基于2-巯基苯甲酸及其原位氧化配体的两种金属配位聚合物的合成、结构及发光性质
2016-12-01杨芳董宝霞唐萌刘文龙扬州大学化学化工学院扬州225002
杨芳 董宝霞 唐萌 刘文龙(扬州大学化学化工学院,扬州225002)
基于2-巯基苯甲酸及其原位氧化配体的两种金属配位聚合物的合成、结构及发光性质
杨芳董宝霞唐萌刘文龙*
(扬州大学化学化工学院,扬州225002)
由水热法得到2个新颖配位聚合物[Cd(2-mba)(bimb)]n(1)和[Pb(2-sb)]n(2)(H2(2-mba)=2-巯基苯甲酸,bimb=4,4-双(1-咪唑基)联苯,sb2-=2-亚磺基苯甲酸酯)通过元素分析、红外光谱、X射线粉末衍射和单晶衍射对其进行了表征。配合物1为二维网状结构,由一维的[Cd(2-mba)]n链与bimb连接形成。在配合物2中配体2-巯基苯甲酸在溶剂热的条件下通过原位反应被氧化成2-亚磺基苯甲酸酯,其结构为一种由配体2-亚磺基苯甲酸酯中的羧基与亚磺基桥联金属铅离子形成的二维(4,4)连接的层状结构。此外,2个配合物展示了良好的热稳定性和光致发光性能。
镉(Ⅱ);铅(Ⅱ);配位聚合物;原位溶剂热合成;晶体结构;荧光
The construction of novel metal-organic coordination polymers(CPs)continues to attract great interest due to their potential applications in many fields,such as catalysis,material science,luminescence and magnetochemistry,as well as their intriguing variety of architectures and topologies[1-5].The selection of organic ligands plays crucial roles as even small changes in conformation or symmetry of the ligands can result in a remarkable diversity of architectures and functions[6-7].The ligands containing O atoms,such asorganic aromatic polycarboxylate and sulfonate ligands, have been extensively employed in the construction of a rich variety of coordination polymers with fascinating structures and potential applications[8-10], due to their versatile coordination conformations and coordination ability.
However,the systematic investigations of coordination polymers based on bifunctional S and O donors are rare[11-14].Recently,the ligands with S and O donors have been paid more and more attentions to construct coordination polymers because such ligands can adopt versatile conformations.2-Mercaptobenzoic acid[H2(2-mba)]which has both hard(carboxylate-O) and soft(thiolate-S)donor sets,is an ideal building block for the formation of coordination polymers since the organothiolato-carboxylates in particular offer the possibility of variable coordination and chelation modes towards metal ions.Up to now,a series of complexes with the 2-mercaptobenzoic acid ligand were successfully synthesized and documented[15-22]. However,only 2-mercaptobenzoic acid ligand is used in these complexes,the incorporation of second ligand into coordination polymers of 2-mercaptobenzoic acid is rare[23-24].
Furthermore,the thiolate-S of 2-mercaptobenzoic acid may transform into other oxidation forms and form 2-sulfinobenzoate ligand in situ;this may lead to some novel hybrid materials through new coordination patterns.Sulfinate,including an S atom with low oxidation state,possesses a trigonal pyramid configuration in comparison with planar for carboxylate and the tetrahedron for sulfonate.While compared to the many carboxylate and sulfonate bridged species,the sulfinate group may possess different binding angles and strong coordination ability relative to the carboxylate and sulfonate group to construct interesting architecture[25].However,to our knowledge,few compounds incorporating the sulfinate ligand have previously been reported[26],those reported metal-sulfinate complexes are all discrete species or one dimensional coordination polymers[27-28].The synthesis of high dimensional compounds bridged with sulfinate ligand is still a challenge.
Considering the points mentioned above,we report here the hydrothermal synthesis and structures of two Cd(Ⅱ)/Pb(Ⅱ)coordination polymer based on 2-mercaptobenzoic acid and its in situ oxidized ligand 2-sulfinobenzoate,[Cd(C7H4O2S)(C18H14N4)]n(1)and[Pb (C7H4O4S)]n,(2),and their thermal stabilities and fluorescent properties are discussed.
