Electrochemically Determining Dopamine and Uric Acid by Modified Glassy Carbon Electrode
2016-12-12LinaAbdullahALSHAHRANILIXiNANJunminTANJuanjuanGUFenglong
Lina Abdullah ALSHAHRANI, LI Xi, NAN Junmin, TAN Juanjuan, GU Fenglong*
(1.Key Laboratory of Theoretical Chemistry of Environment, Ministry of Education, School of Chemistry and Environment,South China Normal University, Guangzhou 510006, China; 2. School of Chemical Engineering and Life Science, Wuhan University of Technology, Wuhan 430070, China)
Electrochemically Determining Dopamine and Uric Acid by Modified Glassy Carbon Electrode
Lina Abdullah ALSHAHRANI1, LI Xi2, NAN Junmin1, TAN Juanjuan1, GU Fenglong1*
(1.Key Laboratory of Theoretical Chemistry of Environment, Ministry of Education, School of Chemistry and Environment,South China Normal University, Guangzhou 510006, China; 2. School of Chemical Engineering and Life Science, Wuhan University of Technology, Wuhan 430070, China)
[Cu(sal-β-Ala)(3,5-DMP2)] is modified on the surface of the single-walled carbon nanotubes (SWCNTs) of glass carbon electrode (GCE). The modified electrode shows impressive detection ability of dopamine (DA) and uric acid (UA). The modified electrode has a good electrocatalytic effect on DA and UA. The detection linear range of [Cu(sal-β-Ala)(3,5-DMP2)] to DA is 10.00 to 210 mmol/L and the detection limit is 7.29 μmol/L. While, the detection of [Cu(sal-β-Ala)(3,5-DMP2)] to UA has a good linear range from 1 to 86 mmol/L and the detection limit is 1.5 μmol/L. In this work Simultaneous Differential Pulse Voltammetric (DPV) is employed to determine DA and UA. The DPV method with [Cu(sal-β-Ala)(3,5-DMP2)]/SWCNTs/GCE has a good sensitivity and resolution rate.
copper (II) Schiff base complex; single-walled carbon nanotubes; modified electrode; dopamine; uric acid; electrochemical detection
Chinese library classification:O646 Document code:A Article ID: 1000-5463(2016)06-0099-07
1 Introduction
Dopamine (DA) is a neurotransmitter in the central nervous system, and has important functions in cardiovascular, kidney, hormones, and other important systems. The abnormal metabolism of DA in the nervous system would lead to a variety of diseases such as epilepsy, aging, dementia, and Parkinson’s[1-3]. In addition, uric acid (UA) is the metabolite of purine and exists in biological fluids such as blood and urine. Monitoring of the DA and UA concentrations in blood or urine is important because these concentrations can be used as an effective early warning sign for central nervous system or kidney diseases. Generally, the DA concentration is very low (0.01~1 μmol/L) in human blood, while UA concentrations are 100~1 000 times higher than that of DA[4]. Sensitive techniques are needed for the simultaneous determination of DA and UA mixture. The conventional detection methods of DA and UA include titration[5], HPLC,[6]and UV[7]etc. All these methods have their own advantages, but also associated with some disadvantages such as expensive equipment, complicated process, and low sensitivity. It’s necessary to develop a quick, simple, and effective method for detecting these compounds.
Due to the predominance such as low cost, easiness, real-time field investigation and high selectivity, electrochemical detection exhibits an important approach for detecting biomolecules. However, the conventional electrodes have the disadvantages of low sensitivity, easy to be contaminated by the oxidation products of DA and UA. In order to improve the detection sensitivity, many materials were used to modify the electrode, for examples, metal complexes, nanoparticles, carbon nanotubes, graphene, conductive polymer[8-10]. Therefore, carbon nanotubes (CNTs) modified electrode has become a hot research topic because of their large specific surface area, excellent electrochemical properties, and high stability[11-13]. The potential applications of CNTs in fabricating electrochemical sensors have been previously applied[14-15], and CNTs also have been applied for selective detection of DA and UA[16-17]. At the same time, the Schiff base complex has a very stable amino (RCN) structure, it can easily coordinate with transition metal to display a number of features, such as interaction with DNA, antibacterial and anticancer activity, ability of catalyzing the hydroquinone, sulfur cytosine, and ascorbic acid. When the electrode is modified by the transition metal complexes, the electron transfer rate can be increased, the oxidation potential will be reduced, and the peak current will be increased, thus improving the detection sensitivity, and acting as a catalyst in the electrode surface[18-20]. As a transition metal complex, Cu(sal-β-Ala)(3,5-DMP2) possesses unique composition and structure, and can be expected as a promising modifier for electrode. In this paper, [Cu(sal-β-Ala)(3,5-DMP2)] and SWCNTs modified glass carbon electrode (GCE) are prepared, which exhibits impressive sensitivity for simultaneously determining DA and UA.
