两个基于β-二酮席夫碱配体构筑的银配合物的合成、晶体结构和荧光性质
2016-12-06张奇龙徐红冯广卫黄亚励贵州医科大学化学教研室贵阳550004
张奇龙 徐红 冯广卫 黄亚励(贵州医科大学化学教研室,贵阳550004)
两个基于β-二酮席夫碱配体构筑的银配合物的合成、晶体结构和荧光性质
张奇龙*徐红冯广卫黄亚励
(贵州医科大学化学教研室,贵阳550004)
在常温的条件下,分别将2个双β-二酮席夫碱配体与银盐进行配位反应得到2个银配合物,{[Ag(L1)](SbF6)}n(1)和[Ag2(L2)2] (BF4)2(2)(L1=1,3-bis(4-methylamino-pentan-2-one)phenyl,L2=1,3-bis(3-methylamino-1-phenyl-butan-1-one)phenyl),并通过元素分析、红外光谱、粉末X射线衍射分析、单晶X射线衍射等对其结构进行了表征。结构分析表明,在配合物1中,银离子与配体L1中的2个碳原子和2个氧原子配位,形成一维链状结构,而配合物2中银离子与L2配体中的2个氧原子和2个碳原子配位,最终得到双核二聚体结构。化合物1和2都通过阴离子与结构单元之间的C-H…F作用,最终形成三维超分子结构。此外,我们还研究了化合物1、2以及配体的荧光性质。
银配合物;晶体结构;荧光性质;氢键作用
0 Introduction
As an outstanding representative of inorganicorganic hybrid materials,the coordination complexes have drawn much attention in last two decades for their diverse structures as well as tunable applications[1-5].The acetylacetone Schiff bases,which holding semi-enclosed structure,generally contain carbonyl groups and secondary nitramine groups together,can act as the hydrogen bonds donors and acceptors in theassembly of coordination complexes[6-8].Owing to their strong coordination ability and multiple coordination modes,the acetylacetone Schiff bases have been widelyusedintheassemblyofcoordination complexes,which show potential applications in the field of gas storage and separation,heterogeneous catalysis,magnetism,luminescence,and so on[9-10].
Inspired by the above-mentioned points,we were interested in the syntheses and properties of bis(βdiketone)Schiff bases coordination complexes and reported two novel silver coordination complexes, {[Ag(L1)](SbF6)}n(1)and[Ag2(L2)2](BF4)2(2)(L1=1,3-bis(4-methylamino-pentan-2-one)phenyl,L2=1,3-bis(3-methylamino-1-phenyl-butan-1-one)phenyl).In complex 1,the center Ag(Ⅱ)ions connected with two C atoms and two O atoms to form a one-dimensional chain, while the complex 2 is a binuclear silver complex. Moreover,the luminescence investigation shows that complexes 1 and 2 emitted green and blue fluorescence, respectively.
1 Experimental
1.1Materials and methods
Allchemicalswerecommerciallyobtained without further purification.IR spectra were measured on a Nicolet 740 FTIR Spectrometer at the range of 400~4 000 cm-1.Elemental analyses were carried out on a CE instruments EA 1110 elemental analyzer. Fluorescence spectra were performed on a Hitachi F-4500 fluorescence spectrophotometer at room temperature.Powder X-ray diffraction(PXRD)analyses were performed on an X-ray diffractometer(D/max 2500 PC, Rigaku)with Cu Kα radiation(λ=0.154 06 nm).
1.2Synthesis
1.2.1Synthesis of{[Ag(L1)](SbF6)}n(1)
AgSbF6(34.4 mg,0.1 mmol)in ethanol(20 mL) was added dropwise with stirring to L1(30.4 mg,0.1 mmol)in ethanol(20 mL)and the mixture was stirred at room temperature for several days.Slow evaporation of this solution yielded colorless block crystals that proved suitable for X-ray analysis.The precipitate that formed was collected by filtration,and dried at room temperature to give 1 in 48%yield based on Ag. Anal.Calcd.for C18H24AgF6N2O2Sb(%):C,33.57;H, 3.76;N,4.35.Found(%):C,33.31;H,3.58;N,4.41. IR(KBr pellet,cm-1):3 438(s),3 149(m),1 617(vs), 1 519(s),1 401(s),1 303(m),1 169(w),1 074(s),808 (m),592(m),472(m).