1 Experimental
1.1Instrum ents and M aterials
The ligand bimb was prepared according to the literature method[29].All other reagents and solvents employed were commercially available and used as received without further purification.Elemental analyses for C,H,N and S were carried out with a Perkin-Elmer 240 elemental analyzer.The IR spectra were obtained as KBr pellets on a Bruker VECTOR 22 spectrometer in the 4 000~400 cm-1region. Thermogravimetric analyses(TGA)were performed on a Perkin Elmer TG/DTA 6300 thermal analyzer from room temperature to 750℃with a heating rate of 10℃·min-1in nitrogen environment.The powder XRD data were collected on a Bruker D8 diffractometer with Cu Kα radiation(λ=0.154 18 nm)over the 2θ range of 5°~40°at room temperature.Luminescence spectra for the solid samples were recorded with a Perkin-Elmer LS55 fluorescence spectrophotometer.
1.2Synthesis and Characterization
[Cd(C7H4O2S)(C18H14N4)]n(1):To 5 mL of a DMF-water(3∶2,V/V)solution was added CdO(12.8 mg, 0.10 mmol),2-mercaptobenzoic acid(15.4 mg,0.1 mmol),and bimb(28.6 mg,0.10 mmol)with stirring at room temperature.After a few minutes,the cloudy solution was sealed in a 25 mL Teflon-lined stainless steel vessel,which was heated at 160℃for 3 d.After the mixture was slowly cooled to room temperature over 24 h,colourless block crystals of 1 were obtained with 52%yield based on CdO.Elemental analysis calculated for C25H18CdN4O2S(%):C,54.50;H,3.29;N 10.17;S,5.82.Found(%):C,54.32;H,3.41;N 10.09; S,5.73.IR data(KBr pellet,cm-1):3 044(w),1 567(s), 1 513(s),1 431(m),1 384(s),1 257(w),1 123(w), 1 068(m),857(m),814(m),732(s),648(m),464(m).
[Pb(C7H4O4S)]n(2):A mixture of Pb(NO3)2(0.033 1 g,0.10 mmol),2-mercaptobenzoic acid(0.015 4 g, 0.10 mmol),MeOH(3 mL),H2O(2 mL)was sealed in a 25 m l Teflon-lined stainless steel vessel and heated at 80℃for 2d.After the mixture had been slowly cooled to room temperature over 24 h,block colourless crystals of 2 suitable for X-ray analysis were obtained with 45%yield based on Pb.Elemental analysis calculated for C7H4O4PbS(%):C,21.48;H, 1.03;S,8.19.Found(%):C,21.36;H,1.11;S,8.15. IR data(KBr pellet,cm-1):3 040(w),1 569(m),1 506 (s),1 434(w),1 376(s),1 281(w),1 114(w),1 055(m), 1 008(s),858(s),750(s),709(w),652(m),591(w),552 (m),476(w).In the product 2 the ligand 2-mercaptobenzoic acid was oxidized into 2-sulfintobenzoate in situ by O2in solvent medium,which is similar to that in previous reports[30-31].
1.3X-ray crystallography
Single-crystal X-ray diffraction analyses of 1 and 2 were carried out on a Bruker SMART APEXⅡCCD diffractometer with graphite-monochromated Mo Kα radiation(λ=0.071 073 nm)at 296(2)K.Empirical absorption corrections were applied by using the SADABS program[32].
The structures were solved by direct methods and refined by the full matrix least-squares based on F2using SHELXTL program package[33-34].All nonhydrogen atoms were refined anisotropically.The hydrogen atoms attached to ligands were generated geometrically and refined using a riding model.In 1, the O2 atom of 2-mercaptobenzoic acid ligand is disordered over two sites with an occupancy factor of 0.436/0.564 for O2/O2′.The crystal data and the details of the structure determination for complexes 1 and 2 are listed in Table 1.Selected bond lengths and angles are given in Table 2.
CCDC:1056796,1;1056797,2.

