Recognition of a multiple antigen peptide containing sequence from mimotope of the dengue type 3 virus NS4B protein by human antibodies
2016-11-29NevisAminMaritzaPupoAliciaAguilarSusanazquezYamiraCaballeroRolandoOchoaMarGuzmArmandoAcosta
Nevis Amin, Maritza Pupo, Alicia Aguilar, Susana Vázquez, Yamira Caballero, Rolando Ochoa, María G Guzmán, Armando Acosta
1Implementation and Design of Clinical trials, Clinical Research and Impact Evaluation Direction, Finlay Institute, 11600 Havana, Cuba
2‘Pedro Kourí’ Tropical Medicine Institute (IPK), PAHO/WHO Collaborating Center for the Study of Dengue and its Vector, 17100 Havana, Cuba
Recognition of a multiple antigen peptide containing sequence from mimotope of the dengue type 3 virus NS4B protein by human antibodies
Nevis Amin1*, Maritza Pupo2, Alicia Aguilar1, Susana Vázquez2, Yamira Caballero2, Rolando Ochoa1, María G Guzmán2, Armando Acosta1
1Implementation and Design of Clinical trials, Clinical Research and Impact Evaluation Direction, Finlay Institute, 11600 Havana, Cuba
2‘Pedro Kourí’ Tropical Medicine Institute (IPK), PAHO/WHO Collaborating Center for the Study of Dengue and its Vector, 17100 Havana, Cuba
ARTICLE INFO
Article history:
Received 15 November 2015
Received in revised form 20 December 2015
Accepted 15 January 2016
Available online 20 February 2016
MAP
Antibodies
NS4B
Dengue virus
Objective: To evaluate the recognition of NS4B mimotope, as multiple antigen peptide (MAP), by dengue antibodies presents in serum samples from patients with different serotype infections. Methods: A MAP containing mimotope sequence was synthesized and used to evaluate the recognition of NS4B mimotope as MAP by a panel of 66 human sera from dengue cases by an indirect ELISA assay. Results: The MAP differentiated between sera from dengue viruses infected patients and sera from healthy individuals and the best reactivity was shown by serum from dengue type 3 virus patients. The recognition was more intense with serum from patients with secondary infection. Conclusions: The findings suggest the potential use of NS4B mimotope on the development of a multi-epitope diagnostic tool. These results are important for further immunogenicity studies.
1. Introduction
Dengue viruses (DENV) belong to the family Flaviviridae, and the genome encodes 3 structural proteins: the capsid (C), precursor membrane (prM), envelope (E) and 7 non-structural proteins (NS1-NS5). Robust antibody responses are generated to 3 proteins: E, which contains 3 distinct domains, Ⅰ, Ⅱ, and Ⅲ (potent neutralizing activity); prM, which augments infectivity of poorly infectious immature virions; and NS1, which directs complementmediated lysis of infected cells[1]. Serological studies in humans have suggested, that after a DENV infection, people develop serum antibodies against some of the non-structural proteins (NS1, NS3, NS5)[2]. However, few studies have been carried out to characterize the immune response to others non-structural proteins[3].
The advent of the phage-displayed peptide technology, in which large, complex libraries of filamentous bacteriophage bearing random peptide sequences on their coat proteins can be generated and screened with antibodies, has provided a new approach to identify previously unknown antigens and/or new epitopes on already known antigens[4]. In this approach, phage-displayed peptide libraries are screened with sera from patients who have suffered a particular disease or pathological condition to discover peptide epitopes that are specifically recognized by patient’s sera[5-7].
The present study extends previous results obtained with the mimotope of the non-structural NS4B DENV protein. The NS4 protein is composed of two distinct domains: NS4A and NS4B which may play a role in viral replication[8], however, the role in humoral immunity of NS4B has been poorly studied. Nevertheless, detection of antibody specific to purified recombinant GST-NS4B antigen was reported in serum samples from dengue patients[9]. Here we evaluate the recognition of NS4B mimotope as MAP by dengue antibodies in serum samples from patients with different dengue serotype infections.
2. Materials and methods
2.1. Serum samples
All the serum specimens used in the study came from a human sera collection stored at -20 ℃. To obtain these sera, all procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 [Cuadernos de Bioética (Bs As) 2010;15/16:289-348].
Sera collected from 66 dengue confirmed adult patients and stored at the Dengue Sera Bank of the Virology Department at the ‘Pedro Kouri’ Tropical Medicine Institute of Havana in Cuba were analyzed in this study. Dengue diagnostics was confirmed by virus isolation and identification, RT-PCR and IgM-IgG determination. Cases were classified in primary or secondary infection according to anti-dengue IgG level[10,11]. Serum samples were collected five to seven days or two months after the onset of fever. Sera from healthy donors were obtained from the Cuban National Blood Bank and used as a negative control.
