碳源和盐度对好氧反硝化细菌脱氮特性的影响
2016-12-12廖绍安黄捷畅王安利高方舟相晨曦罗年滔李永锋蓝宗坚
廖绍安, 黄捷畅, 王安利*, 高方舟, 相晨曦, 罗年滔, 李永锋, 蓝宗坚
(1. 华南师范大学生命科学学院, 广州 510631; 2. 广东水产健康安全养殖重点实验室, 广州 510631;3. 广东普通高校生态与环境科学重点实验室, 广州 510631; 4. 清远市水产研究所, 清远 511500)
碳源和盐度对好氧反硝化细菌脱氮特性的影响
廖绍安1,2,3, 黄捷畅1,2,3, 王安利1,2,3*, 高方舟1,2,3, 相晨曦1,2,3, 罗年滔4, 李永锋4, 蓝宗坚4
(1. 华南师范大学生命科学学院, 广州 510631; 2. 广东水产健康安全养殖重点实验室, 广州 510631;3. 广东普通高校生态与环境科学重点实验室, 广州 510631; 4. 清远市水产研究所, 清远 511500)
采用16S rDNA序列分析对菌株LZX301进行了初步鉴定,在150 r/m摇瓶好氧培养,探讨了碳源及盐度对菌株好氧反硝化特性的影响. 结果表明,该菌株16S rDNA序列与PseudomonasstutzeriATTC 17594(AY905607.1)等3株施氏假单胞菌序列相似度为99%,系统发育树分析显示菌株LZX301与P.stutzeri的关系比同属的P.aeruginosa和P.putida更近,因此初步确定菌株LZX301为P.stutzeri. 培养液初始含7 mg/L亚硝酸盐和28 mg/L硝酸盐、C/N比为10∶1条件下,以葡萄糖、乙酸钠和蔗糖为碳源时无机氮去除率分别为79.1%、67.9%和38.8%,氨氮积累量分别为1.978、1.224、0.727 mg/L. 以葡萄糖为唯一碳源时,在5‰、15‰、25‰等3个盐度下无机氮总去除率分别为73.2%、85.8%和78.7%,其中硝酸盐去除率分别为89.8%、86.1%和76.5%,亚硝酸盐去除率分别为36.2%、94.7%和96.4%,氨氮质量浓度分别为2.117、0.691、0.595 mg/L. 研究结果表明菌株LZX301在盐度5‰~25‰ 范围内具有较强的好氧反硝化能力,以葡萄糖为碳源脱氮效果最好,对该菌株的应用具有指导意义.
好氧反硝化; 脱氮; 碳源; 盐度; 假单胞菌
日益严重的氮污染是最重要的环境问题之一,反硝化作为主要的脱氮途径长期以来被深入研究.传统上认为在厌氧或缺氧条件下反硝化才会发生,ROBERTSON等[1]首次发现具有好氧反硝化能力的Thiosphaerapantotropha改变了这一认识. 好氧反硝化菌在溶解氧较高水平下也具有较高的反硝化活性,可以实现硝化过程和好氧反硝化菌代谢在时空上有效耦合,为提高脱氮效率提供新思路和新工艺,因此,好氧反硝化菌的研究得到了较高重视. 目前已从不同环境中分离获得了多种好氧反硝化菌,主要包括假单胞菌属(Pseudomonas)、产碱杆菌属(Alcaligenes)、副球菌属(Paracoccus)和微小菌属(Microvirgula)[2-3]. 碳源在细菌生长、呼吸作用和反硝化代谢中提供电子供体必不可少,其种类和碳氮比(C/N比)对脱氮有极为重要的影响;另外,盐度也是影响反硝化效率的重要因素[4-5].
本研究对源于对虾养殖池中的一株好氧反硝化菌LZX301的16S rDNA进行了同源性分析,并研究了碳源和盐度对其脱氮性能的影响,为其在水产养殖系统中氮污染控制提供必要的基础.