Table 1 Crystal data and structure refinements for compounds 1 and 2
Tab le 2 Selected bond lengths(nm)and angles(°)for 1 and 2
2 Results and discussion
2.1Crystal structure of complex 1
X-ray crystallographic analysis reveals that 1 crystallizes in the triclinic system,P1 space group. The asymmetric unit of 1 consists of one independent Cd(Ⅱ)cation,one 2-mba2-ligand,and one bimb auxiliary ligand.Cd1 adopts a distorted octahedral coordination geometry,and is coordinated by two O and two S atoms from two different 2-mba2-ligands, and two N atoms from two different bimb ligands(Fig. 1a).The Cd1-O distances are 0.233 5(3)and 0.239 5(3) nm;the Cd1-N bond lengths are 0.233 1(3)and 0.234 6(3)nm;the Cd1-S distances are 0.272 51(12) and 0.268 43(11)nm,respectively.
The 2-mba2-ligand adopts μ3-1κ1S:22κS,O:3κ1O coordination mode,adjacent Cd(Ⅱ)cations are bridged by pairs of carboxylate group and thiolate-S of 2-mba2-ligands in bridging-monodentate modes to generate a 1D chain in the ac-plane,which contains two alternate four-membered rings Cd2S2and Cd2O2(Fig.1b).The Cd…Cd separations in the rings are 0.391 41(7)and 0.376 56(6)nm,respectively.Each bimb ligand links the adjacent 1D chains to afford a 2D network with(4, 4)topology(Fig.1c).The two imidazolyl groups and the biphenyl groups of bimb in 1 are almost linear. The 2D networks are held together further by nonclassical hydrogen bonds between H atoms of imidazolyl rings and carboxylate-O and thiolate-S (C10-H10…S1vi0.393 4(5)nm;C25-H25…O2vii0.328 0(3)nm;Symmetry codes:vi-x,-y+1,-z+2;viix,y,z-1)resulting in a 3D supramolecular framework (Fig.1d).
2.2Crystal structure of complex 2
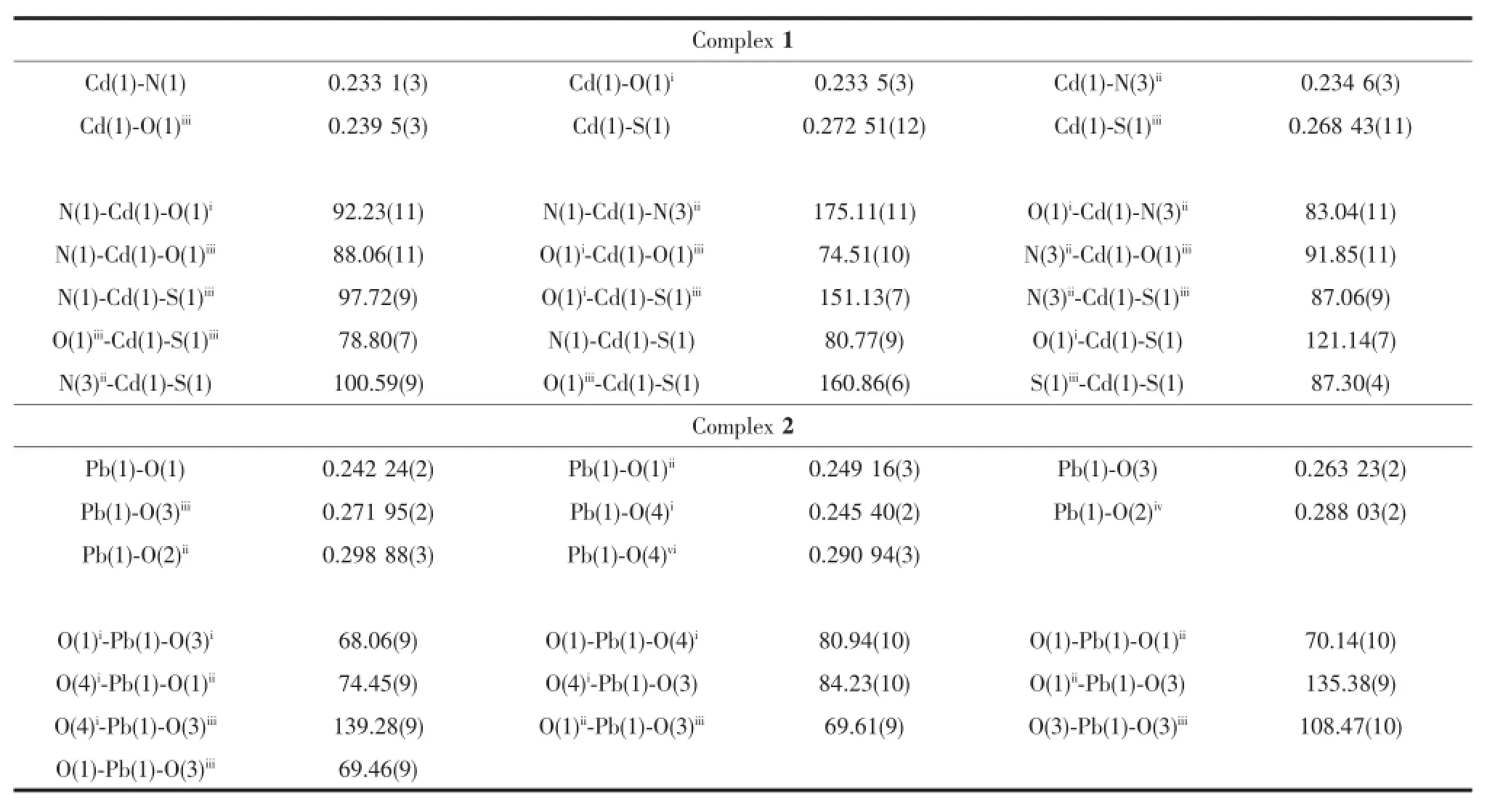
Complex 2 crystallizes in the monoclinic space group P21/c and its asymmetric unit contains one Pb(Ⅱ)cation and one sb2-.As shown in Fig.2a,each Pb(Ⅱ)cation is hemidirected and five-coordinated by five oxygen atoms in a distorted square-pyramid geometry. In the five-coordination,two sites were occupied by a carboxylate-O and a sulfinate-O from one 2-sulfobenzoate,and three others were furnished by two carboxylate-O and one sulfinate-O from three different 2-sulfobenzoate ligands.The