. Nat Rev Immunol, 2002,2(10):725-734.
[14] Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-κB signaling[J]. Cell Res, 2011,21(1):103-115.
[15] Kaltschmidt B, Baeuerle PA, Kaltschmidt C. Potential involvement of the transcription factor NF-κB in neurological disorders[J]. Mol Aspects Med, 1993,14(3),171-190.
[16] Matsuzawa A, Ichijo H. Stress-responsive protein kinases in redox-regulated apoptosis signaling[J]. Antioxidants Redox Signaling, 2005,7(3-4):472-481.
[17] Borsello T, Forloni G. JNK signalling: a possible target to prevent neurodegeneration[J]. Curr Pharm Des, 2007, 13(18):1875-1886.
[18] Paul A, Wilson S, Belham CM, et al. Stress-activated protein kinases: activation, regulation and function[J]. Cellular Signalling, 1997,9(6):403-410.
[19] Wang YR, Cui YT, Cao FY, et al. Ganglioside GD1a suppresses LPS-induced proinflammatory cytokines in RAW264.7 macrophages by reducing MAPKs and NF-κB signaling pathways through TLR4[J]. Int Immunopharmacol, 2015,28:136-145.
[20] Mattson MP, Meffert MK. Roles for NF-kappaB in nerve cell survival, plasticity, and disease[J]. Cell Death Differ, 2006,13:852-860.
[21] Schmitz ML. Function and activation of the transcription factor NF-kappa B in the response to toxins and pathogens[J]. Toxicol Lett, 1995(11),82-83:407-411.
[22] Schroder K, Tschopp J. The inflammasomes[J]. Cell, 2010,140:821-832.
[23] Kruszewski M, Schwarze PE, Skuland T, et al. Comparison of non-crystalline silica nanoparticles in IL-1β release from macrophages[J]. Part Fibre Toxicol, 2012,9:32.
[24] Wang M, Zhu J, Pan Y, et al. Hydrogen sulfide functions as a neuromodulator to regulate striatal neurotransmission in a mouse model of Parkinson’s disease[J]. J Neurosci Res, 2014,3:117-186.
[25] Cai DS. Neuroinflammation and neurodegeneration in overnutritioninduced diseases[J]. Trends Endocrinol Metab, 2013,24(1):40-47.
[26] Sun J, Zhang SS, Zhang X, et al. IL-17A is implicated in lipopolysaccharideinduced neuroinflammation and cognitive impairment in aged rats via microglial activation[J]. J Neuroinflammation, 2015,12:165-167.
[27] Holmes C, Cunningham C, Zotova E, et al. Systemic inflammation and disease progression in Alzheimer disease[J]. Neurol, 2009,73:768-774.
[28] Harris MA, Tsui JK, Marion SA, et al. Association of Parkinson’s disease with infections and occupational exposure to possible vectors[J]. Mov Disord, 2012,27:1111-1117.
[29] D’Amelio M, Ragonese P, Morgante L, et al. Longterm survival of Parkinson’s disease: a population-based study[J]. J Neurol, 2006,253:33-37.
[30] Felderhoff-Mueser U, Schmidt OI, Oberholzer A, Buhrer C, Stahel PF. IL-18: a key player in neuroinflammation and neurodegeneration?[J] Trends Neurosci, 2005,28(9):487-493.
[31] Moynagh PN. The interleukin-1 signalling pathway in astrocytes: a key contributor to inflammation in the brain[J]. J Anat, 2005,207(3):265-269.
[32] Yoo CG, Lee CT, Kim YW, et al. Effect of acetylsalicylic acid on endogenous I kappa B kinase activity in lung epithelial cells[J]. Am J Physiol Lung Cell Mol Physiol, 2001,280:L3-L9.
This study was supported in part by Nanjing Medical Science and Technology Development Foundation (No. ZKX12037, No. YKX13129), and in part by National Natural Science Foundation of China (No. 81271418).
✉s: Prof. Yingdong Zhang, Department of Neurology, Nanjing First Hospital affiliated to Nanjing Medical University, Nanjing, Jiangsu 210006, China (E-mail: zhangyingdong@aliyun.com);
Dr. Li Zhang, Department of Geriatrics, Nanjing Brain Hospital affiliated to Nanjing Medical University, Nanjing, Jiangsu 210029, China (E-mail: neuro_zhangli@163.com).
25 October 2015, Revised 23 November 2015, Accepted 13 March 2016, Epub 15 April 2016
R742, Document code: A
The authors declared no financial conflict of interests.
猜你喜欢
杂志排行
THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Lipid transport to avian oocytes and to the developing embryo
- Role of stromal cell-derived factor 1α pathway in bone metastatic prostate cancer
- A pilot exome-wide association study of age-related cataract in Koreans
- Dynamic monitoring of menopause hormone therapy and defining the cut-off value of endometrial thickness during uterine bleeding
- Polycystic ovary syndrome patients with high BMl tend to have functional disorders of androgen excess: a prospective study
- Effect of vitamin D3 on production of progesterone in porcine granulosa cells by regulation of steroidogenic enzymes