1 材料与方法
1.1 实验菌株
实验菌株来源于对虾淡化养殖池生物膜,为本实验室保存菌株,编号为LZX301,具有较强的好氧反硝化能力.
1.2 菌株活化培养
基本培养基:醋酸钠(1.64 g),葡萄糖(5.4 g),NH4Cl(1.07 g),NaNO3(0.85 g),K2HPO4(1.4 g),KH2PO4(2.7 g),pH 7.6,人工海水(盐度10‰)1 000 mL,115 ℃灭菌30 min.
取超低温冰箱保存的菌株LZX301在LB琼脂平板培养基上活化培养后,挑取菌落接种到基本培养基中液体纯培养24 h.
1.3 菌株16S rDNA序列分析
用1.5 mL小离心管收集24 h培养的菌液,12 000 r/m离心2 min,弃上清液,收集菌体. 使用TAKARA细菌基因组提取试剂盒提取菌株基因组DNA,操作按照说明书进行. 电泳检测提取的基因组DNA.
所采用的细菌通用引物27F(5′-AGAGTTTGATCCTGGCTCAG-3′)/1492R(5′-GGTTACCTTGTTACGACTT-3′)[6]由上海生工生物工程有限公司合成,对16S rDNA进行PCR扩增. 扩增体系:2× Premix Taq (Ex TaqTMVersion,TaKaRa公司) 25 μL,引物各0.5 μmol/L,模板1 μL,灭菌去离子水补至50 μL. 采用PE2400 PCR扩增仪,反应程序为:94 ℃变性5 min;94 ℃变性30 s,60 ℃退火30 s,72 ℃延伸1 min,32个循环;72 ℃延伸10 min,4 ℃保存. PCR产物以含0.15 mg/L溴化乙锭的1%琼脂糖凝胶电泳检测,凝胶图像分析系统(Bio-Rad Gel Doe 2000)记录结果. 用Microcon-PCR Purification Kit(Millipore)纯化扩增产物,由上海生工生物有限公司采用ABI 377 DNA测序仪完成测序. 测序结果在数据库GenBank采用BLASTN程序进行同源性检索. 在NCBI上搜索参考序列,利用软件MEGA 6对菌株LZX301及参考序列进行遗传关系分析,采用Neighbour-joint法构建系统发育树.
1.4 摇瓶培养
不同碳源培养基制备. 培养基基本组分:NaNO30.17 g,NaNO20.035 g,K2HPO41.4 g,KH2PO42.7 g,pH 7.6,人工海水(盐度10‰)1 000 mL. 基本培养基配制3份,各份按C/N比均为10∶1分别加入葡萄糖、蔗糖、乙酸钠等3种碳源,配制不同碳源培养基. 调节pH 7.6,各碳源培养基分装250 mL各3瓶,115 ℃灭菌30 min.
不同盐度培养基制备. 培养基基本组分:NaNO30.17 g,NaNO20.035 g,K2HPO41.4 g,KH2PO42.7 g,pH 7.6,葡萄糖0.875 g. 人工海水盐度设置3个梯度,分别为5‰、15‰、25‰. 调节pH 7.6,每个盐度的培养基分装250 mL各3瓶,115 ℃灭菌30 min.
将LZX301液体培养物离心去上清液,用盐度为10‰的无菌海水重悬浮,制备浓度约为5.0×108cells/mL的菌悬液. 每瓶液体培养基接入制备好的菌悬液5 mL,32 ℃下摇床培养,转速150 r/min.
1.5 样品分析方法及仪器
定时取样,用上海元析UV-5500PC紫外可见分光光度计在波长600 nm下检测OD600,氨氮浓度采用纳氏试剂分光光度法测定[7],亚硝酸盐浓度和硝酸盐浓度分别采用N- (1-萘基)-乙二胺分光光度法和锌镉还原法测定(GB/T 12763.4-2007)[8],盐度采用折射仪ATAGO MASTER-S/Mill α测定.