2 Experiments
2.1 Materials and chemical reagents
DA, UA, and Cu(sal-β-Ala) (3,5-DMP2) were bought from the Sigma Company (St. Louis, MO,USA). Other chemical reagents are analytical grade and directly used without further purification. The acetate buffer solution (ABS, pH6.0, 0.1 mol/L) was prepared with NaAc and HAc. All solutions were prepared with double distilled water. Single-walled carbon nanotubes (SWCNTs) were purchased form Shenzhen Nanotech Port Co., Ltd (Shenzhen, China). According to the literature’s method, a certain amount of SWCNTs was added in the mixed acidV(H2SO4)∶V(HNO3)=3∶1 and refluxed for 2 h. Then, the concentrated hydrochloric acid was added and refluxed for another hour. After the reaction completed, the product was filtered, and dried in an oven at 80 ℃. The as-prepared SWCNTs (10 mg) was dispersed in 100 mL H2O, and then ultrasonic treated for 10 min, and sealed for use.
2.2 Preparation of [Cu(sal-β-Ala)(3,5-DMP2)]/SWCNTs/GCE
GCE was polished to a mirror on the surface of Al2O3of 1.0 and 0.3 μm, and then ultrasonic treatment in acetone and water for 2 min. To constitute a three-electrode system, the GCE was used as the working electrode, Pt wire electrode as the counter electrode, and a saturated calomel electrode as the reference electrode. The Cyclic Voltammetry (CV) test was in 0.5 mol/L H2SO4solution, and the potential was -0.35~1.50 V, scanning at 100 mV/s. After the CV curves become stable, the pretreatment of GCE was completed. The GCE was placed in a DMSO solution and mixed with Cu(sal-β-Ala)(3,5 -DMP2) and 0.1 mol/L NaNO3, and then treated with CV experiment between -0.8~1.2 V. The electrode was washed with water for several times, and the modified electrode was thus obtained, marked as [Cu(sal-β-Ala) (3,5-DMP2)]/SWCNTs/GCE.
2.3 Electrochemical measurements
Electrochemical measurements were conducted using three-electrode system. The saturated calomel electrode was used as the reference electrode, a platinum electrode as the auxiliary electrode, and the [Cu(sal-β-Ala)(3,5-DMP2)]/SWCNTs/GCE as working electrode. All electrochemical experiments were carried out using the CHI660D Electrochemical workstation (Shanghai Chenhua China) electrochemical workstation. The electrochemical impedance spectroscopy (EIS) was carried in an equimolar amount of 5 mmol/L [Fe(CN)6]3-/ [Fe(CN)6]4-solution, using 0.1 mol/L KCl as supporting electrolyte, and the frequency sweep range is 0.01~103 kHz.
3 Results and discussion
3.1 Characterization of the modified electrode
The surfaces of GCE, SWCNTs/GCE, and [Cu(sal-β-Ala)(3,5-DMP2)]/SWCNTs/GCE were investigated by SEM (Figure 1). By the comparison of Figure 1A and 1B, it can be seen that the SWCNTs were distributed on the GC electrode. After Cu(sal-β-Ala) (3,5-DMP2) was electro-polymerized on the surface of SWCNTs/GCE, the image became cloudy because of the formation of [Cu(sal-β-Ala)(3,5-DMP2)] film (Figure 1C). It is indicated that the Cu(sal-β-Ala) (3,5-DMP2) film has been successfully grown on the electrode.