1.2.2Synthesis of[Ag2(L2)2](BF4)2(2)
The synthesis of 2 is similar to 1 using AgBF4(19.4 mg,0.1 mmol)and L2(42.8 mg,0.1 mmol)instead of AgSbF6and L1.Colorless block.Yield:54%yield based on Ag.Anal.Calcd.for C56H56Ag2B2F8N4O4(%): C,54.31;H,4.56;N,4.52.Found(%):C,54.37;H, 4.61;N,4.56.IR(KBr pellet,cm-1):3 440(s),3 129 (m),1 587(vs),1 544(m),1 436(m),1 393(s),1 319 (m),1 223(w),1 061(vs),1 008(m),857(w),782(m), 707(m),569(w),523(w).
1.3X-ray crystallography
The single-crystal X-ray diffraction was performed on a Bruker Smart ApexⅡCCD diffractometer. Intensities of reflections were measured using graphite -monochromatized Mo Kα radiation(λ=0.071 073 nm) at 296(2)K with the data collection θ ranging from 1.67°to 26°for 1,and at 293(2)K with the data collection θ ranging from 1.73°to 25.01°for 2, respectively.The structurewassolvedbydirect methods using the SHELXS program of the SHELXTL package and refined with SHELXL[11].Anisotropic thermal factors were assigned to all the non-hydrogen atoms.H atoms attached to C were placed geometrically and allowed to ride during subsequent refinement with an isotropic displacement parameter fixed at 1.2 times Ueqof the parent atoms.H atoms bonded to O or N atoms were first located in difference Fourier maps and then placed in the calculated sites and included in the refinement.Crystallographic data parameters for structural analyses are summarized in Table 1.Selected bond lengths and angles for complex 1 and 2 are listed in Table 2 and Table 3,respectively.
CCDC:1469231,1;1469232,2.
2 Results and discussion
2.1Crystal structure of{[Ag(L1)](SbF6)}n(1)
Complex 1 crystallizes in the triclinic space group P1,and its asymmetric unit contains onecrystallographically independent Ag(Ⅱ)ion,one L1ligand,and one SbF6-anion.As shown in Fig.1,the Ag(Ⅱ)ion is four-coordinated by two oxygen atoms (O1iiand O2i),and two carbon atoms(C10 and C15) from three different L1ligands.The central Ag(Ⅱ)ion located in a distorted AgC2O2tetrahedral coordination geometry,with the τ4=0.70(τ4=[360°-(α+β)]/141°,in which α and β are the two largest bond angles in the four-coordinate complex)[12].Besides,the Ag-C bond lengths are 0.234 0(9)and 0.236 7(1)nm,while the Ag-Odistancesare 0.237 1(4)and 0.274 4(9)nm (Table 2).Except the weak Ag1-O2iiinteractions,other bond lengths are in the normal ranges of Ag-O bonds in silver complexes[13].In the complex 1,two acetylacetone units of the L1ligand bridge three Ag(Ⅱ)centres through Oacetylacetoneand Cacetylacetone,extending in the direction of a axis,leaving a one-dimensional polymeric chain finally,with the nearest Ag…Ag distances being 0.527 7(6)and 0.565 3(2)nm(Fig.2). The one-dimensional polymeric chains interacted with the SbF6-anions through C-H…F hydrogen bonds (Table 4),finally gave a three-dimensional supramolecular network(Fig.3).