five Pb-O distances rangefrom 0.242 2(3)to 0.271 9(3)nm(Table 2).However, when the Pb-O length limit extends from 0.288 03(3) to 0.298 88(3)nm,the potential weak bonds of Pb-O (Pb1-O2iv0.288 03(2)nm,Pb1-O4vi0.290 94(3)nm, Pb1-O2ii0.298 88(3)nm)can be drawn,and the coordination number of Pb(Ⅱ)will increase from five to eight.As a result,the coordination geometry of Pb1 will change from hemidirected into holodirected.In fact,long Pb-O weak bonds have also been reported in other lead complexes[35-36].

Fig.1(a)The coordination environments of the Cd(Ⅱ)ion in compound 1 with the ellipsoid of 50%probability;(b)The 1D structure viewed along b axis;(c)Formation of 2D network in compound 1;(d)A view of the three-dimensional supramolecular structure of 1 with blue dashed lines representing the hydrogen bonds
Fig.2(a)The coordination environment of the Pb(Ⅱ)in 2 showing the atom numbering scheme;(b)A view of the two-dimensional layer of 2,extending along the bc plane;(c)A view of the 12-membered tetranuclear lead metallamacrocycle(green lines) and 2D(4,4)net(blue lines)in 2
The sb2-ligand adopts μ4-1κ1O:2κ2O,O′:3κ1O′: 4κ1O″coordination mode,and the carboxylate group ofeach sb2-ligand adopts a bridging-bidentate and bridging-monodentate mode to connect three Pb(Ⅱ)cations.The sulfinate group adopts a bridgingmonodentate mode to connect two Pb(Ⅱ)cations.Pb(Ⅱ)cation is chelated by one 2-sulfobenzoate ligand via a carboxylate-O and a sulfinate-O forming a sevenmembered O,O-donor chelate ring.Two sulfinate and two carboxylate groups from four different ligands bridged two Pb(Ⅱ)cations,giving a binuclear motif [Pb2(SO2)2(CO2)2]with Pb…Pb separations of 0.402 22(4) nm.The binuclear units are further linked by the bridging-monodentate carboxylate oxygen atoms to give rise to a two-dimensional layer(extending along the bc plane)(Fig.2b).Interestingly,in the layer,four bridging-bidentate/monodentate carboxylate groups ligands connect four Pb(Ⅱ)cations to constitute a 12-membered tetranuclear lead metallamacrocycle(Fig. 2c).If the binuclear unit is considered as a single node and sb2-ligand as a linear linker,then complex 2 has a(4,4)net,as shown in Fig.2c.