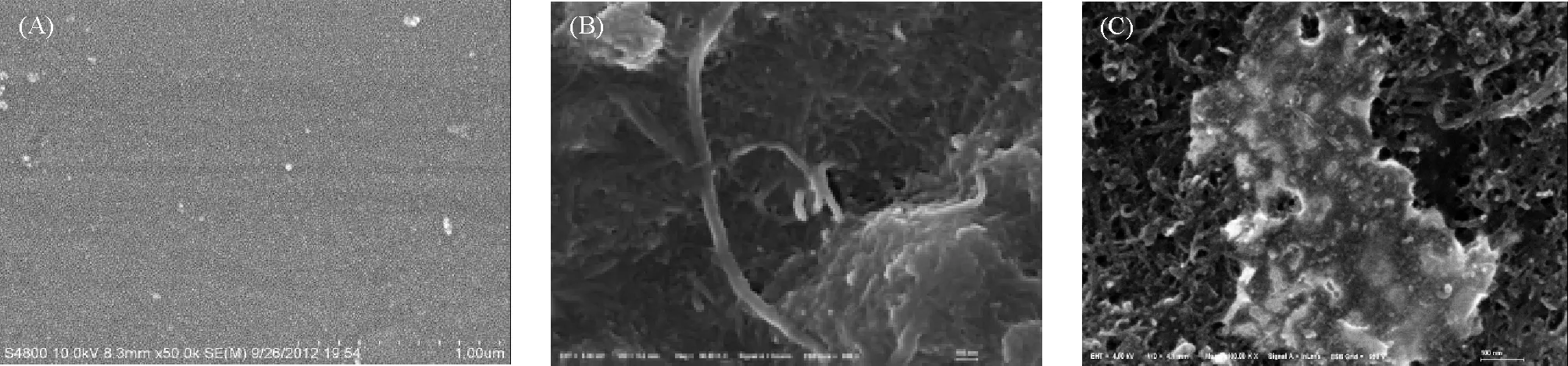
Figure 1 SEM images of bare GCE (A), SWCNTs/GCE (B), and Cu(sal-β-Ala)(3,5-DMP2)/SWCNTs/GCE (C)
3.2 Electrochemical behavior and detection of DA
The electrocatalysis of DA on bare GCE, SWCNTs/GCE, and Cu(sal-β-Ala)(3,5-DMP2)/SW CNTs/GCE was firstly investigated in a buffer solution at pH 7.0, The cyclic voltammogram of DA at bare GCE shows an irreversible redox behavior with weak oxidation current (23.36A) atEpc=0.24 V. Whereas, with SWCNTs and [Cu(sal-β-Ala)(3,5-DMP2)] on the GCE, DA exhibits obviously enhanced voltammetric response (Figure 2,scan rate=100 mV/s). The results indicate that the electrocatalytic activity of the modified electrode can be applied to the determination of DA. The effects of scan rate on the peak current of DA at the [Cu(sal-β-Ala)(3,5-DMP2)]/SWCNTs/GCE are shown in Figure 3. The anodic peak current of DA is proportional to the square root of scan rate in the range of 10~300 mV/s, which indicates that the electrocatalytic oxidation of DA at the [Cu(sal-β-Ala)(3,5-DMP2)]/SWCNTs/GCE is a diffusion controlled process. The linear regression equations of modified electrode isIpa(μA) = -50.958+19.731U1/2(mV/s)1/2with the correlation coefficients (R2) of 0.998 (Figure 4).

Figure 2 Cyclic voltammograms of 1 mmol/L DA at different electrodes in PBS solution (pH 7.0) containing 0.1 mol/L KCl supporting electrolyte

Figure 3 Cyclic voltammograms of the [Cu(sal-b-Ala)(3,5-DMP2)]/SWCNTs/GCE at different scan rates (from 30 to 300 mV/s) in PBS solution (pH 7.0) containing 1 mmol/L DA

Figure 4 Linear dependence of peak currents on the square root of the scan rate
In the optimal experimental conditions, amperometric was used to determine the oxidation peak current and DA concentration relationship. A stirred solution of chronoamperometry contained 0.1 mol/L KCl in 0.2 mol/L of PBS (pH 7.0) was added to achieve different concentrations of DA, the voltage was fixed at 0.22 V, as shown in Figure 5.