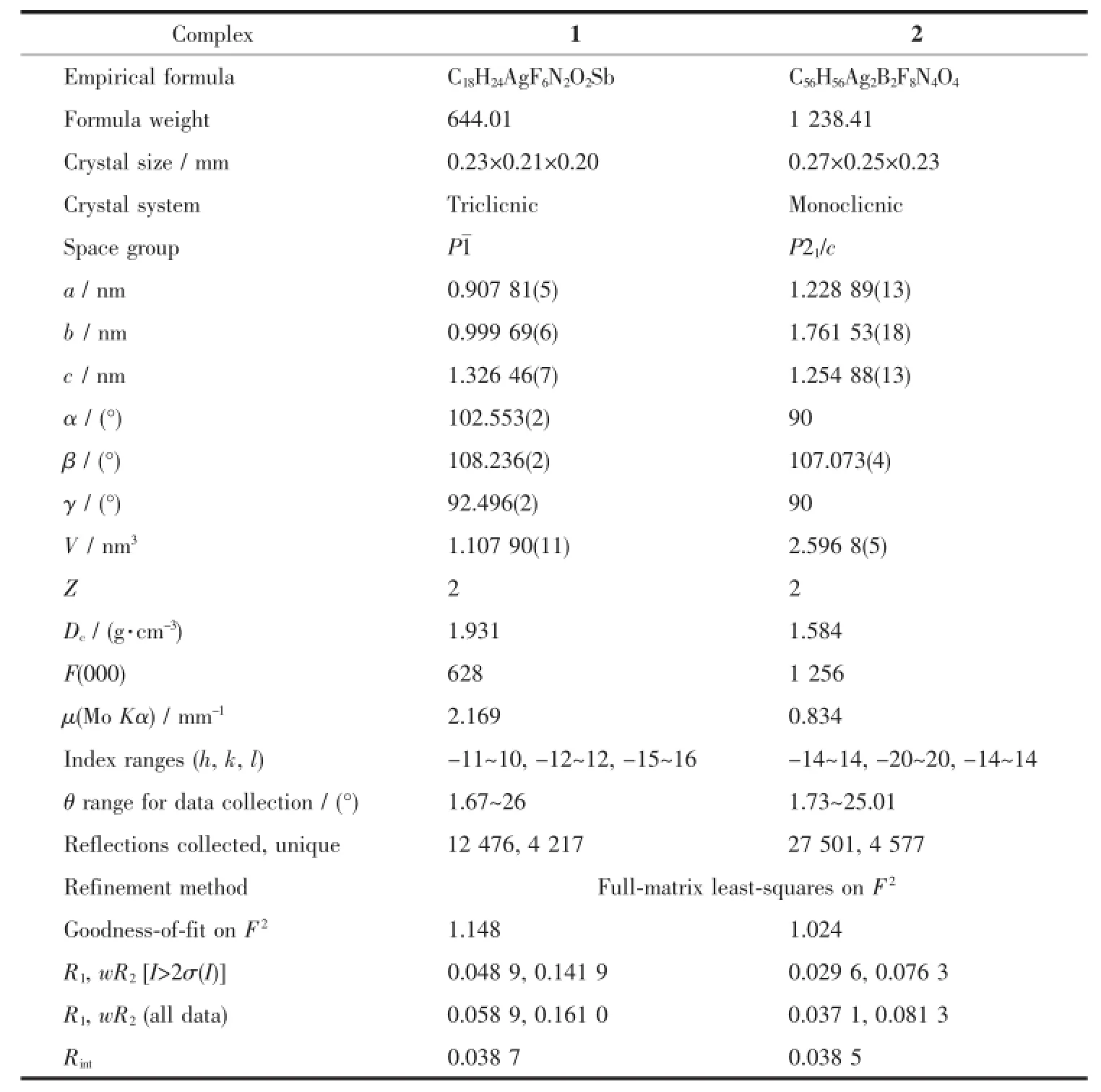
Table 1Crystal structure parameters of complexes 1 and 2
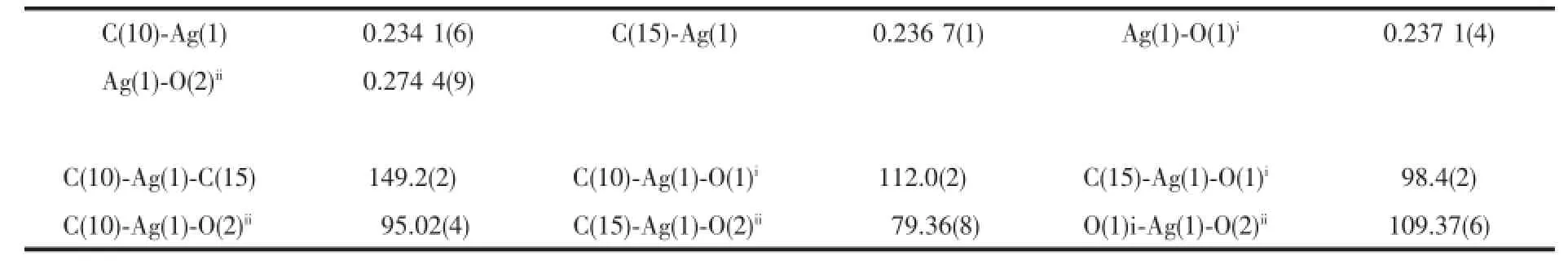
Table 2Selected bond lengths(nm)and bond angles(°)for 1
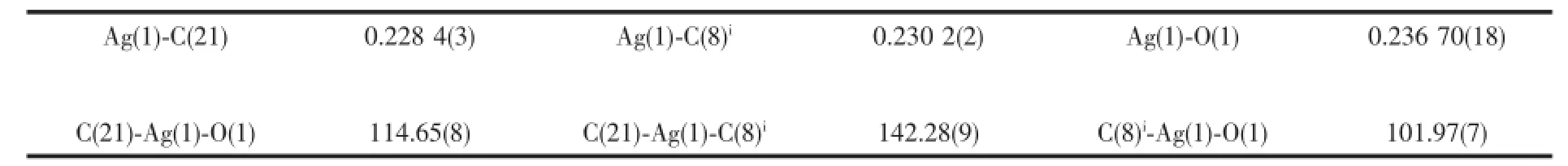
Table 3Selected bond lengths(nm)and bond angles(°)for 2
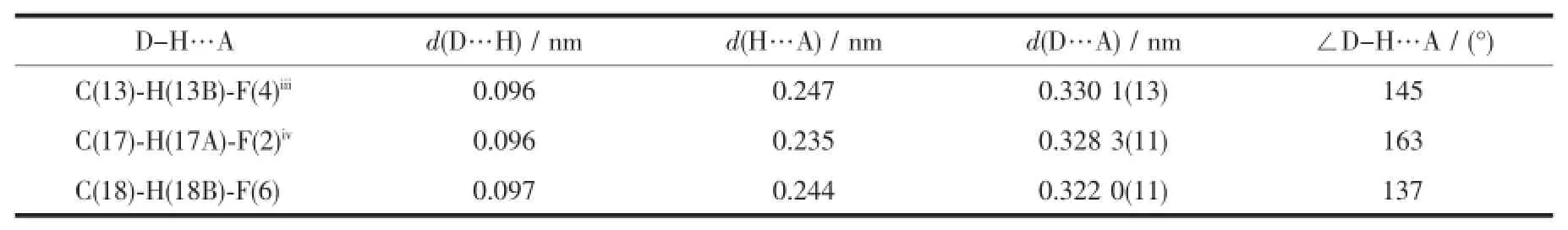
Table 4Hydrogen bond geometry of complex 1
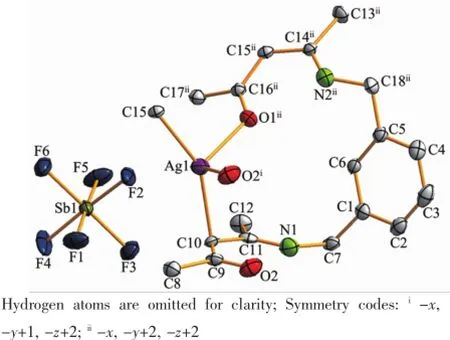
Fig.1Asymmetric unit of complex 1 with 30% probability level
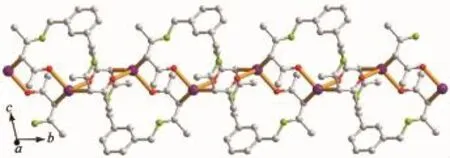
Fig.2One-dimensional polymeric chain structure of complex 1
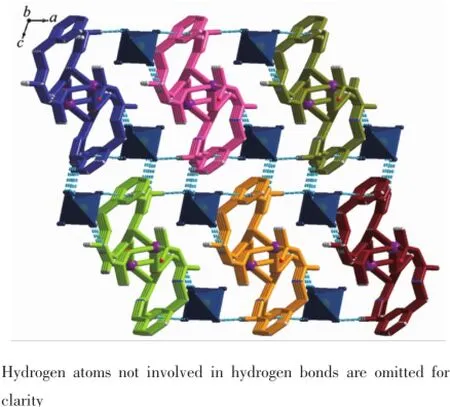
Fig.3Three-dimensional supramolecular framework of complex 1
2.2Crystal structure of[Ag2(L2)2](BF4)2(2)
In complex 2,the steric effects of phenyl group in L2ligand make it impossible to form a similar 1D polymeric chain structure like 1.Complex 2 crystallizes in the monoclinic space group P21/c.There are one crystallographically independent silver