Preventive and therapeutic effect of N-Acetyl-L-cysteine on infection-associated preterm labor in mice
2016-11-29LingJiangQianYanRongHuiLiuLuZhang
Ling Jiang, Qian Yan, Rong-Hui Liu, Lu Zhang
Yantaishan Hospital Affiliated to Taishan Medical University, Yantai, Shandong 264000, China
Preventive and therapeutic effect of N-Acetyl-L-cysteine on infection-associated preterm labor in mice
Ling Jiang, Qian Yan*, Rong-Hui Liu, Lu Zhang
Yantaishan Hospital Affiliated to Taishan Medical University, Yantai, Shandong 264000, China
ARTICLE INFO
Article history:
Received 15 November 2015
Received in revised form 20 December 2015
Accepted 15 January 2016
Available online 20 February 2016
N-Acetyl-L-cysteine
Infection-associated preterm labor
AP-1
MCP-1
NF-κBp65
TNF-α
Objective: To study the preventive and therapeutic effect of N-Acetyl-L-cysteine on infectionassociated preterm labor in mice. Methods: A total of 66 C57BL/6 inbred strain pregnant mice were selected and randomly divided into groups A, B and C, with 22 cases in each group. Group A, B and C were regarded as model group, prevention group and treatment group, respectively. The model of infection-associated preterm labor was built by intraperitoneal injection of Escherichia coli. Ten mice of each group were taken and observed the preterm birth rates and live birth rates, respectively. Three mice of each group were killed at 3 h, 6 h, 12 h and 24 h after building the model. Their uterus tissues were collected and the expressions of the AP-1 and MCP-1 in those tissues were assayed with immunohistochemical method and the expressions of NF-κBp65 and TNF-α protein in the placenta tissues of those mice were also detected with immunohistochemical method. Results: The preterm birth rates of mice in groups B and C were significantly lower than that in group A, while their live birth rates were distinctly higher than that in group A (P<0.05); the expressions of the AP-1 and MCP-1 in the uterus tissues and NF-κBp65 and TNF-α protein in the placenta tissues of mice in groups B and C were evidently lower than those in group A (P<0.05); the comparison of the expressions of the NF-κBp65 and TNF-α between group B and C showed no statistical differences (P>0.05). Conclusions: N-Acetyl-L-cysteine can lower the incidence rate of infection-associated preterm labor by prohibiting the activation of the protein AP-1/MCP-1 and decreasing the expression of NF-κBp65 and TNF-α in the pregnant tissues of premature mice to reduce the inflammatory reactions.
1. Introduction
Preterm birth is a significant cause of the neonatal death. In recent years, the preterm birth rate is increasing globally[1]. According to the statistics[2,3], infants of preterm birth has accounted for 11% of the total number of new-born infants. There are many factors inducing preterm birth in clinic. Infections play a very important role in the incidence of preterm birth and it is also one of the most common causes inducing preterm birth and the neonatal death[4].The placenta is the intermediary organ maintaining the mother and the fetus. Before the full development of the placenta, any tiny pathogenic factor invading the placenta can cause pathological inflammatory responses. Hence, inflammatory signaling pathways play a vital role in the incidence of preterm birth[5-7]. N-Acetyl-L-cysteine (NAC) is a classic apophlegmatisant with rather strong anti-inflammatory and antioxidant properties, which has good curative effect on the treatment of various diseases[8]. It is reported that NAC processes certain preventive and therapeutic effect on infection-associated preterm labor[9]. In this study, in order to study the preventive and therapeutic effect of NAC and explore its related mechanism on infection-associated preterm labor, a number of C57BL/6 inbred strain pregnant mice were selected and an infection-associated preterm labor was established. Then, NAC was used for treatment and prevention, and the preterm birth and live birth rates were observed. Now the results are reported as follows.