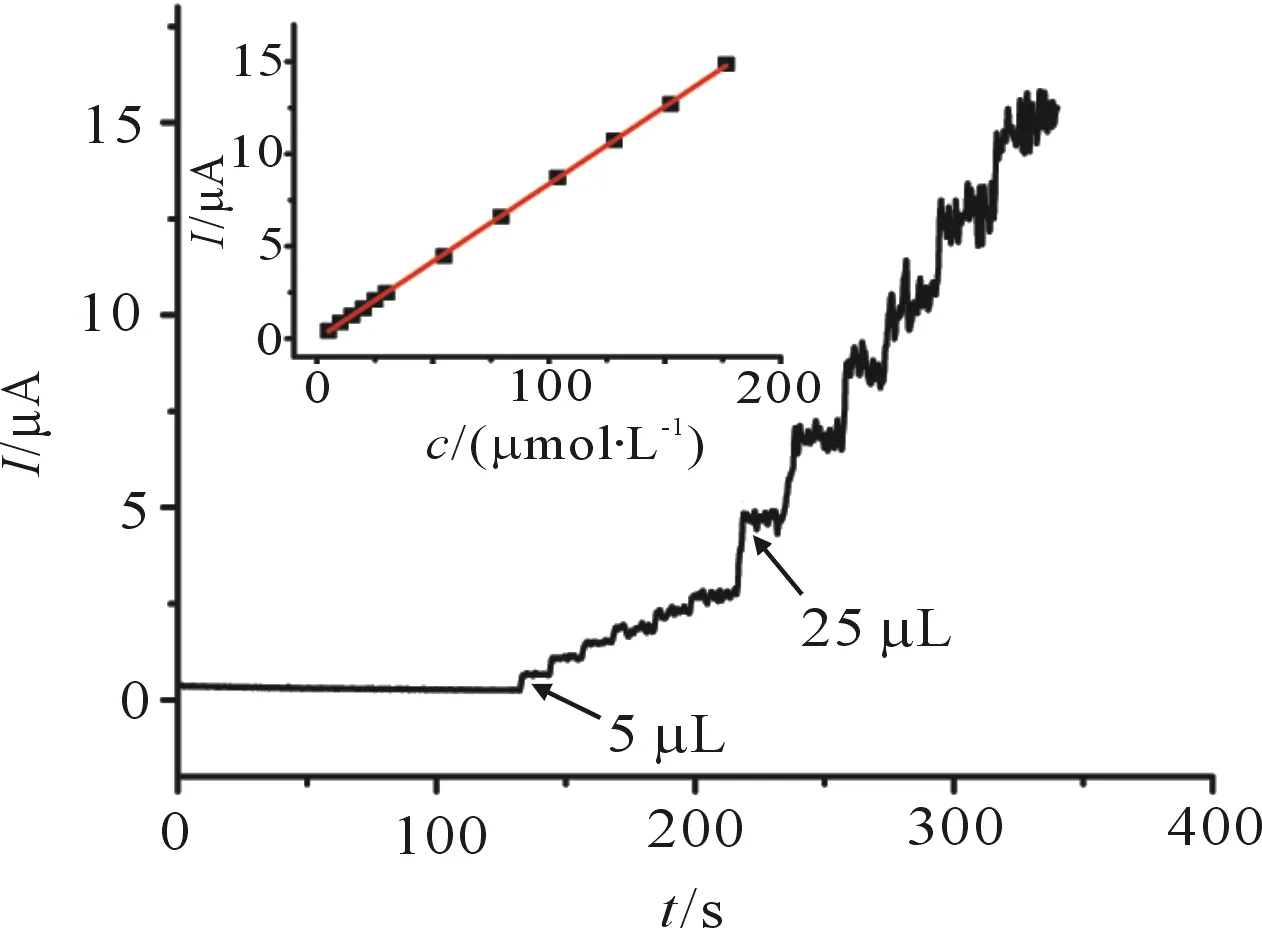
Figure 5 Amperometric response curves of [Cu(sal-β-Ala)(3,5-DMP2)]/SWCNTs/GCE when successive additions of different concentration DA. Inseted: calibration curve of amperometric response I vs. DA concentration.
