饲粮粗蛋白质水平对猪胃肠道钙敏感受体基因表达、胃肠激素分泌及胃功能性酶活性的影响
2016-12-01县怡涵赵秀英丁立人孟祥龙朱伟云杭苏琴
县怡涵 赵秀英 丁立人 孟祥龙 王 超 朱伟云 杭苏琴
(南京农业大学消化道微生物研究室,南京210095)
饲粮粗蛋白质水平对猪胃肠道钙敏感受体基因表达、胃肠激素分泌及胃功能性酶活性的影响
县怡涵 赵秀英 丁立人 孟祥龙 王 超 朱伟云 杭苏琴*
(南京农业大学消化道微生物研究室,南京210095)
本试验旨在研究不同饲粮粗蛋白质水平对猪胃肠道钙敏感受体(CaSR)基因表达、胃肠激素分泌及胃功能性酶(H+-K+-ATP酶、胃蛋白酶)活性的影响,探讨小肠CaSR基因表达量与血清胃肠激素浓度以及胃CaSR基因表达量与H+-K+-ATP酶和胃蛋白酶活性的关系。选择35日龄、初始体重为(9.57±0.64) kg的“杜×长×大”杂交断奶仔猪18头,随机分为3组,分别为NP组(NRC标准粗蛋白质水平组)、MP组(较标准蛋白质水平组降低3%粗蛋白质水平)和LP组(较标准粗蛋白质水平组降低6%粗蛋白质水平),每组6个重复,每个重复1头仔猪。根据猪不同生长阶段的营养需要,分别于仔猪阶段(35~80日龄)饲喂粗蛋白质水平为20%(NP组)、17%(MP组)和14%(LP组)的饲粮,生长猪阶段(81~110日龄)饲喂粗蛋白质水平为18%(NP组)、15%(MP组)和12%(LP组)的饲粮,肥育猪阶段(111~160日龄)饲喂粗蛋白质水平为16%(NP组)、13%(MP组)和10%(LP组)的饲粮,平衡饲粮的赖氨酸(Lys)、蛋氨酸(Met)、苏氨酸(Thr)和色氨酸(Trp)水平,试验期125 d。试验结束后采集前腔静脉血液,屠宰全部试验猪后取胃食糜、胃、十二指肠、空肠和回肠组织及黏膜,测定血清胃肠激素浓度、胃食糜中胃蛋白酶和胃黏膜中H+-K+-ATP酶活性以及胃肠道各段组织中CaSR基因表达量。结果表明:1)LP组猪胃CaSR基因表达量显著高于NP和MP组(P<0.05),MP和LP组十二指肠及空肠CaSR基因表达量均显著低于NP组(P<0.05),而各组回肠CaSR基因表达量无显著差异(P>0.05)。2)与NP组相比,MP和LP组猪血清酪酪肽(PYY)和葡萄糖促胰岛素肽(GIP)浓度显著降低(P<0.05),MP组血清胆囊收缩素(CCK)浓度显著升高(P<0.05),LP组胃黏膜中H+-K+-ATP酶与胃食糜中胃蛋白酶活性显著升高(P<0.05)。3)胃CaSR基因表达量与胃黏膜中H+-K+-ATP酶和胃食糜中胃蛋白酶活性均呈显著正相关(P<0.05);十二指肠CaSR基因表达量与血清GIP浓度呈显著正相关(P<0.05),与血清PYY浓度呈显著正相关趋势(0.05≤P<0.10);空肠CaSR基因表达量与血清GIP和PYY浓度均呈显著正相关(P<0.05);回肠CaSR基因表达量与血清PYY浓度呈显著正相关趋势(0.05≤P<0.10)。综上所述,饲粮粗蛋白质水平降低影响猪胃肠道CaSR基因的表达,从而影响胃功能性酶活性及胃肠激素分泌。
猪;粗蛋白质水平;钙敏感受体;胃肠激素;H+-K+-ATP酶;胃蛋白酶