2.2. Synthetic peptides
Peptides consisting of the mimotope from NS4B DENV protein, mimotope from E DENV protein (as positive control of antigen)[12] and mimotopes from Hepatitis A virus (HAV) (as negative control of antigen)[13] were synthesized at the Synthetic Peptide Laboratory at the Center for Genetic Engineering and Biotechnology (Havana City) (Table 1). The peptides were named NS4B MAP, E peptide and MAP 46-56, respectively.
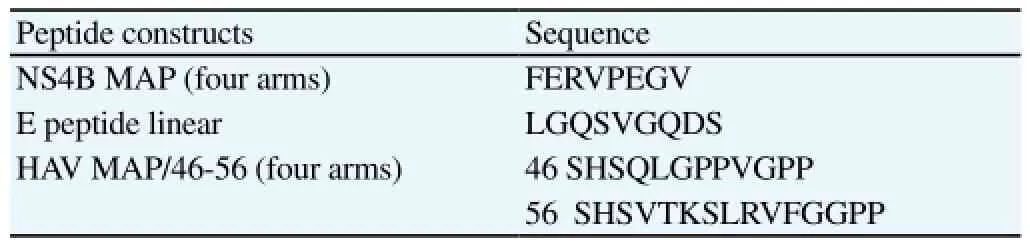
Table 1Sequence of mimotopes contained in synthetic antigens.
2.3. Indirect ELISA-NS4 MAP and controls
Multi-well plates (Nunc Maxisorp F8, Life Technologies Limited, Paisley, UK) were coated with 100 µL/well of NS4B MAP: 5 µg/ mL, E peptide: 20 µg/mL or MAP 46-56: 5 µg/mL diluted in 0.05 M sodium carbonate buffer pH 9.6 (coating buffer), and incubated overnight at 4 ℃. Plates were washed 3 times with phosphate buffered saline in 0.05% Tween 20 (PBS/T) and blocked with skimmed milk (3% w/v) in PBS/T (blocking solution) for 1 h at 37 ℃. Human serum samples diluted in blocking solution (1:20, 100 µL/well) were added to the plates and incubated 1 h at 37 ℃. After repeating the washes, bound antibodies were detected with HRP-conjugated anti-human IgG antibodies (Sigma-Aldrich, UK), H2O2and o-phenylenediamine dihydrochloride (Sigma). The reaction was stopped with 0.1 M H2SO4. The absorbance at 492 nm was measured by an automated ELISA reader. All samples were run in duplicate and results were expressed as mean values of optical density (OD) ratios.
Control ELISAs were run in parallel, in which the E peptide and HAV MAP were used. Each ELISA was performed twice.
Positivity criteria: The formula ratio=P/N where P is the OD obtained in the sample and N is the OD mean values obtained with sera of healthy donors for the screening of the serum sample with NS4B peptide was employed. A sample with a ratio≥2 was considered positive.
2.4. Statistical analysis
The GraphPad Prism statistical software (San Diego, CA, USA) was used. The Mann Whitney test was used to compare the results of OD ratios obtained in each serotype and values of P<0.05 were considered significant.
3. Results
To analyse the potential of NS4B mimotope as MAP to behave as antigen, their ability to detect anti-DENV antibodies was assessed. ELISA screening was performed with human sera collected from dengue patients with NS4B MAP. The reactivity of 30 serum samples from secondary cases to DENV-1 (n=10), DENV-3 (n=10) and DENV-4 (n=10) is shown in Figure 1. Serum samples of DENV-2 infection were not available for the study. The OD ratio values for DENV-3 were statistically significant when compared with DENV-1 (P=0.03) and DENV-4 (P=0.04).
Dengue antibodies from the studied samples were not detected with MAP 46-56 used as control, demonstrating a specific recognition of NS4B MAP by antibodies produced in response to the natural dengue infection. Figure 1 shows the results obtained with dengue 3 sera against MAP 46-56.

Figure 1. ELISA reactivity of human sera to NS4B mimotope as MAP.
The unpaired sera of 48 patients from DENV-3 positive cases (20 serum samples collected between five to seven days, and 28 collected after two months of fever onset) were used to compare the response to NS4B antibodies by ELISA-NS4B MAP (Figure 2). These patients were classified in primary or secondary type of infection. NS4B MAP was recognized strongly by samples collected at five to seven days in secondary infection (70%) and only 2 were positive in primary cases. Sera collected≥two months showed the highest percentage of recognition in both primary (93%) and secondary cases (100%); however, the OD ratios were lowest in relation with secondary cases (samples five to seven days). A greater reactivity to E peptide, used as positive control, was observed in sera collected at the convalescent phase of illness (data not shown).

