猪氨基酸代谢节俭机制新假说
2016-12-01孙志洪许庆庆印遇龙朱伟云江青艳黄飞若
孙志洪 李 貌 许庆庆 印遇龙 朱伟云 江青艳 黄飞若
(1. 西南大学生物饲料与分子营养实验室,重庆400715;2.中国科学院亚热带农业生态研究所,长沙410125;3.南京农业大学动物科技学院,南京210095;4.华南农业大学动物科技学院,广州510642;5.华中农业大学动物科技学院,武汉430070)
猪氨基酸代谢节俭机制新假说
孙志洪1李 貌1许庆庆1印遇龙2朱伟云3江青艳4黄飞若5
(1. 西南大学生物饲料与分子营养实验室,重庆400715;2.中国科学院亚热带农业生态研究所,长沙410125;3.南京农业大学动物科技学院,南京210095;4.华南农业大学动物科技学院,广州510642;5.华中农业大学动物科技学院,武汉430070)
猪尿氮排放量为总氮排放量的60%~70%,而尿素是尿液中的主要含氮物,其合成速率在很大程度上决定着尿氮以及总氮的排放量。因此,降低猪肝脏尿素合成速率是减少氮排放量的根本途径。本文首先介绍了当前猪氮减排常用的营养调控技术,然后分别就肝脏尿素合成的直接前体物(氨)与间接前体物(如甘氨酸和丙氨酸)以及氨基酸代谢燃料功能替代机制进行论述,在此基础上提出猪氨基酸代谢节俭机制新假说,即促进丙酮酸/葡萄糖等物质的供能效率,以降低谷氨酸等氨基酸的代谢速率,从而达到减少门静脉尿素前体物净流量、肝脏尿素合成以及尿氮排放量的目的。
猪;氮排放;氨基酸;代谢节俭;丙酮酸脱氢酶
近年来,氮排放引发的环境污染随畜禽养殖规模和集约化程度的不断扩大而日趋严重。目前,全球畜禽氮排放量的估计值高达8 900万~16 400万t;我国畜禽氮排放量约为3 000万t,其中单胃动物(主要是猪)的氮排放量约占总氮排放量的60%。与此同时,蛋白质资源紧缺是全世界共同面临的问题;2014年,中国蛋白质饲料原料的进口量约为4 000万t,鱼粉和大豆的进口依存度达到70%。因此,如何提高蛋白质的利用效率、减少氮排放量已成为我国畜禽养殖业尤其是养猪业迫切需要解决的科学问题。
1 猪氮减排常用的营养调控技术
目前围绕生猪氮排放已经开展了大量研究,包括以理想氨基酸模式为基础配制饲粮[1]、降低饲粮蛋白质含量并补充限制性氨基酸[2-7]、增加饲粮中可发酵性碳水化合物的比例[2,8-9]以及添加酶制剂、益生素和有机酸等添加剂[10-11]。尽管大量研究已经证实低蛋白质饲料可显著降低猪的氮排放量[2,6,12-13],但这一营养调控措施尚未成为养猪生产业的通用技术,尤其是在以获取快速生长为目标的集约化生产体系中;其他营养调控技术也只能在一定程度上减少猪的氮排放量。
鉴此,有必要深入研究猪的氮排放机制以明确关键调控靶点。猪尿氮排放量占总氮排放量的比例为60%~70%[9,14-15],而尿素是尿液中的主要含氮物,其合成速率在很大程度上决定了尿氮以及总氮的排放量。因此,降低猪肝脏尿素合成速率是减少氮排放量的重要策略,而明确尿素前体物的种类与来源则是开展氮减排研究的首要前提。
2 尿素前体物
2.1 氨——尿素的直接前体物