2.3XRPD measurem ents,FTIR and therm al analysis
The phase purity of the complexes was independently confirmed by X-ray powder diffraction (XRPD)at room temperature.All the XRPD patterns measured for the as-synthesized samples were in good agreement with the XRPD patterns simulated from the respective single-crystal X-ray data using the Mercury 2.4 program.On the basis of the XRPD results it can be established that the single crystal selected is a good representative of the bulk compound(Fig.3).
The IR spectra of complexes 1 and 2 show weak bands at 3 040 and 3 044 cm-1,which can be assigned to C-H stretching frequency of the ligands. There are no peaks observed over 1 700 and 2 500 cm-1indicating that the carboxyl group and mercapto group are completely deprotonated,which are good agreement with the crystallographic data.The bands in the 1 281~1 114 cm-1region correspond to the C-C stretching vibrations,while the peaks at 1 434,1 431 cm-1and in the range of 750~648 cm-1attribute to the C-H in-plane and out-plane bending vibrations, respectively.The intense peaks at 1 569,1 506,1 376 cm-1for 1 and 1 567,1 513,1 384 cm-1for 2 were observed,These should be characteristic bands of carboxyl groups for the antisymmetric stretching and symmetric stretching,which also showing the deprotonated carboxylate groups coordinate to the metal atoms in bridging fashion[37].The peaks observed at 1 055,1 008,858 cm-1for 2 can be attributed to the characteristic vibrations of antisymmetric and symmetric S-O stretching of sulfinate[38].
In order to examine the thermal stabilities of 1 and 2,thermal gravimetric analysis(TGA)were carried out between 25 and 750℃under nitrogen atmosphere at the heating rate of 10℃·min-1,as shown in Fig.4.The TGA curve of 1 showed the crystal can keep stable until 295℃.Then there is a sharp weight loss occurring in the temperature range of 295~479℃for degradation of the bimb ligand (Calcd.51.98%,Found 51.08%).The second weight loss of 26.31%in the range of 479~720℃approximately amounts to the loss of the 2-mba2-ligand and the residue accounts for 23.65%,which isnearly in agreement with the calculated value of 23.31%by assuming that the final product is CdO. There is one major step in the TG curve of 2,which remains intact up to 350℃.A subsequent mass loss of 42.4%in the temperature range of 350~590℃corresponds to the decomposition of the sb2-ligand (Calcd.43.0%).Finally,the residue corresponds to PbO(Calcd.57.0%,Found 57.5%).
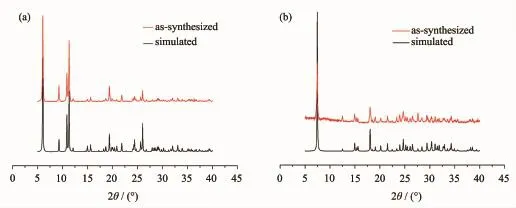
Fig.3 Simulated and experimental PXRD patterns for compound 1(a)and compound 2(b)

Fig.4 TG curves of complexes 1 and 2
2.4Photolum inescent Properties
Complexes with Cd and Pb centers and organic ligands are promising candidates for photoluminescent materials[39-40].Thus,the photoluminescent properties of complexes 1 and 2 in the solid state have been investigated at room temperature.As shown in Fig.5, upon excitation at 398 nm,a very strong and broad emission peak is observed at 479 nm in 1.Compared with the emission spectrum of H2(2-mba)of 456 nm (λex=389 nm),red shift of 23 nm in 1 was observed. Complex 2 shows a slightly strong emission peak at 410 nm with excitation at 235 nm.The emissions of the compounds may be ascribed to intraligand charge transfer emission of the ligand due to their remarkable resemblance of the emission bands[41].