Figure 2. Recognition of NS4B MAP of serum samples from patients with primary (P 5-7=10 and P≥2 m=13) or secondary (S 5-7=10 and S≥2 m=15) dengue 3 infections by an Indirect ELISA.
4. Discussion
One of the advantages of the phage display technology is to reduce complex protein antigens to small structured peptides that retain immune recognition. Mimotopes can potentially serve as lead compounds to develop low molecular weight substitutes of the template protein for the development of diagnostic assays[6,14]. This approach has been widely used in epitope mapping of flavivirus[15-18]. Recently, a mimotope of NS4B protein of DENV-3 was obtained by screening a solid-phase 9mer random peptide library using human immune sera. The selected sequences mimic the binding properties of natural antigen epitopes[19].
In this study, we detected antibodies against DENV from serum samples of dengue patients using the NS4B mimotope by indirect ELISA. The mimotope was synthesized in the MAP form, because the binding efficiency of an MAP is greater than that of a singlechain peptide. Short synthetic peptides are usually ineffective antigens for solid phase immunoassays owing to their poor ability to attach to solid surfaces. The multimeric nature of MAP constructs have been found to overcome these deficiencies and provide consistently reproducible results in increased surface-binding properties and sensitivity of detection[20]. The high reactivity with DENV-3 can be explained by the fact that the NS4B mimotope was selected with an anti-dengue 3 sera[19]. These data correlate well with the results reported by several authors who identified serotype specific epitopes using the phage-displayed peptide library screening method[15-18,21].
The serum antibody responses are different following primary and secondary DENV infections. In secondary infections, the stimulation of B-cell memory leads to a rapid rise in DENV-specific IgG that is measurable even on the first day of symptoms. Moreover DENV specific serum IgG titers are much higher in secondary compared to primary infections[1]. Valdes et al[22] have demonstrated that the antibody response to NS5 and NS3 proteins in acute-phase samples from secondary cases is greater than in primary cases, including the intensity of the reaction, due to the high levels of IgG antibodies in secondary cases. The present study confirms these results, with the presence of anti-NS4B antibodies in acute-phase samples from secondary cases, using NS4B mimotope as MAP. Previously, NS4B MAP was shown to induce robust humoral response in mice and these antibodies were able to recognize NS4B protein, which is produced in the first step of viral replication[12]. On the other hand,‘in silico’ analysis predicted potential B-cell epitopes on the NS4B protein including the epitope mimic of NS4B[23]. These preliminary data support the usefulness of NS4B MAP to detect antibodies to DENV in human sera suggesting that the NS4B protein of dengue virus could be implicated in the humoral response. Our findings suggest the potential use of NS4B mimotope for the development of a multi-epitope diagnostic tool. These results are important for further immunogenicity studies towards the evaluation of the mimotope as experimental vaccine.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The authors would like to thank Dr. Marta Romano for the critical reading of the manuscript.
[1] Wahala WM, Silva AM. The human antibody response to dengue virusinfection. Viruses 2011; 3(12): 2374-2395.
[2] Mathew A, West K, Kalayanarooj S, Gibbons RV, Srikiatkhachorn A, Green S, et al. B-cell responses during primary and secondary dengue virus infections in humans. J Infect Dis 2011; 204(10): 1514-1522.
[3] Anandarao R, Swaminathan S, Khanna N. The identification of immunodominant linear epitopes of dengue type 2 virus capsid and NS4a proteins using pin-bound peptides. Virus Res 2005; 112(1-2): 60-68.
[4] Paramasivam SK. Diagnostic and immunoprophylactic applications of synthetic peptides in veterinary microbiology. Microbiol Res 2010; 1(1). Doi: 10.4081/mr.2010.e1.
[5] Gazarian KG, Palacios-Rodriguez Y, Gazarian TG, Huerta L. HIV-1 V3 loop crown epitope-focused mimotope selection by patient serum from random phage display libraries: implications for the epitope structural features. Mol Immunol 2013; 54(2): 148-156.
[6] Casey JL, Coley AM, Parisi K, Foley M. Peptide mimics selected from immune sera using phage display technology can replace native antigens in the diagnosis of Epstein-Barr virus infection. Protein Eng Des Sel 2009; 22(2): 85-91.
[7] Houimel M, Dellagi K. Peptide mimotopes of rabies virus glycoprotein with immunogenic activity. Vaccine 2009; 27(34): 4648-4655.
[8] Miller S, Bartenschlager R. Subcellular localization and membrane topology of the dengue virus type 2 non-structural protein 4B. J Biol Chem 2006; 281: 8854-8863.
[9] Lázaro L, Mellado G, García J, Escobar A, Santos L, Gutiérrez B, et al. Analysis of antibody responses in human dengue patients from the Mexican coast using recombinant antigens. Vector Borne Zoonotic Dis 2008; 8(1): 69-79.