氨为尿素的直接前体物,主要来源于氨基酸的分解代谢。门静脉回流组织(portal-drained viscera,PDV)是氨基酸代谢的重要场所,如饲粮中97%的谷氨酸和天门冬氨酸、70%的谷氨酰胺、40%~50%的丝氨酸和甘氨酸、40%的精氨酸和脯氨酸、20%~40%的支链氨基酸以及30%~60%的其他必需氨基酸均在PDV中发生分解代谢[4,16-20]。氨基酸脱氨后转化为乙酰辅酶A、丙酮酸、草酰乙酸、琥珀酰辅酶A、延胡索酸和α-酮戊二酸等物质进入三羧酸(tricarboxylic acid,TCA)循环以氧化供能[21](图1)。氨基酸在PDV中的广泛代谢导致门静脉血氨浓度远高于其他部位,进入肝脏后大部分血氨用于尿素的合成[22]。

Glucose:葡萄糖;pyruvate:丙酮酸;threonine:苏氨酸;cysteine:半胱氨酸;serine:丝氨酸;glycine:甘氨酸;alanine:丙氨酸;tryptophan:色氨酸;lysine:赖氨酸;leucine:亮氨酸;isoleucine:异亮氨酸;tyrosine:酪氨酸;phenylalanine:苯丙氨酸;acetyl-CoA:乙酰辅酶A;citrate:柠檬酸;isocitrate:异柠檬酸;glutamine:谷氨酰胺;histidine:组氨酸;arginine:精氨酸;proline:脯氨酸;glutamate:谷氨酸;α-ketoglutarate:α-酮戊二酸;succinyl-CoA:琥珀酰辅酶A;methionine:蛋氨酸;valine:缬氨酸;succinate:琥珀酸;fumarate:富马酸;malate:苹果酸;oxaloacetate:草酰乙酸;aspartate:天门冬氨酸;NAD+:烟酰胺腺嘌呤二核苷酸nicotinamide adenine dinucleotide;NADH:还原型烟酰胺腺嘌呤二核苷酸reduced nicotinamide adenine dinucleotide;GTP:三磷酸鸟苷guanosine triphosphate;GDP:二磷酸鸟苷guanosine diphosphate;FAD:黄素腺嘌呤二核苷酸flavin adenine dinucleotide;FADH2:还原型黄素腺嘌呤二核苷酸reduced flavin adenine dinucleotide。
图1 氨基酸氧化代谢途径
Fig.1 The oxidative metabolism pathways of amino acids[21]
2.2 甘氨酸和丙氨酸——尿素的间接前体物
前期研究发现,采食粗蛋白质水平为20%、17%和14%饲粮的仔猪门静脉谷氨酸净吸收速率分别为-4.43、-5.65和-6.64 mg/min;门静脉氨的净吸收速率则分别为2.86、2.68和2.38 mg/min[23]。该结果与其他报道一致,即猪PDV中广泛代谢谷氨酸等氨基酸,同时也产生大量的氨[4,17,20]。此外,采食上述3个蛋白质水平饲粮的仔猪门静脉甘氨酸与丙氨酸的净吸收量占总氨基酸净吸收量的比例分别为38.2%、37.3%和37.0%;甘氨酸和丙氨酸在肝脏中的消耗量占总氨基酸代谢量的比例分别为52.0%、49.5%和43.8%。这一氨基酸代谢规律的发现引起人们对甘氨酸和丙氨酸的来源及代谢去路的深入思考。