Amperometric tests demonstrate that the modified electrode has a relatively rapid response time and high sensitivity to DA. The oxidation current increases very fast with the increase of DA concentration and reached the steady-state. The amperometric response is found to be linear to the DA over the range of 10 to 210 μmol/L with the correlation coefficient ofR2=0.999 92. Linear regression equation isIpa=0.086 3+0.006 4c(μmol/L). The limit of detection (LOD), defined as a signal-to-noise ratio of 3∶1 is found to be 7.29 μmol/L . The results indicate that the modified electrode fabricated by the proposed procedure has a good accuracy for the determination of DA. On the other hand, this system is also optimized in the solution with some common ions and small biomolecules for DA determination interference. It was shown that the allowable error range of ±5%, 100 times K+, Na+, Cl-, ascorbic acid, citric acid, glucose, hardly interfere with the determination of DA, indicating that [Cu(sal-β-Ala)(3,5-DMP2)]/SWCNTs/GCE determination of DA has high selectivity.
3.3 Electrochemical behaviors and detection of UA
Using the above optimal experimental conditions, the modified electrode was prepared and used to detect UA. The electrocatalysis of UA on bare GCE, SWCNTs/GCE, and [Cu(sal-β-Ala)(3,5-DMP2)]/SWCNTs/GCE was also investigated in a buffer solution with pH 2.0. As shown in Figure 6~8, it can be seen that on bare GCE, the cyclic voltammogram of UA shows a irreversible redox behavior with weak oxidation current (23.36A) atEpc=0.24 V. When SWCNTs and [Cu(sal-β-Ala)(3,5-DMP2)] were modified on the GCE, UA exhibits obviously enhanced voltammetric response (Figure 6). The results indicate that the electrocatalytic activity of the modified electrode can be applied to the determination of UA. The effect of scan rate on the peak current of UA on the [Cu(sal-β-Ala)(3,5-DMP2)]/SWCNTs/GCE has been investigated. The results are shown in Figure 7. The anodic peak current of UA is proportional to the square root of scan rate in the range of 30~300 mV/s, which indicates that the electrocatalytic oxidation of UA at the [Cu(sal-β-Ala)(3,5-DMP2)]/SWCNTs/GCE is a diffusion controlled process. The linear regression equations of modified electrode wasIpa(μA) = -22.533+12.585U1/2(mV/s)1/2with the correlation coefficients (R2) of 0.996 (Figure 8).
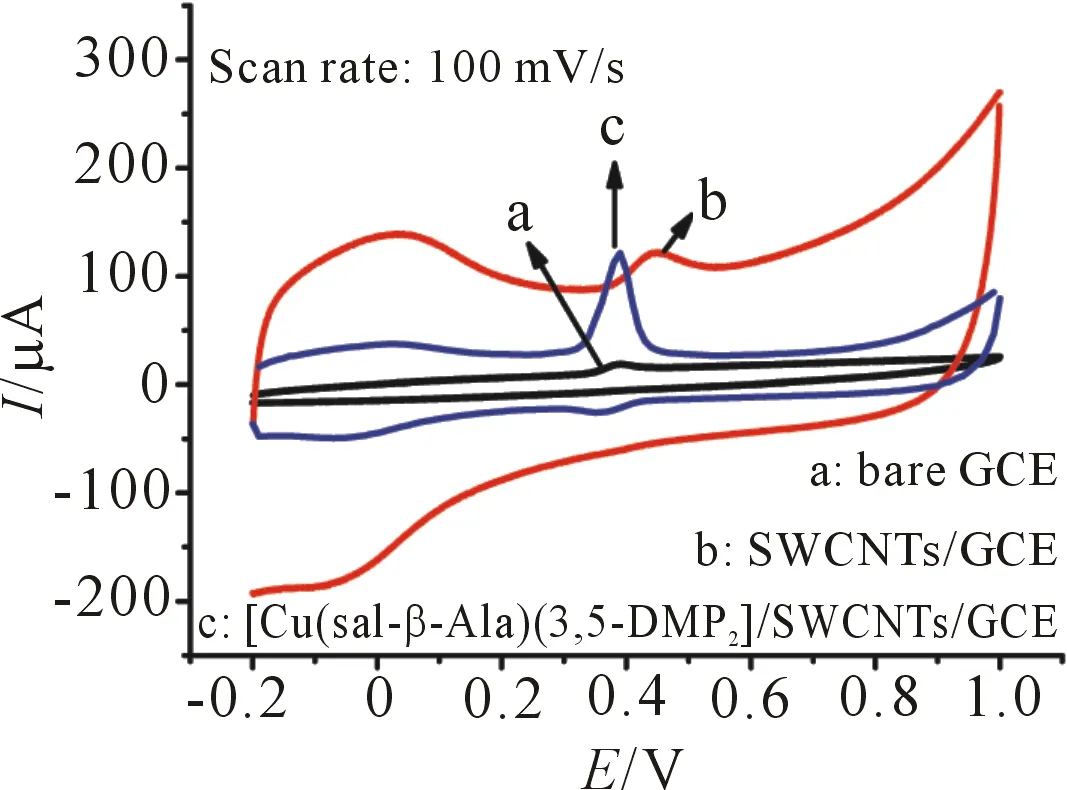
Figure 6 Cyclic voltammograms of 2 mmol/L UA at different electrodes in PBS solution (pH 2.0) containing 0.1 mol/L KCl supporting electrolyte
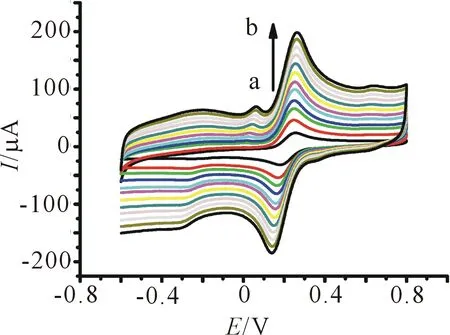
Figure 7 Cyclic voltammograms of the [Cu(sal-b-Ala)(3,5-DMP2)]/SWCNTs/GCE at different scan rates (from 30 to 300 mV/s) in PBS solution (pH 2.0) containing 2 mmol/L UA

Figure 8 Linear dependence of peak currents on the square root of the scan rate