Fig.5 Photoluminescence of 1,2 and free ligand H2(2-mba)in the solid state at room temperature
3 Conclusions
In summary,Two novel Cd(Ⅱ)/Pb(Ⅱ)coordination polymers have been synthesized based on 2-mercaptobenzoic acid,2-sulfintobenzoate and auxiliary N-donor ligand 4,4-bis(1-imidazolyl)bibenzene,which display 2D topological networks.The results of this study not only make clear that the coordination modes and conformations of 2-mercaptobenzoate and 2-sulfintobenzoate ligands,but also give nice example of the N-donor ligands play an important role in the construction of coordination polymers.Especially,2-sulfintobenzoate ligand from the 2-mercaptobenzoic acid precursor has been firstly synthesized in 2 through the in situ ligand oxidation reaction under solvothermal conditions,which also provides a useful strategy to the construction of novel coordination complexes or metalorganic frameworks via in situ ligand synthesis reactions.In addition,the photoluminescence investigation shows that two coordination polymers appear potentially applied as new luminescent materials.
Acknow ledgments:We gratefully acknowledge the financial support by the National Natural Science Foundation of China(Grant No.21371150,21301152)and the Priority Academic Program Development of Jiangsu Higher Education Institutions(Grant No.2014-132).
[1]Furukawa H,Cordova K E,O′Keeffe M,et al.Science, 2013,341:1230444-1-1230444-12
[2]Cui Y,Yue Y,Qian G,et al.Chem.Rev.,2012,112:1126-1162
[3]Dang D B,An B,Bai Y,et al.Chem.Commun.,2013,49: 243-2245
[4]Liu L J,Telfer S G.J.Am.Chem.Soc.,2015,37:3901-3909
[5]Sun H L,Wang Z M,Gao S.Coord.Chem.Rev.,2010,54:1081-1100
[6]Adarsh N N,Kumar D K,Dastidar P.CrystEngComm,2009, 11:796-802
[7]Zheng S T,Wu T,Chou C T,et al.J.Am.Chem.Soc., 2012,134:4517-4520
[8]Khan N A,Jhung S H.Angew.Chem.Int.Ed.,2012,51: 1224-1227
[9]Ren Y X,Zheng X J,Jin L P.CrystEngComm,2011,13: 5915-5923
[10]Wang J,Wang J G,Li X H,et al.Transition Met.Chem., 2013,38:765-770
[11]Sun L,Miyakai T,Seki S,et al.J.Am.Chem.Soc.,2013, 135:8185-8188
[12]Casarin M,Devic T,Famengo A,et al.Inorg.Chem.,2010, 49:4099-4108
[13]Humphrey S M,Mole R A,McPartlin M,et al.Inorg. Chem.,2005,44:5981-5983
[14]Chen H W,Fackler J P,Schussler D P,et al.J.Am.Chem. Soc.,1978,100:2370-2375
[15]Humphrey S M,Mole R A,Rawson J M,et al.Dalton Trans.,2004:1670-1678
[16]Duran N,Clegg W,Cucurull-Sanchez L,et al.Inorg.Chem., 2000,39:4821-4832
[17]Ma C L,Zhang Q F,Zhang R F,et al.Chem.-Eur.J.,2006, 12:420-428
[18]Nomiya K,Noguchi R,Kato C.Chem.Lett.,2000:162-163
[19]Cave D,Gascon J-M,Bond A D,et al.Chem.Commun., 2002:1050-1051
[20]Sun D,Liu F J,Huang R B,et al.Inorg.Chem.,2011,50: 12393-12395
[21]Sun D,Wang D F,Liu F J,et al.CrystEngComm,2011,13: 2833-2836
[22]Sun D,Luo G G,Zhang N,et al.Inorg.Chem.Commun., 2010,13:306-309
[23]Sun D,Yan Z H.Acta Crystallogr.Sect.C,2012,C68:m229-m232
[24]Ge S Z,Liu Q,Deng S,et al.J.Inorg.Organomet.Polym., 2013,23:571-578
[25]Wang H,Huo L H,Deng Z P,et al.CrystEngComm, 2012,14:3501-3508
[26]Zheng S L,Tong M L,Yu X L,et al.Dalton Trans.,2001: 586-592
[27]Begum R A,Farah A A,Hunter H N,et al.Inorg.Chem., 2009,48:2018-2027
[28]Li F Y,Xu L,Gao G G,et al.Dalton Trans.,2007:1661-1664
[29]Fan J,Hanson B E.Chem.Commun.,2005:2327-2329
[30]Jackson W G,Rahman A F M M.Inorg.Chem.,2003,42: 383-388
[31]Yue C Y,Lin Z Z,Chen L F,et al.J.Mol.Struct.,2005, 779:6-22
[32]SADABS,SMART and SAINT,Bruker AXS Inc.,Madison, Wisconsin,USA,2003.