传统观点认为丝氨酸是甘氨酸的主要前体物,而Wu[21]则提出不同的观点,认为仅有10%左右的甘氨酸来源于丝氨酸;丙氨酸的前体物包括丙酮酸、丝氨酸和天门冬氨酸[24]。根据氨基酸的代谢转化途径[21,24](图2),推测PDV中广泛代谢的氨基酸(如谷氨酸、谷氨酰胺和天门冬氨酸等)极有可能是甘氨酸和丙氨酸的重要前体物。为证实这一推测,利用血插管与15N稳定性同位素示踪技术发现,PDV中转化为甘氨酸和丙氨酸的谷氨酸占谷氨酸代谢总量的比例约为30%。这一氨基酸代谢规律实质上反映了机体的一项重要自我保护机制:PDV中氨基酸代谢所产生的氨如果全部直接进入肝脏会造成氨的浓度过高,有可能引起肝损伤,而将其中一部分氨转化为分子质量相对较小的甘氨酸和丙氨酸(分子质量分别为75和89 u,远低于氨基酸的平均分子质量),不仅能有效降低氨的浓度、减轻肝脏的氨负担,同时又能发挥谷氨酸等氨基酸在PDV中的代谢燃料功能。
Berthiaume等[25]和Doepel等[26]先后报道肝脏会代谢大量的甘氨酸和丙氨酸,且甘氨酸是重要的生氨氨基酸[27];丙氨酸会增加饥饿大鼠肝细胞尿素的合成[28],丙氨酸也是甘氨酸代谢过程的重要参与者[29]。以上研究表明,甘氨酸和丙氨酸与肝脏尿素合成密切相关[27-29],但尚未有报道证实甘氨酸和丙氨酸是尿素合成的重要氮来源。结合前人的研究报道,推测在肝脏中多余的甘氨酸和丙氨酸用来合成尿素。为证实这一推测,利用血插管与15N稳定性同位素示踪技术开展了甘氨酸和丙氨酸在肝脏中代谢去路的研究,研究表明甘氨酸和丙氨酸是尿素的重要间接前体物[30]。
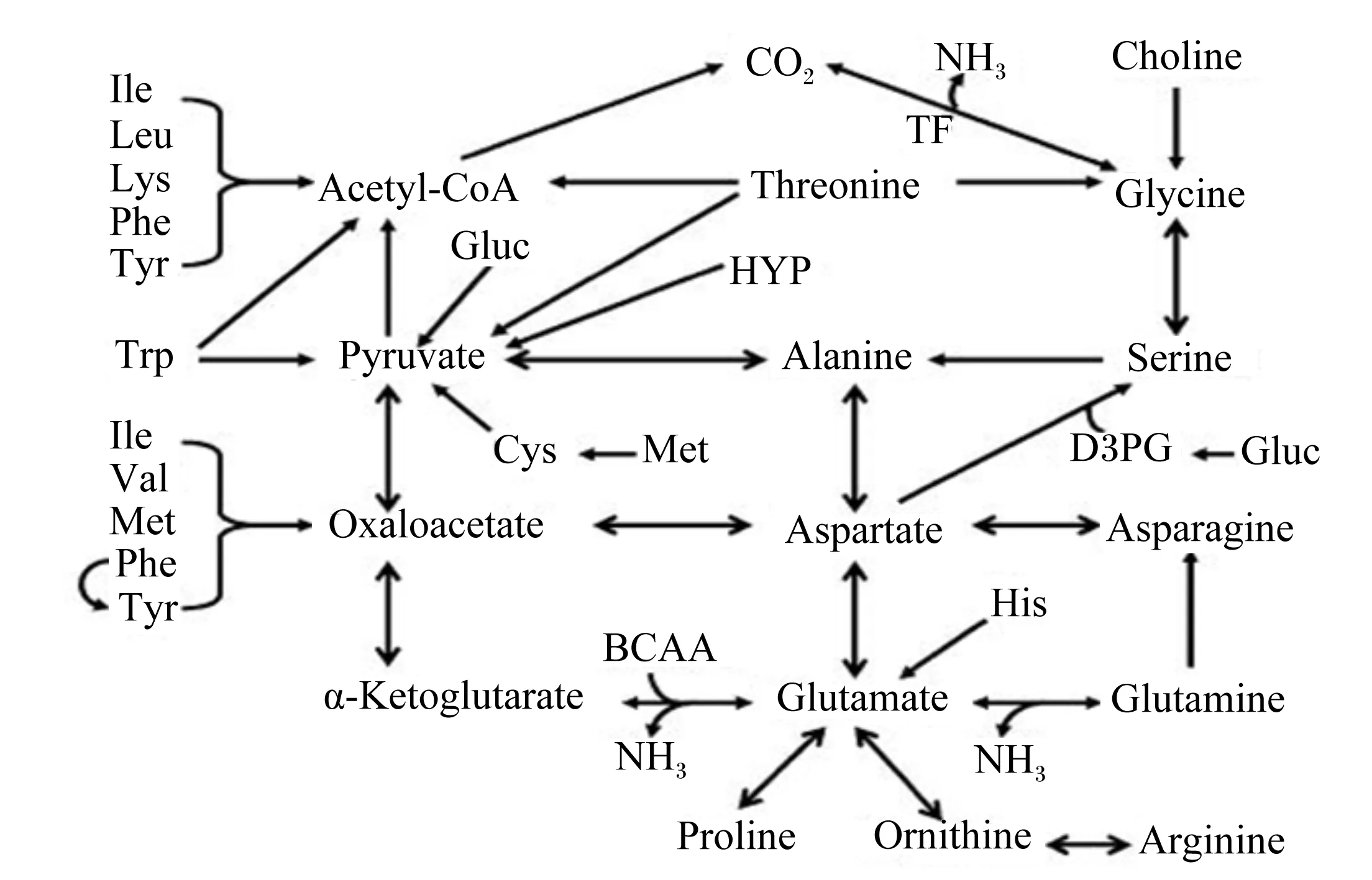
Ile:异亮氨酸isoleucine;Leu:亮氨酸leucine;Lys:赖氨酸lysine;Phe:苯丙氨酸phenylalanine;Tyr:酪氨酸tyrosine;Trp:色氨酸tryptophan;Acetyl-CoA:乙酰辅酶A;CO2:二氧化碳carbon dioxide;NH3:氨ammonia;Choline:胆碱;Threonine:苏氨酸;Glycine:甘氨酸;Serine:丝氨酸;Alanine:丙氨酸;Pyruvate:丙酮酸;Gluc:葡萄糖glucose;Val:缬氨酸valine;Met:蛋氨酸methionine;Oxaloacetate:草酰乙酸;Aspartate:天门冬氨酸;Asparagine:天门冬酰胺;α-Ketoglutarate:α-酮戊二酸;BCAA:支链氨基酸branched-chain amino acids;Glutamate:谷氨酸;His:组氨酸histidine;Glutamine:谷氨酰胺;Proline:脯氨酸;Ornithine:鸟氨酸;Arginine:精氨酸;Cys:半胱氨酸cysteine;D3PG:D-3-磷酸甘油酸D-3-phosphoglycerate;HYP:羟(基)脯氨酸 hydroxyproline;TF:四氢叶酸 tetrahydrofolic acid。
图2 氨基酸的代谢转化途径
Fig.2 The pathways of metabolic transformation between amino acids[21,24]
3 氨基酸代谢燃料功能替代机制
综上所述,减少PDV中尿素前体物(主要包括氨、甘氨酸和丙氨酸)的生成是降低尿素合成以及尿氮排放量的关键,而提供氨基酸代谢燃料替代物以降低氨基酸的氧化代谢速率是实现这一目标的重要途径。有关氨基酸代谢燃料替代物的探索开始于20世纪90年代,但由于研究甚少,迄今为止尚未取得突破性进展。除谷氨酸/谷氨酰胺外,葡萄糖也是各类组织细胞的重要燃料物质,但通常情况下葡萄糖难以抑制谷氨酸/谷氨酰胺的氧化分解[17];不仅如此,谷氨酸/谷氨酰胺还会显著降低葡萄糖的氧化代谢速率[31-33]。因此,如何提高葡萄糖在PDV中的氧化供能效率是猪氮减排研究亟待解决的科学问题。