[10] Vazquez S, Cabezas S, Perez AB, Pupo M, Ruiz D, Calzada N, et al. Kinetics of antibodies in sera, saliva, and urine samples from adult patients with primary or secondary dengue 3 virus infections. Int J Infect Dis 2007; 11(3): 256-262.
[11] Vazquez S, Acosta N, Ruiz D, Calzada N, Alvarez M, Guzman MG. Immunoglobulin G antibody response in children and adults with acute dengue 3 infection. J Virol Methods 2009; 159(1): 6-9.
[12] Amin N, Pupo M, Aguilar A, Camacho F, Alvarez M, Caballero Y, et al. Immunogenicity of NS4b dengue 3 virus mimotope presented to the immune system as multiple antigen peptide system. ISRN Virol 2013; 2013(1). Doi: 10.5402/2013/924057.
[13] Aguilar A, Camacho F, Martínez R, Huerta V, Garay H, Amin N, et al. Study of peptide mimetics of hepatitis a virus conjugated to keyhole limpet hemocyanin and as multiple antigen peptide system. Int J Pept Res Ther 2014; 20(1): 33-42.
[14] Casey JL, Coley AM, Street G, Parisi K, Devine PL, Foley M. Peptide mimotopes selected from a random peptide library for diagnosis of Epstein-Barr virus infection. J Clin Microbiol 2006; 44(3): 764-771.
[15] Jiang L, Zhou JM, Yin Y, Fang DY, Tang YX, Jiang LF. Selection and identification of B-cell epitope on NS1 protein of dengue virus type 2. Virus Res 2010; 150(1-2): 49-55.
[16] Chen YC, Huang HN, Lin CT, Chen YF, King CC, Wu HC. Generation and characterization of monoclonal antibodies against dengue virus type 1 for epitope mapping and serological detection by epitope-based peptide antigens. Clin Vaccine Immunol 2007; 14(4): 404-411.
[17] Wu HC, Huang YL, Chao TT, Jan JT, Huang JL, Chiang HY, et al. Identification of B-cell epitope of dengue virus type 1 and its application in diagnosis of patients. J Clin Microbiol 2001; 39(3): 977-982.
[18] Yao ZJ, Kao MC, Loh KC, Chung MC. A serotype-specific epitope of dengue virus 1 identified by phage displayed random peptide library. FEMS Microbiol Lett 1995; 127(1-2): 93-98.
[19] Amin N, Aguilar A, Chamacho F, Vazquez Y, Pupo M, Ramirez JC, et al. Identification of dengue-specific B-cell epitopes by phage-display random peptide library. Malays J Med Sci 2009; 16(4): 4-14.
[20] Tam JP, Zavala F. Multiple antigen peptide. A novel approach to increase detection sensitivity of synthetic peptides in solid-phase immunoassays. J Immunol Methods 1989; 124(1): 53-61.
[21] Tian Y, Chen W, Yang Y, Xu X, Zhang J, Wang J, et al. Identification of B cell epitopes of dengue virus 2 NS3 protein by monoclonal antibody. Appl Microbiol Biotechnol 2013; 97(4): 1553-1560.
[22] Valdes K, Alvarez M, Pupo M, Vazquez S, Rodriguez R, Guzman MG. Human dengue antibodies against structural and nonstructural proteins. Clin Diagn Lab Immunol 2000; 7(5): 856-857.
[23] Amin N, Reyes F, Calero R, Camacho F, Acosta A. T and B epitope prediction of NS4B protein of dengue virus type 3. Vaccimonitor 2013; 22(3): 14-21.
ent heading
10.1016/j.apjtm.2016.01.019
*Corresponding author: Nevis Amin, Implementation and Design of Clinical trials, Clinical Research and Impact Evaluation Direction, Finlay Institute. Ave 27, No 19805, La Lisa, AP 16017, 11600 Havana, Cuba.
Tel: 53 7 2080984
Fax: 53 7 2086075
E-mail: namin@finlay.edu.cu
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Preventive and therapeutic effect of N-Acetyl-L-cysteine on infection-associated preterm labor in mice
- First histopathological study in kidneys of rodents naturally infected with Leptospira pathogenic species from Yucatan, Mexico
- Anti-Alzheimer's disease potential of coumarins from Angelica decursiva and Artemisia capillaris and structure-activity analysis
- Dengue virus non-structural 1 protein interacts with heterogeneous nuclear ribonucleoprotein H in human monocytic cells
- Effect of roselle calyx extract on in vitro viability and biofilm formation ability of oral pathogenic bacteria
- Evaluation of anti-tubercular activity of linolenic acid and conjugatedlinoleic acid as effective inhibitors against Mycobacterium tuberculosis