2 结果与分析
2.1 菌株16S rDNA序列分析
对菌株LZX301的16S rDNA扩增和测序,得到1 488个碱基的部分片段序列, 提交GenBank的登记号为KX685271. Blast比对结果显示,该菌株与多株施氏假单胞菌(Pseudomonasstutzeri)菌株16S rDNA序列相似性均达99%,表1列出了菌株LZX301与其中3株的相似度结果. 16S rDNA 序列分析可以作为细菌鉴定的手段,利用该方法90%以上的可以鉴定到属,65%~83%的可以鉴定到种[9]. 一般情况下16S rDNA 序列相似度高于99%被认为是同一个种[10].

表1 菌株LZX301的16S rDNA部分序列同源检索结果
根据BLAST结果[11],确认LZX301菌株属于Pseudomonas属,从γ-Proteobacteria类群中搜索与LZX301相似或相近的参考序列,构建系统发育树(图1). 菌株LZX301与高相似度的P.stuzeriDQ1、P.stuzeriA1501和P.stuzeriATTC 17594归为同类,且可信度达到100%;而与同属的P.aeruginosa和P.putida亲缘关系较远. 因此,初步认定菌株LZX301为P.stuzeri.
目前已发现的好氧反硝化菌中属水平上假单胞菌的最多,其中又以施氏假单胞菌为主[2,12],如P.stutzeriSU2[13]、P.stutzeriYZN-001[14]、P.stutzeriTR2[15]、P.stutzeriPCN-1[16]、P.stutzeriKTB[17]、P.stuzeriC3[18]和P.stutzeriYG-24[19].
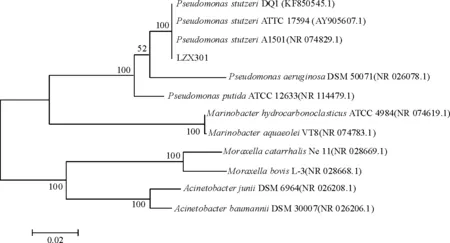
图1 基于菌株LZX301的16S rDNA部分序列与参考序列用N-J法构建的系统发育树
Figure 1 Phylogenetic tree constructed by the Neighbour Joining method on the basis of partial 16S rDNA sequence of the strain LZX301 and the reference strains
2.2 不同碳源对菌株LZX301脱氮特性的影响

图2 葡萄糖、蔗糖和乙酸钠为碳源时培养液中菌体浓度、氨氮浓度、亚硝酸盐浓度和硝酸盐浓度的变化
Figure 2 Concentrations of strain LZX301,ammonia,nitrite and nitrate using glucose,sucrose,and sodium acetate as the carbon source
2.3 不同盐度对菌株LZ301脱氮特性的影响


图3 不同盐度培养液中菌体浓度以及氨氮、亚硝酸盐、硝酸盐质量浓度的变化
3 结论
在水环境氮污染治理中反硝化菌发挥着极为重要的作用,筛选高效反硝化菌是科研工作者的一项重要任务;另外,反硝化代谢需要利用碳源作为还原剂异化还原高价态的氮,不同的反硝化菌对碳源的需求存在差异,因此,比较不同碳源对反硝化菌脱氮特征的影响可以为反硝化菌的应用提供极为重要的指导. 本文对菌株LZX301的研究分析,得到以下结论:
(1)经16S rDNA序列的相似性比较和系统发育分析,初步确定好氧反硝化细菌菌株LZX301为施氏假单胞菌(Pseudomonasstutzeri).
(2)碳源和盐度对其生长和脱氮特性的影响实验结果表明,在C/N比为10∶1、无机氮为亚硝酸盐氮和硝酸盐氮时,葡萄糖为碳源时菌株LZX301的脱氮效果最好、乙酸钠次之、蔗糖最差,且有轻微的氨氮积累. 在以葡萄糖为唯一碳源时,盐度5‰~25‰范围内,48 h LZX301的无机氮去除率介于73.2%~85.8%,具有较高的脱氮活性,但是也出现氨氮积累.