胃肠道不仅可以消化和吸收饲粮中的营养物质,还可以分泌胃肠激素,对维持机体的消化功能和稳态有重要作用。胃肠道散在分布多种内分泌细胞,包括胃壁细胞、D细胞及胃窦G细胞等[1],这些细胞能表达多种营养素感应受体[2],对糖类、脂肪酸、氨基酸及肽等营养素进行感应,调控胃肠激素的分泌,进而影响胃肠道的消化和吸收功能[3]。钙敏感受体(calcium sensing receptor,CaSR)基因能够感应氨基酸和多肽,尤其是芳香族L-氨基酸[如L-苯丙氨酸(L-Phe)和L-色氨酸(L-Try)][4-8],且在胃肠内分泌细胞中有广泛的表达[3-4]。CaSR基因和H+-K+-ATP酶在胃壁细胞中均有表达,且H+-K+-ATP酶的活性受CaSR基因调控。Busque等[9]研究发现,大鼠胃壁细胞的CaSR基因能介导氨基酸,从而增强与胃酸分泌相关的H+-K+-ATP酶活性。Mace等[10]试验发现,L-氨基酸能激活CaSR基因,从而促进体外培养的大鼠小肠葡萄糖促胰岛素肽(glucose insulinotropic peptide,GIP)、酪酪肽(peptide tyrosine tyrosine,PYY)和胰高血糖素样肽-1(glucagon-like peptide-1,GLP-1)的分泌。Liou等在研究小鼠I细胞[11]和Hira等[12]在研究小鼠STC-1细胞时均发现,L-Phe能够激活CaSR基因,从而促进胆囊收缩素(cholecystokinin,CCK)的分泌。目前为止,关于蛋白质及氨基酸对胃肠道中CaSR基因的表达及功能影响的研究主要集中在人、小鼠和大鼠等,且主要为体外试验,而在猪的体内研究尚未见报道。鉴此,本试验设计了不同粗蛋白质水平的饲粮,对猪进行长期饲喂,研究其对猪胃肠道CaSR基因表达、胃肠激素分泌及胃功能性酶活性的影响。
1 材料与方法
1.1 试验动物与试验设计
选择35日龄、初始体重为(9.57±0.64) kg的“杜×长×大”杂交断奶仔猪18头,根据饲粮粗蛋白质水平随机分为3组,分别为NP组(NRC标准粗蛋白质水平组)、MP组(较标准粗蛋白质水平组降低3%蛋白质水平)和LP组(较标准粗蛋白质水平组降低6%蛋白质水平),每组6个重复,每个重复1头仔猪,单栏饲养,自由饮水和采食。根据NRC标准(2012)配制玉米-豆粕型基础饲粮,正式试验前预饲3 d;预饲结束后,分别于仔猪阶段(35~80日龄)饲喂粗蛋白质水平为20%(NP组)、17%(MP组)和14%(LP组)的饲粮,生长猪阶段(81~110日龄)饲喂粗蛋白质水平为18%(NP组)、15%(MP组)和12%(LP组)的饲粮,肥育猪阶段(111~160日龄)饲喂粗蛋白质水平为16%(NP组)、13%(MP组)和10%(LP组)的饲粮,平衡赖氨酸(Lys)、蛋氨酸(Met)、苏氨酸(Thr)和Trp,试验期125 d。试验饲粮组成及营养水平见表1。试验于中国农业科学院亚热带农业研究所猪代谢室进行。试验前对猪舍环境、用具等进行清洗、熏蒸、浸泡消毒处理。试验期间按时进行消毒、驱虫、免疫等工作。
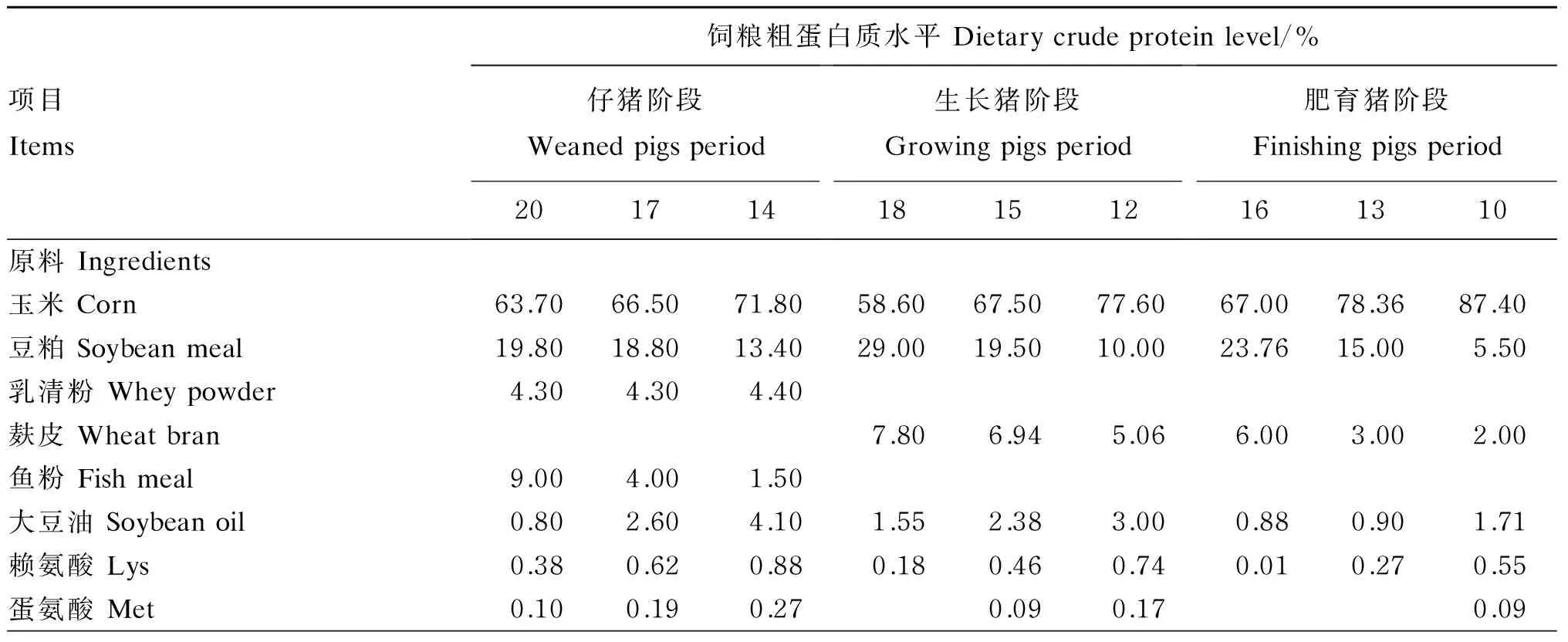
表1 试验饲粮组成及营养水平(饲喂基础)