ion,one L2ligand,and one BF4-anion in the asymmetric unit of 2 (Fig.4).The central Ag(Ⅱ)ion is three-coordinated by one oxygen atom(O1),and two carbon atoms(C21 and C8i)from two different L2ligands.And the Ag-C bond lengths are 0.228 3(7)and 0.230 1(6)nm,the Ag-O distance is 0.236 7(0)nm,respectively(Table 3).The units interacted with the adjancent ones and the BF4-anions through the C-H…F andC-H…O hydrogen bond interactions(Table 5),and finally give a stable three-dimensional supramolecular architecture (Fig.5).
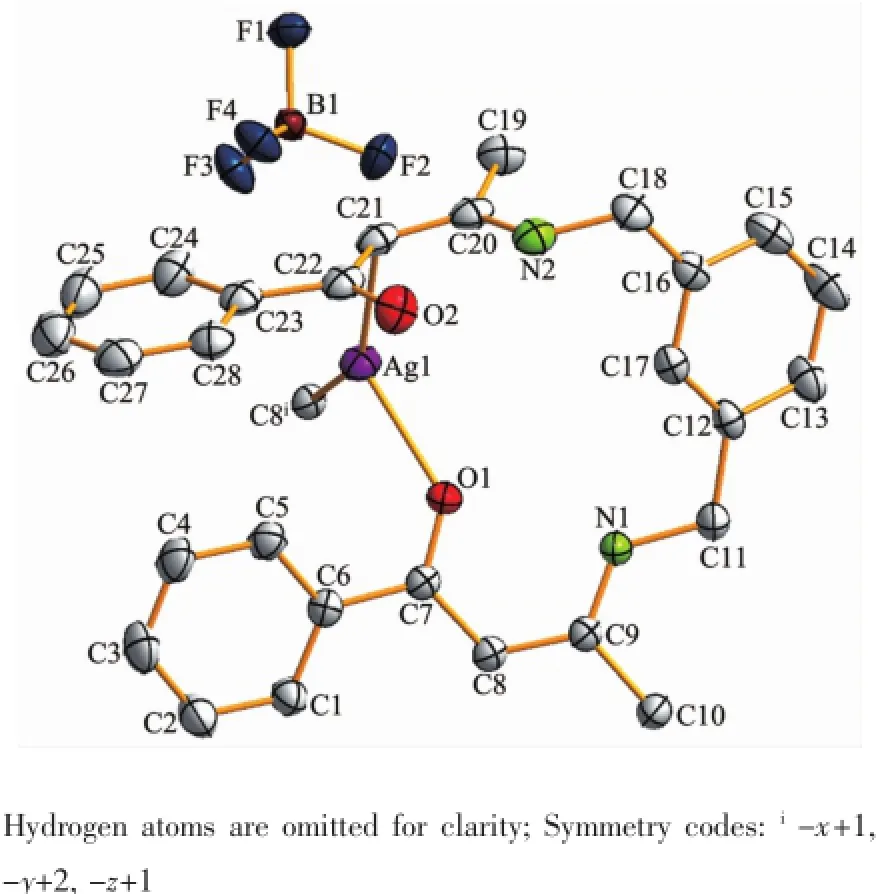
Fig.4Molecular structure of complex 2 with 30% probability level

Table 5Hydrogen-bond geometry of complex 2
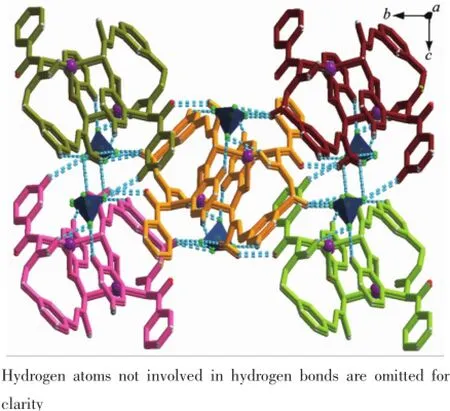
Fig.5Three-dimensional supramolecular framework ofcomplex 2
2.3Powder X-ray diffraction
In order to check the phase purity of the complexes,the PXRD patterns of title complexes were determined at room temperature.As shown in Fig.6, the peak positions of