2. Materials and methods
2.1. Animals
A total of 66 sexually mature C57BL/6 inbred strain pregnant mice of clean grade weighing from 17 g to 23 g were selected. They were provided by the Experimental Animal Center of Taishan Medical University and raised at (23±3) ℃. They could take food and water freely. The management of the experimental animals in this study was carried out by following the Laboratory Animal Administration Rules strictly and approved by the Ethical Committee of the medical college.
2.2. Instruments and reagents
The Anke TDL-40B centrifuge was produced by Shanghai Anting Scientific Instrument Factory; the SANYO light microscope, OLYMPUS high-speed centrifuge with low temperature (Japan), constant temperature bath oscillator and spectrophotometry were all manufactured by Chongqing Tested Instrument Factory; the FOTODYNE gel image analysis system was from PE (USA); NAC was provided by Minsheng Pharmaceutical Group Co. Ltd. (approved by the state, No. H20051788); rabbit-anti-rat TNF-α polyclonal antibody, mouse anti-human NF-κBp65 monoclonal antibody and DAB color kits were purchased from Beijing Zhongshan Jinqiao Biotechnology Co. Ltd. The bacterial liquid of Escherichia coli (E. coli) was made. Standard strains of E. coli were collected a day before the experiment and inoculated in the culture medium at 37 ℃ overnight. Afterwards, they were diluted to a suspension with a concentration of 104cfu/mL for the standby application at the day of experiment.
2.3. Modeling methods and animal groups
A total of 66 C57BL/6 inbred strain pregnant mice were selected and randomly divided into groups A, B and C, with 22 cases in each. Group A, B and C were regarded as the model group, prevention group and treatment group, respectively. The model of infectionassociated preterm labor was built by intraperitoneal injection of E. coli. The experimental mice were given intraperitoneal injection of 100 uL E. coli suspension at day 16 after the pregnancy to make an intrauterine infection-associated preteim labor model. Mice in group A received intraperitoneal injection of equivalent normal saline treatment after the model was built; mice in group B were given intraperitoneal injection of 100 mg/kg of NAC preventive treatment at 1 h before building the model; mice in group C were treated with intraperitoneal injection of 100 mg/kg of NAC at 2 h after completing the mode.
2.4. Observation methods
Ten mice of each group were taken and observed the preterm birth rates and live birth rates for 48 h, respectively. Three mice of each group of the rest were killed at 3 h (T1), 6 h (T2), 12 h (T3) and 24 h (T4) after an intervention treatment. The uterus tissues were collected and the expressions of the AP-1 and MCP-1 in those tissues were assayed with immunohistochemical method, and the expressions of NF-κBp65 and TNF-α protein in the placenta tissues of those mice which were sacrificed at the last minute were also detected with immunohistochemical method.
2.5. Statistical methods
SPSS13.0 was applied for data analysis. The comparison of the measurement data was analyzed with One-way ANOVA and represented by mean±SD, while the enumeration data were tested by Chi-square test and expressed as proportions. P<0.05 meant that differences had statistically significance
3. Results
3.1. Comparison of preterm birth rates and live birth rates of three groups
The preterm birth rates of groups B and C within 48 h were significantly lower than that in group A, while the live birth rates of groups B and C were observably higher than that in group A. There were significant differences between two groups (P<0.05) (Table 1).

Table 1Comparison of preterm birth rates and live birth rates of three groups (%).
3.2. Protein expressions of AP-1 and MCP-1 in uterus tissues of mice in three groups at different time periods
The protein expressions of the AP-1 and MCP-1 in the uterus tissues of mice in groups B and C at T1, T2, T3and T4were all significantly lower than those in group A, and the inter-group differences had statistical significances (P<0.05) (Table 2).