续表2项目Items饲粮粗蛋白质水平Dietarycrudeproteinlevel/%仔猪阶段Weanedpigsperiod201714生长猪阶段Growingpigsperiod181512肥育猪阶段Finishingpigsperiod161310苏氨酸Thr0.090.210.330.010.140.260.060.19色氨酸Try0.010.040.080.020.070.010.06磷酸氢钙CaHPO40.000.741.150.690.780.900.500.550.65石粉Limestone0.520.700.790.870.890.900.550.550.55食盐NaCl0.300.300.300.300.300.300.300.300.30预混料Premix1)1.001.001.001.001.001.001.001.001.00合计Total100.00100.00100.00100.00100.00100.00100.00100.00100.00营养水平Nutrientlevels2)消化能DE/(MJ/kg)14.6014.6014.6014.2014.2014.2014.2014.2014.20粗蛋白质CP20.0517.0914.0918.2715.1612.3516.3013.1710.26赖氨酸Lys1.251.251.250.970.970.940.720.720.73蛋氨酸+半胱氨酸Met+Cys0.620.630.620.560.560.540.500.420.42苏氨酸Thr0.760.750.760.610.600.600.560.500.49色氨酸Try0.200.200.200.170.170.170.170.130.13精氨酸Arg1.090.930.701.080.820.570.940.700.44组氨酸His0.440.370.300.410.330.250.390.310.22异亮氨酸Ile0.700.590.450.640.490.350.600.450.30亮氨酸Leu1.521.321.111.351.140.941.321.130.91苯丙氨酸Phe0.810.700.560.770.620.460.710.570.41缬氨酸Val0.720.640.540.660.560.440.610.500.36必需氨基酸EAA8.117.386.497.226.265.266.525.434.41非必需氨基酸NEAA8.727.475.988.977.265.498.026.334.61必需氨基酸/总氨基酸EAA/TAA0.480.500.520.450.460.490.450.460.49
1)预混料为每千克饲粮提供 Premix provided the following per kg of diets:VA 10 800 IU,VD34 000 IU,VE 40 IU,VK34 mg,VB16 mg,VB212 mg,VB66 mg,VB120.05 mg,生物素 biotin 0.2 mg,叶酸 folic acid 2 mg,烟酸 nicotinic acid 50 mg,D-泛酸钙D-calcium pantothenate 25 mg,Fe (as FeSO4) 100 mg,Cu (as CuSO4) 150 mg,Mn (as MnO2) 40 mg,Zn (as ZnO) 100 mg,I (as KI) 0.5 mg,Se (as Na2SeO3) 0.3 mg。
2)消化能、粗蛋白质为计算值,其他营养水平为实测值。DE and CP were calculated values, while other nutrient levels were measured values.