氨基酸、脂肪、葡萄糖的氧化路径虽不同,但最后都汇聚于同一点,即TCA循环[34](图3)。乙酰辅酶A、丙酮酸、草酰乙酸、琥珀酰辅酶A、延胡索酸和α-酮戊二酸是氨基酸进入TCA循环的中间产物[21],其中丙酮酸在三大物质的代谢联系中起重要的枢纽作用,若丙酮酸代谢发生异常将会导致众多疾病的发生,包括糖尿病、肥胖[35]、线粒体功能紊乱[36]、心脏衰竭[37]、神经退行性疾病[38]和癌症[39]。研究表明,丙酮酸是氨基酸氧化代谢的重要调控因子[40-42]。鉴于丙酮酸在三大物质代谢过程中所发挥的重要作用,推测丙酮酸有可能是氨基酸和葡萄糖代谢的共同调控靶点,促进丙酮酸在PDV中的氧化分解有望增加葡萄糖的氧化代谢速率、抑制氨基酸的代谢燃料功能,从而降低尿素前体物(氨、甘氨酸和丙氨酸)的生成以及尿素的合成。

Lipids:脂类;fatty acids:脂肪酸;acyl-CoA:酰基辅酶A;acetyl-CoA:乙酰辅酶A;carbohydrates:碳水化合物;glucose:葡萄糖;ADP:二磷酸腺苷adenosine diphosphate;ATP:三磷酸腺苷adenosine triphosphate;glycolysis:醣酵解;pyruvate:丙酮酸;proteins:蛋白质;amino acids:氨基酸;transamination:转氨基;deamination:脱氨;CO2:二氧化碳carbon dioxide;TCA cycle:三羧酸循环;β-oxidation:β-氧化;NADH:还原型烟酰胺腺嘌呤二核苷酸reduced nicotinamide adenine dinucleotide;FADH2:还原型黄素腺嘌呤二核苷酸reduced flavin adenine dinucleotide;Pi:磷酸基;ETC:电子传递链electron transfer chain。
图3 三大营养物质氧化代谢途径
Fig.3 The oxidative metabolism pathways of three major nutrients[34]
哺乳动物细胞中,丙酮酸脱氢酶复合体(pyruvate dehydrogenase complex,PDC)负责催化丙酮酸转化为乙酰辅酶A。PDC由3种酶[丙酮酸脱氢酶(pyruvate dehydrogenase,PDH)、二氢硫辛酰转乙酰基酶、二氢硫辛酸脱氢酶]和6种辅助因子[焦磷酸硫胺素、硫辛酸、黄素腺嘌呤二核苷酸(flavin adenine dinucleotide,FAD)、烟酰胺腺嘌呤二核苷酸(nicotinamide adenine dinucleotide,NAD)、辅酶A(coenzyme A,CoA)和Mg2+]组成。PDH上游调控因子主要包括丙酮酸脱氢酶激酶(pyruvate dehydrogenase kinase,PDK)和丙酮酸脱氢酶磷酸酶(pyruvate dehydrogenase phosphatase,PDP),调控机制如图4所示[43]。PDK1通过磷酸化PDH分子上的丝氨酸残基(包括Ser-293、Ser-300、Ser-232)抑制其活性,而PDP则通过去磷酸化恢复PDH以及PDC的活性[44]。酪氨酸磷酸化将分别激活PDK活性和抑制PDP活性[45]。综上所述,PDK/PDP/PDH轴极有可能是葡萄糖/氨基酸的调控靶点。