Similarly, amperometric was used to determine the oxidation peak current and UA concentration relationship. A stirred solution of chronoamperometry contained 2 mol/L KCl in 0.2 mol/L of PBS (pH 6.0) was added to achieve different concentrations of UA, the voltage was fixed at 0.22 V. Amperometric tests demonstrate that the modified electrode has a relatively rapid response time and high sensitivity to UA (Figure 9). The oxidation current is increased very rapidly with the increase of UA concentration and reached the steady-state. The amperometric response is found to be linear to the UA over the range of 10 to 86 μmol/L with the correlation coefficient ofIpa=0.8743+613.82c(μmol/L) andR2=0.999 7. The limit of detection (LOD) defined as a signal-to-noise ratio of 3∶1, is found to 1.5 μmol/L. The results indicate that the modified electrode fabricated by using the proposed procedure has a good accuracy for the determination of UA.

Figure 9 Amperometric response curves of [Cu(sal-β-Ala)(3,5-DMP2)]/SWCNTs/GCE when successive additions of different concentration UA. Inserted: calibration curve of amperometric response I vs.UA concentration
3.4 Simultaneous determination of DA and UA
In accordance with the best electrolyte analyses experimental conditions and instrument parameters, Differential Pulse voltammetry (DPV) experiments with different concentrations of DA and UA have be carried out.
Figure 10 shows the typical CVs of DA and UA at the bare GCE, SWCNTs/GCE, and [Cu(sal-β-Ala) (3,5-DMP2)]/SWCNTs/GCE. At the bare GCE, DA and UA show broader oxidation peaks and overlapped. At the SWCNTs/GCE, the oxidation of DA and UA appears at 0.26 V and 0.39 V, respectively. After electrodeposited [Cu(sal-β-Ala)(3,5-DMP2)] on the electrode, the oxidation peaks current increased, and peak separation between DA and UA is 0.13 V, indicating that the simultaneous determination of the two species is feasible.

Figure 10 CVs in 0.2 mol/L PBS (pH 6.0) containing 1.0 mmol/L DA and 0.2 mmol/L UA in each case
DPV using [Cu(sal-β-Ala)(3,5-DMP2)]/SWCNTs/CCE as working electrode was used as a highly sensitive electrochemical method with very low detection limit to determine the trace of DA and UA. Figure 11 shows typical DPVs of different concentrations of DA in the existence of 0.1 mmol/L UA, using [Cu(sal-β-Ala)(3,5-DMP2)]/SWCNTs/CCE as working electrode. The oxidation peak current of UA is positively proportional to its concentration (1.998~122.947 μmol/L), with theIpa(UA)=-0.006 8+0.038 9c(μmol/L) and the correlation coefficient ofR2=0.998. The limit of detection (LOD) is found to be 1.42 μmol/L (S/N=3), The results indicate that the modified electrode fabricated by the proposed procedure has a good accuracy for the determination of DA.