1.2 样品采集与处理
1.2.1 血清样品
试验结束前所有试验猪禁食24 h,自由饮水。试验第125天,采集所有试验猪的前腔静脉血液100 mL,待血液凝固后离心取上层血清,-20 ℃保存,用于胃肠激素CCK、GIP和PYY浓度的测定。
1.2.2 胃肠道样品
采血后处死试验猪,剖开腹腔,立即取出整个消化道,分离、结扎各部位。将胃食糜充分混匀后尽快取出,-20 ℃保存,用于胃蛋白酶活性分析。冰上剪取胃、十二指肠、空肠、回肠组织及黏膜,在磷酸盐缓冲液(PBS)中漂去内容物后,放入液氮中保存,用于H+-K+-ATP酶活性分析及CaSR基因表达量检测。
1.3 测定指标及方法
1.3.1 血清胃肠激素浓度
利用酶联免疫吸附法(ELISA)检测试验猪前腔静脉血清中胃肠激素CCK、PYY和GIP的浓度,猪CCK(FU-Z044;CCK8 antibody,orb10260,biorbyt)、PYY(FU-Z240,PYY antibody,LS-C191185-400,LifeSpan BioSciences)及GIP(FU-A192;GIP antibody GTX37687 GeneTex)检测试剂盒均购自北京方程生物科技有限公司。
1.3.2 胃功能性酶活性
严格按照猪胃蛋白酶和H+-K+-ATP酶的试剂盒说明书检测胃食糜中胃蛋白酶及胃黏膜中H+-K+-ATP酶活性,胃蛋白酶(A080-1)和H+-K+-ATP酶(A069)试剂盒均购自南京建成悦浩科技有限公司。
1.3.3CaSR基因表达量

1.4 数据统计与分析
试验数据经Excel 2010初步整理后,采用SPSS 20.0软件中的单因素方差(one-way ANOVA)模型进行分析,S-N-K test进行差异显著性检验,P<0.05为差异显著,P<0.01为差异极显著;采用GraphPad Prism 5分析胃肠道中CaSR基因表达量与胃黏膜中H+-K+-ATP酶、胃食糜中胃蛋白酶活性及血清中胃肠激素浓度的相关关系,P<0.05为显著相关,0.05≤P<0.10为呈显著相关趋势。
2 结 果
2.1 饲粮粗蛋白质水平对猪胃肠道CaSR基因表达量的影响
由图1可以看出,在胃中,LP组CaSR基因表达量显著高于NP和MP组(P<0.05),NP与MP组差异不显著(P>0.05);十二指肠及空肠中,MP和LP组CaSR基因表达量均显著低于NP组(P<0.05),MP与LP组差异不显著(P>0.05);而回肠各组CaSR基因表达量无显著差异(P>0.05)。

同一组织数据柱形标注无字母或相同字母者表示差异不显著(P>0.05),不同小写字母者表示差异显著(P<0.05)。
Data columns of the same tissue with no letter or the same letter superscripts mean no significant difference (P>0.05), while with different small letter superscripts mean significant difference (P<0.05).