施氏假单胞菌(P.stutzeri)是常见的好氧反硝化菌,已报道的好氧反硝化菌属于该种的菌株最多. 从本文的研究结果来看,实验菌株LZX301可用于中低盐度废水,特别是海水养殖水体的脱氮修复或是海水养殖废水的净化.
[1] ROBERTSON L A,KUENEN J G,KLEIJNTJENS R. Aerobic denitrification and heterotrophic nitrification byThiosphaerapantotropha[J]. Antonie Van Leeuwenhoek,1985,51(4):445-445.[2] 梁炜,詹颖. 好氧反硝化菌的分离及应用研究进展[J]. 广东化工,2016,12:105-107.
LIANG W,ZHAN Y. Research progress on isolation and application of aerobic denitrifier[J]. Guangdong Chemical Industry,2016,12:105-107.
[3] 郭焱,张召基,陈少华. 好氧反硝化微生物学机理与应用研究进展[J]. 微生物学通报,2016,DOI:10.13344/j.microbiol.china.160001.
GUO Y,ZHANG Z J,CHEN S H. Microbiology and potential application of arobic denitrification:a review[J]. Microbiology China,2016,DOI:10.13344/j.microbiol.china.160001.
[4] 鲜思淑,陈茂霞,熊蓉,等. 异养硝化-好氧反硝化影响因素研究进展[J]. 水处理技术,2016(1):1-6.
XIAN S S,CHEN M X,XIONG R,et al. Research advances of heterotrophic nitrification-aerobic denitrification under different factors[J]. Technology of Water Treatment,2016(1):1-6.
[5] 乔森,刘雪洁,周集体. 异养硝化-好氧反硝化在生物脱氮方面的研究进展[J]. 安全与环境学报,2014(2):128-135.
QIAO S,LIU X J,ZHOU J T. Research progress of heterotrophic nitrification- aerobic denitrification in biological denitrification[J]. Journal of Safety and Environment,2014(2):128-135.
[6] EDWARDS U,ROGALL T,BLÖCKER H,et al. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA[J]. Nucleic Acids Research,1989,17(19):7843-7853.
[7] 国家环保总局. 水和废水监测分析方法 [M]. 4版. 北京:中国环境科学出版社,2002:279.
[8] 国家质量监督检验检疫总局,国家标准化管理委员会. 海洋调查规范 第四部分:海水化学要素调查:GB/T 12763.4-2007 [S]. 北京:中国标准出版社,2007:17.
[9] JANDA J M,ABBOTT S L. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory:pluses,perils,and pitfalls[J]. Journal of Clinical Microbiology,2007,45(9):2761.
[10] 朱诗应,戚中田. 16S rDNA扩增及测序在细菌鉴定与分类中的应用[J]. 微生物与感染,2013,8(2):106.
ZHU S Y,QI Z T. Application of bacterial 16S rDNA amplification and sequencing in the classification and identification of bacteria[J]. Journal of Microbes and Infections,2013,8(2):106.
[11]AGOSTINO M. Introduction to the BLAST Suite and BLASTN[M]∥AGOSTINO M. Practical Bioinformatics,[S. l.]:Garland Science,2012:47.
[12]JI B,YANG K,ZHU L,et al. Aerobic denitrification:a review of important advances of the last 30 years[J]. Biotechnology and Bioprocess Engineering,2015,20(4):643-651.[13]SU J J,LIU B Y,LIU C Y. Comparison of aerobic denitrification under high oxygen atmosphere byThiosphaerapantotropha,ATCC 35512 andPseudomonasstutzeri,SU2 newly isolated from the activated sludge of a piggery wastewater treatment system[J]. Journal of Applied Microbiology,2001,90(3):457-462.
[14]ZHANG J B,WU P X,HAO B,et al. Heterotrophic nitrification and aerobic denitrification by the bacteriumPseudo-monasstutzeriYZN-001[J]. Bioresource Technology,2011,102(21):9866-9869.