Figure 11 DPV view of DA at different concentrations in the existence of 0.1 μmol/L UA
Figure 12 shows that the peak current of UA is increased with an increase in UA concentration, when the solution contained constant 0.05 mmol/L of DA, The oxidation peak current of UA is positively proportional to its concentration; while the oxidation peak current of DA does not change. It is found to be linear to the UA over the range of 10.00 to 122.95 μmol/L with the correlation coefficient ofR2=0.998,Ipa(UA)=0.67+0.017 8c(μmol/L). The LOD is found to be 3.7 μmol/L. The results indicate that the modified electrode fabricated by using the proposed procedure has good accuracy for the determination of UA.

Figure 12 DPA response curues of UA at different concentrations
4 Conclusion
The Cu(sal-β-Ala)(3,5-DMP2)/SWCNTs/GCE electrode was prepared and its electrochemical properties on DA and UA shows a good catalytic effect. The concentration of DA and UA and the peak current of oxidation are in linear relationship in some range. Its impressive catalytic properties indicate its potential use in the detection of DA and UA. The [Cu(sal-β-Ala)(3,5-DMP2)]/SWCNTs modified electrode shows good catalytic effect on DA and UA for cyclic voltammetry comparison purposes. DPV method is used as the simultaneous determination of DA and UA. In a mixed solution, when changing the concentration of a substance, the oxidation peak current showed a linear relationship with its concentration over a wide range, these results demonstrate that the modified electrode has high sensitivity.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (51273155), the Fundamental Research Funds for the Central Universities of China (2012-Ia-022 and 2014-Ia-030), and the Science and Technology Planning Project of Guangdong Province (2013B051000074). Thanks also to the Ministry of Higher Education of Saudi Arabia for the financial support to L.A.A.
[1] SHANKARAN D R,IIMURA K,KATO T. Simultaneous determination of ascorbic acid and dopamine at a sol-gel composite electrode[J]. Sensors & Actuators B Chemical,2003,94(1):73-80.
[2] WANG R,HONG Q L,LI N B. Simultaneous voltammetric measurement of ascorbic acid,epinephrine and uric acid at a glassy carbon electrode modified with caffeic acid[J]. Biosensors & Bioelectronics,2006,21(7):1086-1092.
[3] WIGHTMAN R M,MAY L J,MICHAEL A C. Detection of Dopamine Dynamics in the Brain[J]. Analytical Chemistry,1988,60(60):141-152.
[4] MO J W,OGOREVC B. Simultaneous measurement of dopamine and ascorbate at their physiological levels using voltammetric microprobe based on overoxidized poly(1,2-phenylenediamine)-coated carbon fiber[J]. Analytical Chemistry,2001,73(6):1196-1202.
[5] DUTT V V,HA M. Determination of uric acid at the microgram level by a kinetic procedure based on a “pseudo-induction” period[J]. Analytical Chemistry,1974,46(12):1777-1781.
[6] STAMFORD J A,JR J J. Probing brain chemistry[J]. Analytical Chemistry,1996,68(11):359A-363A.
[7] KIRK S,SAWYER R. Pearson’s composition and analysis of foods[M]. Pearsons Composition & Analysis of Foods,Longman:Willey,1991:507-544.
[8] WAGNER E S,LINDLEY B,COFFIN R D. High-performance liquid chromatographic determination of ascorbic acid in urine : Effect on urinary excretion profiles after oral and intravenous administration of vitamin C[J]. Journal of Chromatography A,1979,163(2):225-229.
[9] KHAN A,KHAN M I,IQBAL Z,et al. A new HPLC method for the simultaneous determination of ascorbic acid and aminothiols in human plasma and erythrocytes using electrochemical detection[J]. Talanta,2011,84(3):789-801.