Gluconeogenesis:糖异生;cytosol:细胞溶质;glucose:葡萄糖;PEP:磷酸烯醇式丙酮酸phosphoenolpyruvate;pyruvate:丙酮酸;glycolysis:糖酵解;lipid biosynthesis:脂类生物合成;acetyl-CoA:乙酰辅酶A;CO2:二氧化碳carbon dioxide;H+:氢离子;NAD+:烟酰胺腺嘌呤二核苷酸nicotinamide adenine dinucleotide;NADH:还原型烟酰胺腺嘌呤二核苷酸reduced nicotinamide adenine dinucleotide;mitochondrial matrix:线粒体基质;PDC active:有活性的丙酮酸脱氢酶复合体active pyruvate dehydrogenase complex;PDC inactive:无活性的丙酮酸脱氢酶复合体inactive pyruvate dehydrogenase complex;PDK:丙酮酸脱氢酶激酶pyruvate dehydrogenase kinase;isoenzymes:同功异构酶;PDP:丙酮酸脱氢酶磷酸酶pyruvate dehydrogenase phosphatase;insulin:胰岛素:Ca2+:钙离子;ATP:三磷酸腺苷adenosine triphosphate;P1-3:磷酸基1-3;TCA cycle:三羧酸循环。
图4 丙酮酸脱氢酶复合体调节机制
Fig.4 The regulatory mechanisms of pyruvate dehydrogenase complex[43]
丙酮酸氧化代谢速率随PDC活性的升高而提高[46]。小分子物质二氯乙酸(dichloroacetate,DCA)具有诱导细胞自噬、降低细胞增殖的重要功能。此外,研究表明DCA通过抑制PDK活性来激活PDH活性,从而降低糖酵解比例、提高葡萄糖的氧化代谢速率[47-48]。谷氨酰胺氧化代谢速率随葡萄糖氧化代谢速率的升高而降低[49]。研究表明,促进丙酮酸的氧化代谢将导致谷氨酸脱氢酶的活性降低,从而降低来源于谷氨酰胺的乙酰辅酶A的生成[49]。由此可见,通过调控丙酮酸/葡萄糖氧化代谢速率来抑制氨基酸代谢燃料功能是可行的。
4 小 结
综上所述,在PDV中异常增加的甘氨酸和丙氨酸归因于谷氨酸等氨基酸的过度代谢,甘氨酸和丙氨酸是肝脏尿素合成的重要前体物。降低氨基酸的氧化代谢速率是减少尿素合成前体物和肝脏尿素合成的关键。促进丙酮酸/葡萄糖在猪PDV中的供能效率有望增加葡萄糖的氧化代谢速率、抑制氨基酸的代谢燃料功能,从而减少尿素前体物的生成以及尿氮排放量,而PDK/PDP/PDH轴可能是丙酮酸氧化代谢的调控靶点。虽然在体外试验、老鼠试验以及人类临床试验上已经证实通过促进丙酮酸/葡萄糖的氧化代谢速率来降低氨基酸的供能效率是可行的,但猪体代谢与细胞、老鼠和人类相比差异极大,且研究目的不同,因此这一假说需要开展大量的体内和体外试验进行验证。
[1] BOISEN S,HVELPLUND T,WEISBJERG M R.Ideal amino acid profiles as a basis for feed protein evaluation[J].Livestock Production Science,2000,64(2/3):239-251.