the simulated and experimental PXRD patterns are in agreement with each other, demonstrating the good phase purity of the complexes. The dissimilarities in intensity may be due to the preferredorientationofthecrystallinepowder samples.
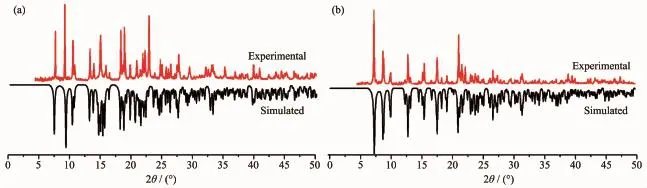
Fig.6PXRD patterns of 1(a)and 2(b)
2.4Photoluminescent properties
Many coordination complexes have been extensively studied due to their potential applications as luminescent materials[14-16].The solid-state luminescent properties of the complexes and the ligands were investigated at room temperature.The photoluminescence spectra of two organic ligands and complex 1 and 2 are shown in Fig.7.The L1and L2display photoluminescence with max emission peak at 518 nm (λex=488 nm),and 483 nm(λex=398 nm),respectively. It can be presumed that the peak originate from the π*→n or π*→π transitions[17-18].It can be observed that intense emissions occur at 514 nm(λex=486 nm) for 1,and 481 nm(λex=396 nm)for 2,respectively. The resemblance of the emissions between the organic ligandsandtheirbasedcoordinationcomplexes indicates that the emissions of the two complexes are probably attributed to the π→π*transitions[19].
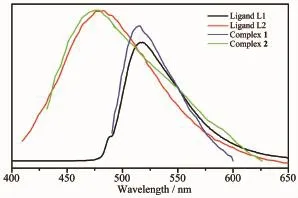
Fig.7Solid-state emission spectra of 1 and 2,and two free ligands at room temperature
3 Conclusions
In summary,two bis(β-diketone)Schiff bases silver coordinationcomplexes,{[Ag(L1)](SbF6)}n(1),and [Ag2(L2)2](BF4)2(2)have been reported.In complex 1, the center Ag(Ⅱ)ions connected with two C atoms and two O atoms to form a one-dimensional chain,while the complex 2 is a binuclear silver complex.Expanded by the weak C-H…F interactions between the units and anions and C-H…O hydrogen bonds between the adjacent units,two complexes formed three-dimensional supramolecularframeworkfinally.Moreover,the luminescence investigation shows that complexes 1 and 2 emitted green and blue fluorescence,respectively.