图1 饲粮粗蛋白质水平对猪胃肠道CaSR基因表达量的影响
Fig.1 Effects of dietary crude protein level onCaSRgene expression level of gastrointestinal tract of pigs
2.2 饲粮粗蛋白质水平对猪血清胃肠激素浓度及胃功能性酶活性的影响
由表2可以看出,与NP组相比,MP和LP组猪血清PYY和GIP浓度显著降低(P<0.05),MP与LP组无显著差异(P>0.05);MP组血清CCK浓度显著升高(P<0.05),LP组无显著变化(P>0.05);LP组胃黏膜中H+-K+-ATP酶与胃食糜中胃蛋白酶活性显著升高(P<0.05),MP组未表现出显著变化(P>0.05)。
2.3 猪胃肠道CaSR基因表达量与血清胃肠激素浓度和胃功能性酶活性相关性分析
由表3可以看出,胃中CaSR基因表达量与胃黏膜中H+-K+-ATP酶和胃食糜中胃蛋白酶活性均呈显著正相关(P<0.05);十二指肠CaSR基因表达量与血清GIP浓度呈显著正相关(P<0.05),与血清CCK浓度无显著相关性(P>0.05),与血清PYY浓度呈显著正相关趋势(0.05≤P<0.10);空肠CaSR基因表达量与血清GIP和PYY浓度均呈显著正相关(P<0.05),与血清CCK浓度无显著相关性(P>0.05);回肠CaSR基因表达量与血清CCK和GIP浓度均无显著相关性(P>0.05),与血清PYY浓度呈显著正相关趋势(0.05≤P<0.10)。
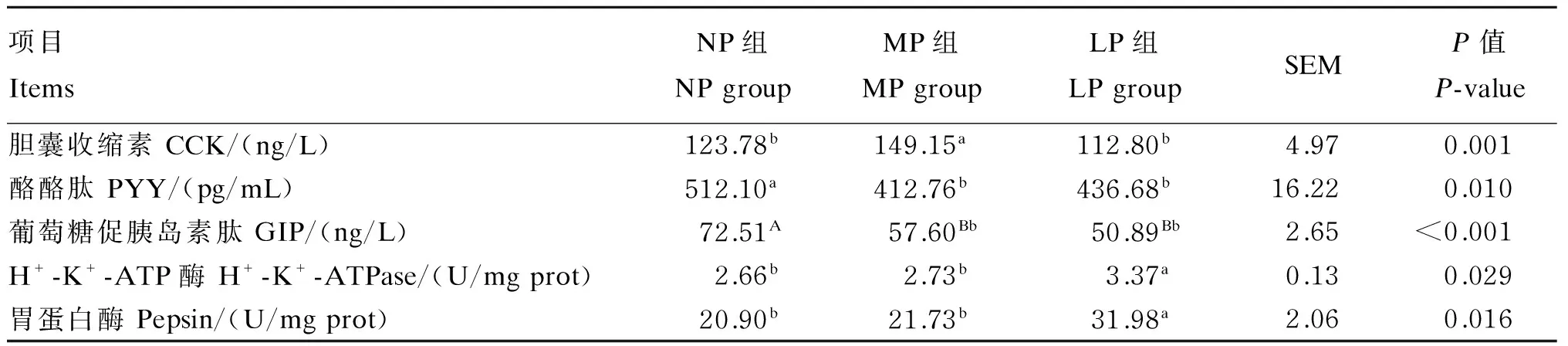
表2 饲粮粗蛋白质水平对猪血清胃肠激素浓度及胃功能性酶活性的影响
同行数据肩标相同或无字母表示差异不显著(P>0.05),不同小写字母表示差异显著(P<0.05),不同大写字母表示差异极显著(P<0.01)。
In the same row, values with the same or no letter superscripts mean no significant difference (P>0.05), while with different small letter superscripts mean significant difference (P<0.05), and with different capital letter superscripts mean significant difference (P<0.01).
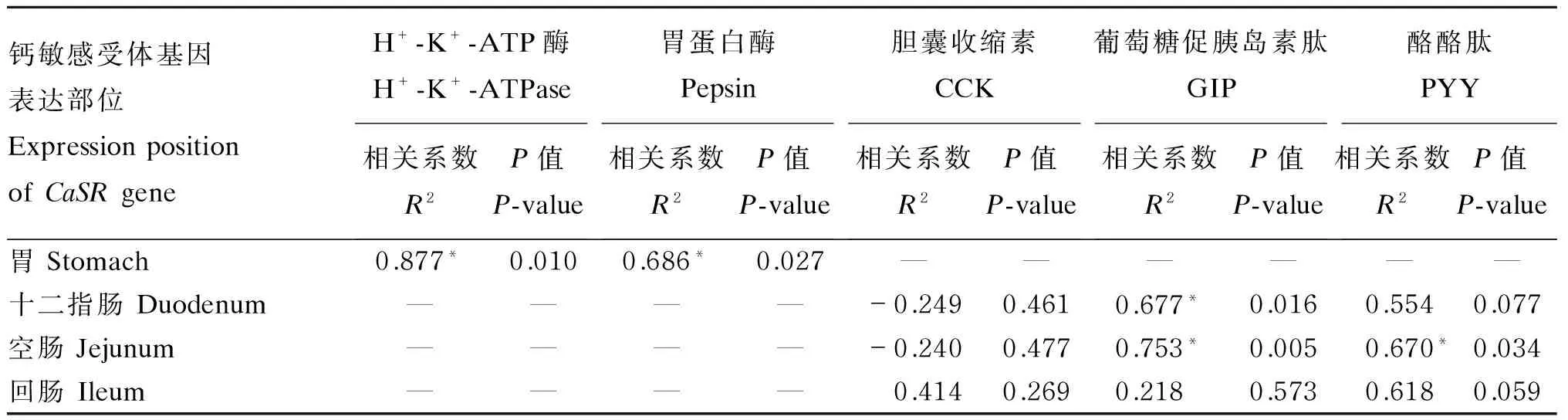
表3 猪胃肠道CaSR基因表达量与血清胃肠激素浓度、H+-K+-ATP酶及胃蛋白酶活性相关性分析
数据肩标*表示显著相关(P<0.05)。
Values with * mean significant correlation (P<0.05).