[2] SHRIVER J A,CARTER S D,SUTTON A L,et al.Effects of adding fiber sources to reduced-crude protein,amino acid-supplemented diets on nitrogen excretion,growth performance,and carcass traits of finishing pigs[J].Journal of Animal Science,2003,81(2):492-502.
[3] LORDELO M M,GASPAR A M,LE BELLEGO L,et al.Isoleucine and valine supplementation of a low-protein corn-wheat-soybean meal-based diet for piglets:growth performance and nitrogen balance[J].Journal of Animal Science,2008,86(11):2936-2941.
[4] YIN Y L,HUANG R L,LI T J,et al.Amino acid metabolism in the portal-drained viscera of young pigs:effects of dietary supplementation with chitosan and pea hull[J].Amino Acids,2010,39(5):1581-1587.
[5] ZHANG G J,SONG Q L,XIE C Y,et al.Estimation of the ideal standardized ileal digestible tryptophan to lysine ratio for growing pigs fed low crude protein diets supplemented with crystalline amino acids[J].Livestock Science,2012,149(3):260-266.
[6] GALLO L,DALLA MONTG,CARRARO L,et al.Growth performance of heavy pigs fed restrictively diets with decreasing crude protein and indispensable amino acids content[J].Livestock Science,2014,161:130-138.
[7] GLOAGUEN M,LE FLOCH N,CORRENT E,et al.The use of free amino acids allows formulating very low crude protein diets for piglets[J].Journal of Animal Science,2014,92(3):637-644.
[8] GALASSI G,COLOMBINI S,MALAGUTTI L,et al.Effects of high fibre and low protein diets on performance,digestibility,nitrogen excretion and ammonia emission in the heavy pig[J].Animal Feed Science and Technology,2010,161(3/4):140-148.
[10] ROTZ C A.Management to reduce nitrogen losses in animal production[J].Journal of Animal Science,2004,82(E-Suppl):E119-E137.
[11] PUIMAN P,STOLL B,MØLBAK L,et al.Modulation of the gut microbiota with antibiotic treatment suppresses whole body urea production in neonatal pigs[J].American Journal of Physiology-Gastrointestinal and Liver Physiology,2013,304(3):G300-G310.
[12] HITOSUGI T,FAN J,CHUNG T W,et al.Tyrosine phosphorylation of mitochondrial pyruvate dehydrogenase kinase 1 is important for cancer metabolism[J].Molecular Cell,2011,44(6):864-877.
[13] NYACHOTI C M,OMOGBENIGUN F O,RADEMACHER M,et al.Performance responses and indicators of gastrointestinal health in early-weaned pigs fed low-protein amino acid-supplemented diets[J].Journal of Animal Science,2006,84(1):125-134.
[14] SHIRALI M,DOESCHL-WILSON A,KNAP P W,et al.Nitrogen excretion at different stages of growth and its association with production traits in growing pigs[J].Journal of Animal Science,2012,90(6):1756-1765.
[15] JØRGENSEN H,PRAPASPONGSA T,VAN THI K V,et al.Models to quantify excretion of dry matter,nitrogen,phosphorus and carbon in growing pigs fed regional diets[J].Journal of Animal Science and Biotechnology,2013,4:42.
[16] KIRCHGESSNER A L.Glutamate in the enteric nervous system[J].Current Opinion in Pharmacology,2001,1(6):591-596.
[17] STOLL B,BURRIN D G.Measuring splanchnic amino acid metabolisminvivousing stable isotopic tracers[J].Journal of Animal Science,2006,84(Suppl):E60-E72.
[19] WU G.Amino acids:metabolism,functions,and nutrition[J].Amino Acids,2009,37(1):1-17.
[20] EL-SABAGH M,SUGINO T,OBITSU T,et al.Effects of forage intake level on nitrogen net flux by portal-drained viscera of mature sheep with abomasal infusion of an amino acid mixture[J].Animal,2013,7(10):1614-1621.
[21] WU G Y.Functional amino acids in growth,reproduction,and health[J].Advances in Nutrition,2010,1:31-37.