References:
[1]Zhang J P,Zhang Y B,Lin J B,et al.Chem.Rev.,2012,112: 1001-1033
[2]Chen X Y,Huang R B,Zheng L S,et al.Inorg.Chem., 2014,53:5246-5252
[3]Xue M,Lü Y C,Sun Q Q,et al.Cryst.Growth Des.,2015,15 (11):5360-5367
[4]Lin G Q,Ding H M,Yuan D Q,et al.J.Am.Chem.Soc., 2016,138(10):3302-3305
[5]Jiang J C,Zhao Y B,Yaghi O M.J.Am.Chem.Soc.,2016, 138(10):3255-3265
[6]Cherutoi J K,Sandifer J D,Pokharel U R,et al.Inorg.Chem., 2015,54(16):7791-7802
[7]ZHANG Qi-Long(张奇龙),WANG Huan-Yu(王焕宇),JIANG Feng(江峰),et al.Chinese J.Inorg.Chem.(无机化学学报), 2016,32:464-468
[8]Zhang Q L,Hu P,Zhao Y,et al.J.Solid State Chem.,2014, 210:178-187
[9]Zhang Q,Ma J P,Wang P,et al.Cryst.Growth Des.,2008,8 (7):2581-2587
[10]Pariya C,Fronczek F R,Maverick A W.Inorg.Chem.,2011, 50(7):2748-2753
[11]Sheldrick G M.SHELXS-97,Program for Crystal Structure Refinement,University of Göttingen,Göttingen,Germany, 1997.
[12]Fan L M,Fan W L,Li B,et al.CrystEngComm,2015,17: 9413-9422
[13]ZHANG Qi-Long(张奇龙),FENG Guang-Wei(冯广卫),HU Peng(胡鹏).Chinese J.Inorg.Chem.(无机化学学报),2014, 30:2433-2439
[14]Chen D M,Ma X Z,Shi W,et al.Cryst.Growth Des.,2015, 15(8):3999-4004
[15]ZHANG Qi-Long(张奇龙).Chinese J.Inorg.Chem.(无机化学学报),2015,31:2213-2220
[16]Wang W,Yang J,Wang R M,et al.Cryst.Growth Des., 2015,15(6):2589-2592
[17]LI Guo-Feng(李国峰),LI Xiu-Mei(李秀梅),JI Jian-Ye(纪建业),et al.Chinese J.Inorg.Chem.(无机化学学报),2014, 30:1947-1953
[18]Fan L M,Zhang X T,Li D,et al.CrystEngComm,2013,15: 349-355
[19]Zhang X T,Fan L M,Fan W L,et al.CrystEngComm,2015, 17:6681-6692
Syntheses,Structures and Luminescent Properties of Two Silver Coordination Complexes Based on Bis(β-diketone)Schiff Bases
ZHANG Qi-Long*XU HongFENG Guang-WeiHUANG Ya-Li
(Department of Chemistry,Guizhou Medical University,Guiyang 550004,China)
Two silver complexes,namely{[Ag(L1)](SbF6)}n(1)and[Ag2(L2)2](BF4)2(2),have been obtained by the reaction of silver salts with two different bis(β-diketone)Schiff bases(L1=1,3-bis(4-methylamino-pentan-2-one) phenyl,and L2=1,3-bis(3-methylamino-1-phenyl-butan-1-one)phenyl)in the ethanol solution.In complex 1,the center Ag(Ⅱ)ions connected with two C atoms and two O atoms to form a one-dimensional chain,while the complex 2 is a binuclear silver complex.Expanded by the weak C-H…F interactions between the units and anions and C-H…O hydrogen bonds between the adjacent units,three-dimensional supramolecular structures of two complexes formed finally.Moreover,the luminescent properties of two silver complexes and their based organic ligands have been investigated.CCDC:1469231,1;1469232,2.
silver complex;crystal structure;fluorescent property;hydrogen bonds
O614.122
A
1001-4861(2016)08-1421-06
10.11862/CJIC.2016.151
2016-03-18。收修改稿日期:2016-05-03。
贵州省科技计划课题(黔科合LH字[2014]7090)、贵州省教育厅自然科学研究项目(黔教合KY字[2015]415号)和贵阳市科技局国家自然科学基金培育项目(No.GY2015-41)资助。
*通信联系人。E-mail:gzuqlzhang@126.com