[15]MIYAHARA M,KIM S W,ZHOU S,et al. Survival of the aerobic denitrifierPseudomonasstutzeristrain TR2 during co-culture with activated sludge under denitrifying conditions[J]. Bioscience,Biotechnology,and Biochemistry,2012,76(3):495-500.
[16]ZHENG M S,HE D,MA T,et al. Reducing NO and N2O emission during aerobic denitrification by newly isolatedPseudomonasstutzeriPCN-1[J]. Bioresource Technology,2014,162(6):80-88.
[17]ZHOU M,YE H,ZHAO X. Isolation and characterization of a novel heterotrophic nitrifying and aerobic denitrifying bacteriumPseudomonasstutzeriKTB for bioremediation of wastewater[J]. Biotechnology and Bioprocess Engineering,2014,19(2):231-238.
[18]JI B,YANG K,WANG H,et al. Aerobic denitrification byPseudomonasstutzeriC3 incapable of heterotrophic nitrification[J]. Bioprocess and Biosystems Engineering,2015,38(2):407-409.
[19]LI C,YANG J,WANG X,et al. Removal of nitrogen by heterotrophic nitrification-aerobic denitrification of a phosphate accumulating bacteriumPseudomonasstutzeriYG-24[J]. Bioresource Technology,2015,182:18-25.
[20] 高喜燕,刘鹰,郑海燕,等. 一株海洋好氧反硝化细菌的鉴定及其好氧反硝化特性[J]. 微生物学报,2010,50(9):1166.
GAO X Y,LIU Y,ZHENG H Y,et al. Identification and characteristics of a marine aerobic denitrifying bacterium[J]. Acta Microbiologica Sinica,2010,50(9):1166.
[21] 韩永和,章文贤,庄志刚,等. 耐盐好氧反硝化菌A-13菌株的分离鉴定及其反硝化特性[J]. 微生物学报,2013,53(1).
HAN Y H,ZHANG W X,ZHUANG Z G,et al. Isolation and characterization of the salt-tolerant aerobic denitrifying bacterial strain A-13[J]. Acta Microbiologica Sinica,2013,53(1).
[22]ZHENG H Y,LIU Y,GAO X Y,et al. Characterization of a marine origin aerobic nitrifying-denitrifying bacterium[J]. Journal of Bioscience & Bioengineering,2012,114(1):36.
[23]SU J F,ZHANG K,HUANG T L,et al. Heterotrophic nitrification and aerobic denitrification at low nutrient conditions by a newly isolated bacterium,Acinetobactersp.SYF26[J]. Microbiology,2015,161:833.
[24]LORRAIN M J,TARTAKOVSKY B,PEISAJOVICH-GILKSTEIN A,et al. Comparison of different carbon sources for ground water denitrification[J]. Environmental Technology,2004,25(9):1043.
[25] 胡国山,张建美,蔡惠军. 碳源、C/N和温度对生物反硝化脱氮过程的影响[J]. 科学技术与工程,2016,16(14):75.
HU G S,ZHANG J M,CAI H J. Effect of carbon source,C/N ratio and temperature on biological denitrification process[J]. Science Technology and Engineering,2016,16(14):75.
[26]GUO L,CHEN Q,FEI F,et al. Application potential of a newly isolated indigenous aerobic denitrifier for nitrate and ammonium removal of eutrophic lake water[J]. Bioresource Technology,2013,142(4):49.
[27] 孙雪梅,李秋芬,张艳,等. 一株海水异养硝化-好氧反硝化菌系统发育及脱氮特性[J]. 微生物学报,2012,52(6):687.
SUN X M,LI Q F,ZHANG Y,et al. Phylogenetic analysis and nitrogen removal characteristics of a heterotrophic nitrifying-aerobic denitrifying bacteria strain from marine environment[J]. Acta Microbiologica Sinica,2012,52(6):687.