[10]ZENG W,MARTINUZZI F,MACGREGOR A. Development and application of a novel UV method for the analysis of ascorbic acid[J]. Journal of Pharmaceutical & Biomedical Analysis,2005,36(5):1107-1111.
[11]LI J,LIN X. Simultaneous determination of dopamine and serotonin on gold nanocluster/overoxidized-polypyrrole composite modified glassy carbon electrode[J]. Sensors & Actuators B Chemical,2007,124(2):486-493.
[12]SHAKKTHIVEL P,CHEN S M. Simultaneous determination of ascorbic acid and dopamine in the presence of uric acid on ruthenium oxide modified electrode[J]. Biosensors & Bioelectronics,2007,22(8):1680-1687.
[13]HABIBI B,POURNAGHI-AZAR M H. Simultaneous determination of ascorbic acid,dopamine and uric acid by use of a MWCNT modified carbon-ceramic electrode and differential pulse voltammetry[J]. Electrochimica Acta,2010,55(19):5492-5498.
[14]WANG J. Carbon-nanotube based electrochemical biosensors:a review[J]. Electroanalysis, 2005,17(1):7-14.
[15]WANG M,ZHAO F,LIU Y,et al. Direct electrochemistry of microperoxidase at Pt microelectrodes modified with carbon nanotubes[J]. Biosensors & Bioelectronics,2005,21(1):159-166.
[16]BRITTO P J,SANTHANAM K S V,ANGEL R,et al. Improved Charge Transfer at Carbon Nanotube Electrodes[J]. Advanced Materials,1999,11(11):154-157.
[17]KONG J,FRANKLIN N R,ZHOU C,et al. Nanotube molecular wires as chemical sensors[J]. Science,2000,287(5453):622-625.
[18]SHAHROKHIAN S,ZARE-MEHRJARDI H R. Application of thionine-nafion supported on multi-walled carbon nanotube for preparation of a modified electrode in simultaneous voltammetric detection of dopamine and ascorbic acid[J]. Electrochimica Acta,2007,52(22):6310-6317.
[19]LIU X,PENG Y,QU X,et al. Multi-walled carbon nanotube-chitosan/poly(amidoamine)/DNA nanocomposite modified gold electrode for determination of dopamine and uric acid under coexistence of ascorbic acid[J]. Journal of Electroanalytical Chemistry,2011,654(S1/S2):72-78.
[20]AMIRI S S M. Voltammetric determination of thiocytosine based on its electrocatalytic oxidation on the surface of carbon-paste electrode modified with cobalt Schiff base complexes[J].Journal of Solid State Electrochemistry. 2007,11,1133-1138.
【中文责编:成文 英文责编:李海航】
2016-09-12 《华南师范大学学报(自然科学版)》网址:http://journal.scnu.edu.cn/n
国家自然科学基金项目(21273081);广东高校国际合作创新平台项目(2013gjha0005)
修饰玻碳电极对多巴胺和尿酸的电化学检测
Lina Abdullah ALSHAHRANI1, 李 曦2, 南俊民1, 谭娟娟1, 顾凤龙1*
(1. 华南师范大学化学与环境学院, 理论化学与环境教育部重点实验室, 广州 510631;2. 武汉理工大学化工与生命科学学院, 武汉 430070)
在单壁碳纳米管(SWCNT)表面修饰[Cu(sal-β-Ala)(3,5-DMP2)]玻碳电极(GCE),该修饰电极不仅对多巴胺(DA)和尿酸(UA)具有很好的电化学催化效果,而且对它们有很强的检测能力. [Cu(sal-β-Ala)(3,5-DMP2)] 修饰电极对DA的检测线性范围为10~210 mmol/L,检测极限为7.29 μmol/L;而对UA的检测线性范围为从1~86 mmol/L,检测极限为1.5 μmol/L. 同时,利用微分脉冲伏安法(DPV)来测定DA和UA,相比之下,[Cu(sal-β-Ala)(3,5-DMP2)] 与单壁碳纳米管及修饰玻碳电极结合具有良好的灵敏度和分辨率.
Cu(II)希夫碱配合物; 单壁碳纳米管; 修饰电极; 多巴胺; 尿酸; 电化学检测
*通讯作者:顾凤龙,教授,珠江学者,Email: gu@scnu.edu.cn.