3 讨 论
CaSR基因在啮齿类动物消化道诸多部位中广泛表达,包括食管、胃、小肠[15]。本研究发现CaSR基因在猪胃及小肠各部位均有表达。饲粮粗蛋白质的降解产物主要为小肽和氨基酸[2],这些降解产物能够激活CaSR[10,16]。Conigrave等[17-18]发现,细胞外钙离子处于一定浓度下,芳香族氨基酸可以调节CaSR的活性,且随着氨基酸浓度的升高,CaSR基因表达量也随之升高。本研究发现,当饲粮粗蛋白质水平降低时,胃CaSR基因表达量升高,这可能是因为饲粮中平衡的4种氨基酸(Lys、Met、Thr和Try)中含有可以激活CaSR基因的Try(随着饲粮粗蛋白质水平的降低色氨酸添加量增加),饲粮进入胃中粗蛋白质尚未降解为氨基酸,所以额外添加的Try含量越高,对CaSR的激活作用越强,CaSR基因表达量则越高;十二指肠和空肠的CaSR基因表达量随饲粮粗蛋白质水平的降低而降低,可能是由于饲粮中粗蛋白质在胃中经过消化后产生的部分肽以及氨基酸进入小肠后激活了十二指肠和空肠的CaSR基因而使其表达量升高;而回肠的CaSR基因表达量虽随着粗蛋白质水平的降低而略有降低,但并未表现出显著变化,这可能是因为氨基酸在十二指肠和空肠中已经被大量消化吸收,到达回肠的氨基酸浓度较低,故未能调控CaSR的基因表达量。
研究表明,CaSR基因在胃壁细胞中表达量较高[19-20],L-芳香族氨基酸可以激活CaSR基因,从而增强H+-K+-ATP酶和胃蛋白酶活性[9,21]。本研究中,随着饲粮粗蛋白质水平的降低,色氨酸添加量逐渐增加,胃蛋白酶与H+-K+-ATP酶的活性升高;相关性分析显示,随着猪胃CaSR基因表达量的升高,H+-K+-ATP酶与胃蛋白酶活性随之升高,二者存在显著正相关,与上述研究结果一致。研究还发现,随着粗蛋白质摄入水平的增加,人体内的GIP、PYY等激素的分泌增加[22]。本研究结果表明,随饲粮粗蛋白质水平的降低,胃肠激素PYY、GIP的分泌也降低。研究也表明,氨基酸对大鼠小肠GIP、CCK、PYY的分泌有促进作用,CaSR特异性激活剂NPS R568可以显著增强这种作用,而CaSR特异性抑制剂Calhex 231显著抑制这种作用[11]。据此,本试验将猪小肠CaSR基因表达量与胃肠激素进行了相关性分析,结果显示,除CCK外,其余胃肠激素与十二指肠和回肠的CaSR基因表达量呈显著或趋于显著的正相关关系。当饲粮粗蛋白质水平降低时,小肠中蛋白质水解产生的氨基酸的水平降低,使CaSR对氨基酸的敏感性降低,导致胃肠激素分泌量下降。以上结果初步表明,氨基酸可以通过激活CaSR调节胃肠激素分泌、胃蛋白酶及H+-K+-ATP酶活性。Shi等[23]和Leray等[24]研究表明,饲粮中粗蛋白质水平降低会促进胃肠道CCK的释放。本研究中,蛋白质水平降低3%,小肠CaSR基因表达量降低,但CCK的分泌量有所升高,可能是由于营养摄入不足所产生的代偿性反应,以保证在低蛋白质水平的情况下,机体仍能保持稳定的状态。目前,由于体内环境复杂,影响因素多,有关蛋白质及其水解产物与CaSR对胃肠激素分泌作用的影响在机体内的研究甚少。鉴此,在后续研究中,课题组计划采用体外灌流技术研究猪胃肠道CaSR基因表达量与胃肠激素分泌的关系,并利用免疫组化及免疫荧光技术探究氨基酸激活CaSR调节胃肠激素分泌、胃功能性酶活性的信号通路,揭示猪胃肠道CaSR基因表达量与胃肠激素分泌和胃功能性酶活性之间的关系及其机制。
4 结 论
综上所述,当饲粮中粗蛋白质水平降低时会影响猪胃肠道中CaSR基因的表达量,从而影响部分胃肠激素(GIP、PYY)的分泌以及胃功能性酶(胃蛋白酶、H+-K+-ATP酶)活性。
[1] 陈杰.家畜生理学[M].4版.北京:中国农业出版社,2003:136.
[2] GEIBEL J P,HEBERT S C.The functions and roles of the extracellular Ca2+-sensing receptor along the gastrointestinal tract[J].Annual Review of Physiology,2009,71(1):205-217.
[3] BRENNAN S C,DAVIES T S,SCHEPELMANN M,et al.Emerging roles of the extracellular calcium-sensing receptor in nutrient sensing:control of taste modulation and intestinal hormone secretion[J].British Journal of Nutrition,2014,111(Suppl.1):S16-S22.