[28]MIAO Y,LIAO R,ZHANG X X,et al. Metagenomic insights into salinity effect on diversity and abundance of denitrifying bacteria and genes in an expanded granular sludge bed reactor treating high-nitrate wastewater[J]. Chemical Engineering Journal,2015,277:121.
[29]ZHAO W,WANG Y,LIU S,et al. Denitrification activities and N2O production under salt stress with varying COD/N ratios and terminal electron acceptors[J]. Chemical Engineering Journal,2013,215:252.
[30] 廖绍安,郑桂丽,王安利,等. 养虾池好氧反硝化细菌新菌株的分离鉴定及特征[J]. 生态学报,2006,26(11):3721.
LIAO S A,ZHENG G L,WANG A L,et al. Isolation and characterization of a novel aerobic denitrifier from shrimp pond[J]. Acta Ecologica Sinica,2006,26(11):3721.
[31]SONG Z F,AN J,FU G H,et al. Isolation and characterization of an aerobic denitrifyingBacillussp.YX-6 from shrimp culture ponds[J]. Aquaculture,2011,319(1):191.
【中文责编:庄晓琼 英文责编:李海航】
Effect of Carbon Source and Salinity on Nitrogen Removal of An Aerobic Denitrifier
LIAO Shaoan1,2,3, HUANG Jiechang1,2,3, WANG Anli1,2,3*, Gao Fangzhou1,2,3, XIANG Chenxi1,2,3, LUO Niantao4,LI Yongfeng4, LAN Zongjian4
(1. School of Life Sciences, South China Normal University, Guangzhou 510631, China;2. Key Laboratory of Ecology and Environmental Science in Guangdong Higher Education, Guangzhou 510631, China;3. Key Laboratory of Safe and Healthy Aquaculture in Guangdong Province, Guangzhou 510631, China;4. Qingyuan Fisheries Research Institute, Qingyuan 511500, China)
The aerobic denitrifying bacterium strain LZX301 was analyzed using 16S rDNA sequence. The similarity of 16S rDNA sequences between the aerobic denitrifying bacterium strain LZX301 andPseudomonasstutzeriaccessed in GenBank was 99%, and phylogenetic analysis showed that the strain, formed a monophyletic clade with members ofP.stutzeri, less closely related toP.aeruginosaorP.putida, which indicated that the strain LZX301 be assigned as the type strain ofP.stutzeri. The tests were conducted to study the effect of carbon sources and salinity on the nitrogen removal efficiency when the initial concentrations were 7 mg/L nitrite nitrogen and 28 mg/L nitrate nitrogen in the liquid media with C/N ratio 10, shaking speed at 150 r/min. The inorganic nitrogen removal efficiency was 79.1%,67.9% and 38.8%, and ammonium accumulation was observed to 1.978, 1.224 and 0.727 mg/L for glucose, sodium acetate and sucrose, respectively, which revealed glucose was the most efficient carbon source. With glucose as sole carbon source, nitrate nitrogen removal efficiency was 89.8%,86.1% and 76.5%,nitrite nitrogen removal efficiency was 36.2%, 94.7% and 96.4%, and the accumulated ammonium nitrogen was at 2.117, 0.691 and 0.595 mg/L at salinities of 5‰, 15‰ and 25‰, respectively, which revealed the strain LZX301 had good ability in removing inorganic nitrogen at salinities of 5‰-25‰. These results implied great potential of the strain LZX301 in practical applications.
aerobic denitrification; nitrogen removal; carbon source; salinity; Pseudomonas
2016-03-15 《华南师范大学学报(自然科学版)》网址:http://journal.scnu.edu.cn/n
国家自然科学基金项目(31472302,31172432);广东省海洋渔业科技与产业发展专项(A201401B01);广东省海洋渔业科技推广专项(A201001H02)
Q93
A
1000-5463(2016)06-0030-07
*通讯作者:王安利,教授,Email:wangalok@163.com.