[22] DAM G,KEIDING S,MUNK O L,et al.Branched-chain amino acids increase arterial blood ammonia in spite of enhanced intrinsic muscle ammonia metabolism in patients with cirrhosis and healthy subjects[J].American Journal of Physiology-Gastrointestinal and Liver Physiology,2011,301(2):G269-G277.
[23] 陈澄.日粮蛋白水平对仔猪肝脏氨基酸代谢转化的影响研究[D].硕士学位论文.重庆:西南大学,2015:21-24
[24] REZAEI R,WANG W W,WU Z L,et al.Biochemical and physiological bases for utilization of dietary amino acids by young pigs[J].Journal of Animal Science and Biotechnology,2013,4:7.
[25] BERTHIAUME R,THIVIERGE M C,PATTON R A,et al.Effect of ruminally protected methionine on splanchnic metabolism of amino acids in lactating dairy cows[J].Journal of Dairy Science,2006,89(5):1621-1634.
[26] DOEPEL L,LOBLEY G E,BERNIER J F,et al.Effect of glutamine supplementation on splanchnic metabolism in lactating dairy cows[J].Journal of Dairy Science,2007,90(9):4325-4333.
[27] ROSE C F.Ammonia-lowering strategies for the treatment of hepatic encephalopathy[J].Clinical Pharmacology & Therapeutics,2012,92:321-331.
[28] WIECHETEK M,SOUFFRANT W B,GARWACKI S.Utilization of nitrogen from15NH4Cl and [15N]alanine for urea synthesis in hepatocytes from fed and starved rats[J].International Journal of Biochemistry,1986,18(7):653-657.
[29] KRISTIANSEN R G,ROSE C F,FUSKEVÅG O M,et al.L-ornithine phenylacetate reduces ammonia in pigs with acute liver failure through phenylacetylglycine formation:a novel ammonia-lowering pathway[J].American Journal of Physiology-Gastrointestinal and Liver Physiology,2014,307(10):G1024-G1031.
[30] 杨静.甘氨酸和丙氨酸在肝脏中的代谢去向研究[D].硕士学位论文.重庆:西南大学,2016:27-35.
[31] KIGHT C E,FLEMING S E.Oxidation of glucose carbon entering the TCA cycle is reduced by glutamine in small intestine epithelial cells[J].The American Journal of Physiology,1995,268(6):G879-G888.
[32] DIENEL G A,CRUZ N F.Astrocyte activation in working brain:energy supplied by minor substrates[J].Neurochemistry International,2006,48(6/7):586-595.
[33] TORRES F V,HANSEN F,LOCKS-COELHO L D.Increase of extracellular glutamate concentration increases its oxidation and diminishes glucose oxidation in isolated mouse hippocampus:reversible by TFB-TBOA[J].Journal of Neuroscience Research,2013,91(8):1059-1065.
[34] EL BACHA T,LUZ M,DA POIAN A.Dynamic adaptation of nutrient utilization in humans[J].Nature Education,2010,3(9):8.
[35] DEFRONZO R A,TRIPATHY D.Skeletal muscle insulin resistance is the primary defect in type 2 diabetes[J].Diabetes Care,2009,32(Suppl. 2):S157-S163.
[36] KERR D S.Review of clinical trials for mitochondrial disorders:1997-2012[J].Neurotherapeutics,2013,10(2):307-319.
[37] FILLMORE N,LOPASCHUK G D.Targeting mitochondrial oxidative metabolism as an approach to treat heart failure[J].Biochimica et Biophysica Acta (BBA)-Molecular Cell Research,2013,1833(4):857-865.
[38] YAO J,RETTBERG J R,KLOSINSKI L P,et al.Shift in brain metabolism in late onset Alzheimer’s disease:implications for biomarkers and therapeutic interventions[J].Molecular Aspects of Medicine,2011,32(4/5/6):247-257.
[39] TENNANT D A,DURN R V,GOTTLIEB E.Targeting metabolic transformation for cancer therapy[J].Nature Reviews Cancer,2010,10(4):267-277.
[40] BRICKER D K,TAYLOR E B,SCHELL J C,et al.A mitochondrial pyruvate carrier required for pyruvate uptake in yeast,Drosophila,and humans[J].Science,2012,337(6090):96-100.