[4] 赵秀英,杭苏琴,朱伟云.钙敏感受体介导的信号传导通路及生理功能[J].动物营养学报,2015,27(3):703-714.
[5] CONIGRAVE A D,BROWN E M.Taste receptors in the gastrointestinal tract II.L-amino acid sensing by calcium-sensing receptors:implications for GI physiology[J].American Journal of Physiology-Gastrointestinal and Liver Physiology,2006,291(5):G753-G761.
[6] CONIGRAVE A D,MUN H C,DELBRIDGE L,et al.L-amino acids regulate parathyroid hormone secretion[J].Journal of Biological Chemistry,2004,279(37):38151-38159.
[7] DIAKOGIANNAKI E,PAIS R,TOLHURST G,et al.Oligopeptides stimulate glucagon-like peptide-1 secretion in mice through proton-coupled uptake and the calcium-sensing receptor[J].Diabetologia,2013,56(12):2688-2696.
[8] BUSQUE S M,KERSTETTER J E,GEIBEL J P,et al.L-type amino acids stimulate gastric acid secretion by activation of the calcium-sensing receptor in parietal cells[J].American Journal of Physiology:Gastrointestinal and Liver Physiology,2005,289(4):G664-G669.
[9] MACE O J,SCHINDLER M,PATEL S.The regulation of K-andL-cell activity by GLUT2 and the calcium-sensing receptor CaSR in rat small intestine[J].The Journal of Physiology,2012,590(12):2917-2936.
[10] LIOU A P,SEI Y,ZHAO X L,et al.The extracellular calcium-sensing receptor is required for cholecystokinin secretion in response toL-phenylalanine in acutely isolated intestinal I cells[J].American Journal of Physiology:Gastrointestinal Liver Physiology,2011,300(4):G538-G546.
[11] HIRA T,NAKAJIMA S,ETO Y,et al.Calcium-sensing receptor mediates phenylalanine-induced cholecystokinin secretion in enteroendocrine STC-1 cells[J].The FEBS Journal,2008,275(18):4620-4626.
[12] CHOMCZYNSKI P,SACCHI N.The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction:twenty-something years on[J].Nature Protocols,2006,1(2):581-585.
[13] 张源淑,邹思湘,赵茹茜,等.乳源活性肽对早期断奶仔猪胃泌素mRNA表达的影响[J].农业生物技术学报,2004,12(1):61-65.
[14] CHATTOPADHYAY N,CHENG I,ROGERS K,et al.Identification and localization of extracellular C2+-sensing receptor in rat intestine[J].American Journal of Physiology:Gastrointestinal and Liver Physiology,1998,274(1):G122-G130.
[15] NAKAJIMA S,HIRA T,HARA H.Calcium-sensing receptor mediates dietary peptide-induced CCK secretion in enteroendocrine STC-1 cells[J].Molecular Nutrition & Food Research,2012,56(5):753-760.
[16] CONIGRAVE A D,FRANKS A H,BROWN E M,et al.L-amino acid sensing by the calcium-sensing receptor:a general mechanism for coupling protein and calcium metabolism[J].European Journal of Clinical Nutrition,2002,56(11):1072-1080.
[17] CONIGRAVE A D,QUINN S J,BROWN E M.L-amino acid sensing by the extracellular Ca2+-sensing receptor[J].Proceedings of the National Academy of Sciences of the United States of America,2000,97(9):4814-4819.
[18] CAROPPO R,GERBINO A,DEBELLIS L,et al.Asymmetrical,agonist-induced fluctuations in local extracellular[Ca2+] in intact polarized epithelia[J].The EMBO Journal,2001,20(22):6316-6326.
[19] CHANG W H,SHOBACK D.Extracellular Ca2+-sensing receptors—an overview[J].Cell Calcium,2004,35(3):183-196.
[20] HEBERT S C,CHENG S,GEIBEL J.Functions and roles of the extracellular Ca2+-sensing receptor in the gastrointestinal tract[J].Cell Calcium,2004,35(3):239-247.
[21] BELZA A,RITZ C,SØRENSEN M Q,et al.Contribution of gastroenteropancreatic appetite hormones to protein-induced satiety[J].American Journal of Clinical Nutrition,2013,97(5):980-989.
[22] SHI G,LERAY V,SCARPIGNATO C,et al.Specific adaptation of gastric emptying to diets with differing protein content in the rat:is endogenous cholecystokinin implicated[J].Gut,1997,41(5):612-618.
[23] LERAY V,SEGAIN J P,CHERBUT C,et al.Adaptation to low-protein diet increases inhibition of gastric emptying by CCK[J].Peptides,2003,24(12):1929-1934.