[33]APEX2,Bruker AXS Inc.,Madison,Wisconsin,USA,2005.
[34]Sheldrick G M.Acta Crystallogr.,2008,A64:112-122
[35]Marandi F,Mirtamizdoust B,Soudi A A,et al.Inorg.Chem. Commun.,2007,10:174-177
[36]Mao J G,Wang Z,Clearfield A.Inorg.Chem.,2002,41: 6106-6111
[37]Nakamoto K.Infrared and Raman Spectra of Inorganic and Coordination Compounds.5th Ed.Part B.New York:John Willey&Sons,1997.
[38]Higashi L S,Lundeen M,Hilti E,et al.Inorg.Chem., 1977,16:310-313
[39]Liu W L,Ye L H,Liu X F,et al.CrystEngComm,2008,10: 395-1403
[40]Zhang L,Qin Y Y,Li Z J,et al.Inorg.Chem.,2008,47: 8286-8293
[41]Blasse G,Grabmaier B C.Luminescent Materials.Berlin: Springer Verlag,1994:134-135
Syntheses,Crystal Structures and Photolum inescence of Two M etal Coordination Polymers Constructed from 2-M ercaptobenzoic Acid and Its in situ Oxidized Ligand
YANG Fang DONG Bao-Xia TANG Meng LIU Wen-Long*
(College of Chemistry and Chem ical Engineering,Yangzhou University,Yangzhou,Jiangsu 225002,China)
Two novel coordination polymers,[Cd(2-mba)(bimb)]n(1)and[Pb(2-sb)]n(2)[H2(2-mba)=2-mercaptobenzoic acid,bimb=4,4-bis(1-imidazolyl)bibenzene,sb2-=2-sulfinobenzoate]have been solvothermal synthesized and characterized by elemental analysis,IR,powder X-ray diffraction and single crystal X-ray diffraction.Complex 1 features a two-dimensional(2D)net structure in which 1D[Cd(2-mba)]nchains are interconnected by bimb ligands.In complex 1,the ligand 2-mercaptobenzoic acid was oxidized into 2-sulfinobenzoate under solvothermal conditions via in situ ligand reactions.Complex 2 exhibits a 2D(4,4)-connected framework bridged with carboxylate and sulfinate groups of 2-sulfinobenzoate ligands.The thermal stabilities and solid state photoluminescent properties of two compounds were also investigated.CCDC:1056796,1;1056797,2.
cadmium(Ⅱ);lead(Ⅱ);coordination polymer;solvothermal synthesis in situ;crystal structure;luminescence
O614.24+2;O614.43+3
A
1001-4861(2016)01-0001-08
10.11862/CJIC.2016.017
2015-09-27。收修改稿日期:2015-11-13。
国家自然科学基金(No.21371150,21301152)、江苏省优势学科建设项目(No.2014-132)资助。
*通信联系人。E-mail:liuwl@yzu.edu.cn;Tel:+86 514 87975244;会员登记号:S06N6893M1405。