[41] VACANTI N M,DIVAKARUNI A S,GREEN C R,et al.Regulation of substrate utilization by the mitochondrial pyruvate carrier[J].Molecular Cell,2014,56(3):425-435.
[42] GRAY L R,SULTANA M R,RAUCKHORST A J,et al.Hepatic mitochondrial pyruvate carrier 1 is required for efficient regulation of gluconeogenesis and whole-Body glucose homeostasis[J].Cell Metabolism,2015,22(4):669-681.
[43] PATEL M S,KOROTCHKINA L G.Regulation of mammalian pyruvate dehydrogenase complex by phosphorylation:complexity of multiple phosphorylation sites and kinases[J].Experimental & Molecular Medicine,2001,33:191-197.
[44] ROCHE T E,BAKER J C,YAN X,et al.Distinct regulatory properties of pyruvate dehydrogenase kinase and phosphatase isoforms[J].Progress in Nucleic Acid Research and Molecular Biology,2001,70:33-75.
[45] SHAN C L,KANG H B,ELF S,et al.Tyr-94 phosphorylation inhibits pyruvate dehydrogenase phosphatase 1 and promotes tumor growth[J].Journal of Biological Chemistry,2014,289:21413-21422.
[46] STACPOOLE P W,NAGARAJA N V,HUTSON A D.Efficacy of dichloroacetate as a lactate-lowering drug[J].Journal of Clinical Pharmacology,2003,43(7):683-691.
[47] BONNET S,ARCHER S L,ALLALUNIS-TURNER J,et al.A mitochondria-K+channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth[J].Cancer Cell,2007,11(1):37-51.
[48] SUN Y,LI T,XIE C,et al.Dichloroacetate treatment improves mitochondrial metabolism and reduces brain injury in neonatal mice[J].Oncotarget,2016,doi: 10.18632/oncotarget.9150.
[49] YANG C D,KO B,HENSLEY C T,et al.Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport[J].Molecular Cell,2014,56(3):414-424.
Author, SUN Zhihong, professor, E-mail: sunzh2002cn@aliyun.com
(责任编辑 李慧英)
A New Hypothesis for the Mechanism of Metabolic Saving of Amino Acids of Pigs
SUN Zhihong1LI Mao1XU Qingqing1YIN Yulong2ZHU Weiyun3JIANG Qingyan4HUANG Feiruo5
(1. Laboratory for Bio-Feed and Animal Nutrition, Southwest University, Chongqing 400715, China; 2. Institute of Subtropical Agriculture, the Chinese Academy of Sciences, Changsha 410125, China; 3. College of Animal Science and Technology, Nanjing Agricultural University, Nanjing 210095, China; 4. College of Animal Science and Technology, Huanan Agricultural University, Guangzhou 510642, China; 5. College of Animal Science and Technology, Huazhong Agricultural University,Wuhan 430070, China)
Urinary nitrogen excretion accounts for 60% to 70% of the total nitrogen excretion of pigs. The production rate of urea, which is the main nitrogen-containing substance in the urine, to a large extent determines the urinary nitrogen and total nitrogen excretion. Therefore, declining the production rate of urea in liver of pigs is a fundamental approach for reducing total nitrogen excretion. This review summarized the existing nutrition regulatory measures for reducing nitrogen excretion in pigs, characterized the nitrogen direct precursors (ammonia) and indirect precursors (glycine and alanine) of urea synthesis in liver, and the mechanism of metabolic fuel function substitution of amino acid (AA). On this basis, a new hypothesis for the regulatory mechanism of metabolic saving of AA was proposed, the essence of which is to promote the efficiency of substances like as pyruvate/glucose being as metabolic fuel, decline metabolic rate of AA especially of glutamate, decrease the net flow of nitrogen precursors for urea synthesis in portal vein, urea synthesis in liver and urinary nitrogen excretion.[ChineseJournalofAnimalNutrition, 2016, 28(11):3369-3376]
nitrogen excretion; amino acids; metabolic saving; pyruvate dehydrogenase
2016-04-11
国家重点基础研究发展计划(2013CB127300);农业部"948"项目(2015Z74);国家科技支撑计划课题(2012BAD14B18);重庆市自然科学基金(cstc2012jjA80001)
孙志洪(1975—),男,四川成都人,教授,硕士生导师,博士,从事动物营养研究。E-mail: sunzh2002cn@aliyun.com
10.3969/j.issn.1006-267x.2016.11.001
S828
A
1006-267X(2016)11-3369-08