*Corresponding author, professor, E-mail: suqinhang69@njau.edu.cn
(责任编辑 李慧英)
Effects of Dietary Crude Protein Level on Calcium Sensing Receptor Gene Expression of Gastrointestinal Tract, Gastrointestinal Hormone Secretion and the Activities of Gastric Function Enzymes of Pigs
XIAN Yihan ZHAO Xiuying DING Liren MENG Xianglong WANG Chao ZHU Weiyun HANG Suqin*
(Laboratory of Gastrointestinal Microbiology, Nanjing Agricultural University, Nanjing 210095, China)
The experiment was conducted to investigate the effects of different dietary crude protein (CP) levels on calcium sensing receptor (CaSR) gene expression of gastrointestinal tract, gastrointestinal hormone secretion and the activities of gastric function enzymes (pepsin and H+-K+-ATPase) of pigs, and to explore the relationships betweenCaSRgene expression level of small intestine and the concentration of gastrointestinal hormone in serum,CaSRgene expression level of stomach and the activities of H+-K+-ATPase and pepsin, respectively. Eighteen 35-day-old “Duroc×Landrace×Large White” crossed weaned pigs with initial body weight of (9.57±0.64) kg were randomly assigned into 3 group with 6 replicates per group and 1 pig per replicate, including NP group (NRC standard CP level group), MP group (decreased 3% CP level compared to the standard CP level group) and LP group (decreased 6% CP level compared to the standard CP level group). According to the nutrient needs of pigs in different growth periods, pigs were fed diets contained 20% (NP group), 17% (MP group) and 14% (LP group) CP levels in weaned pigs period (35 to 80 days of age), respectively; diets contained 18% (NP group), 15% (MP group) and 12% (LP group) CP levels in growing pigs period (81 to 110 days of age), respectively; diets contained 16% (NP group), 13% (MP group) and 10% (LP group) CP levels in finishing pigs period (111 to 160 days of age), respectively. The levels of dietary lysine (Lys), methionine (Met), threonine (Thr) and tryptophan (Trp) were balanced. The experimental period lasted for 125 days. Precaval vein blood were collected by the end of the experiment, then all pigs were slaughtered, and gastric chyme, stomach, duodenum, jejunum and ileum tissues and mucosa were collected, to determination of the concentration of gastrointestinal hormones in serum, the activities of pepsin of gastric chyme and H+-K+-ATPase of gastric mucosa andCaSRgene expression level of each tissues of the gastrointestinal tract. The results showed as follows: 1)CaSRgene expression level of stomach of pigs in LP group was significantly higher than that in NP and MP groups (P<0.05), andCaSRgene expression level of duodenum and jejunum in MP and LP groups was significantly lower than that in NP group (P<0.05), but no significant difference was found inCaSRgene expression level of ileum among all groups (P>0.05). 2) Compared with NP group, the concentrations of peptide tyrosine tyrosine (PYY) and glucose insulinotropic peptide (GIP) in serum of pigs in MP and LP groups were significantly decreased (P<0.05), the concentration of cholecystokinin (CCK) in serum in MP group was significantly increased (P<0.05), and the activities of pepsin of gastric chyme and H+-K+-ATPase of gastric mucosa in LP group were significantly increased (P<0.05). 3)CaSRgene expression level in stomach had a significantly positive correlations with the activities of pepsin of gastric chyme and H+-K+-ATPase of gastric mucosa (P<0.05),CaSRgene expression level in duodenum had a significantly positive correlation with the concentration of GIP in serum (P<0.05), and had a positive correlation tendency with the concentration of PYY in serum (0.05≤P<0.10),CaSRgene expression level in jejunum had a significantly positive correlations with the concentrations of GIP and PYY in serum (P<0.05), andCaSRgene expression level in ileum had a positive correlation tendency with the concentration of PYY in serum (0.05≤P<0.10). In conclusion, reduction of dietary CP level affectsCaSRgene expression of gastrointestinal tract, the activities of gastric function enzymes and gastrointestinal hormone secretion.[ChineseJournalofAnimalNutrition, 2016, 28(11):3634-3641]
pigs; crude protein level; calcium sensing receptor; gastrointestinal hormone; H+-K+-ATPase; pepsin
2016-05-12
国家973项目(2013CB127301)资助
县怡涵(1992—),女,甘肃天水人,硕士研究生,从事动物营养与胃肠道健康方面的研究。E-mail: 2014105006@njau.edu.cn
*通信作者:杭苏琴,教授,硕士生导师,E-mail: suqinhang69@njau.edu.cn
10.3969/j.issn.1006-267x.2016.11.033
S828
A
1006-267X(2016)11-3634-08