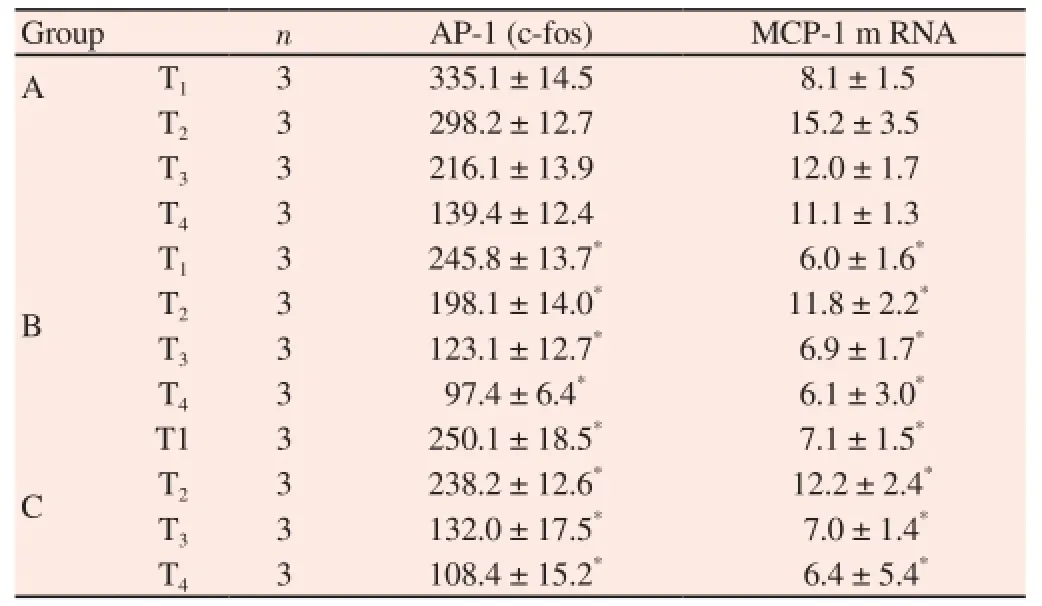
Table 2Protein expressions of AP-1 and MCP-1 in uterus tissues of mice in three groups at different time periods.
3.3. Expressions of NF-κBp65 and TNF-α protein in placenta tissues of mice in three groups
The expressions of NF-κBp65 and TNF-α protein in the placenta tissues of mice in groups B and C at T4were all significantly lower than those in group A, and the inter-group differences had statistical significances (P<0.05) (Table 3).

Table 3Expressions of NF-κBp65 and TNF-α protein in placenta tissues of mice in three groups.
4. Discussion
Preterm birth means that women deliver babies after 28 weeks but less than 37 weeks of the pregnancy, which accounts for about 5%-10% of the total delivery number. Preterm birth becomes an important reason for the newborn deaths[10-13]. According to the statistics[14], the number of premature death accounts for 70% of the total number of newborn death, and those preterm babies who survive fortunately can be prone to suffer from different degrees of sequelae. There are researchers claiming that intrauterine and yeast infections were the vital factors of preterm birth so that if they could be controlled timely and effectively, the incidence rate of preterm birth could be reduced efficaciously[15-17].
The placenta is the intermediary organ maintaining the mother and the fetus. Before the fully development of the placenta, any tiny pathogenic factor invades the placenta can cause pathological inflammatory responses[18]. There are researches showing that inflammatory signaling pathways play a vital role in the incidence of preterm birth[16,19-22]. NF-κB is an important infectious regulating factor with the effect of regulating inflammatory response, chemokines and cytokines which may play an important part in the incidence of preterm birth[23]. NAC, a kind of N-acetylated derivative of cysteine, is a classic apophlegmatisant which can significantly lower the viscosity of mucin. Some studies have shown that NAC can lower the incidence of infection-associated preterm labor by prohibiting the expressions of NF-κBp65 and TNF-α protein in the placenta tissues of preterm mice to reduce the inflammatory reaction[24]. In this study, the expressions of NF-κBp65 and TNF-α protein in the placenta tissues of mice in groups B and C were all significantly lower than those in group A, which was consistent with the related literature[24]. It was concluded that NAC did decease the expressions of NF-κBp65 and TNF-α protein in the placenta tissues in mice of infection associated preterm labor and relieve the inflammatory reactions. In addition, the preterm birth rates of groups B and C were observably lower than those in group A, while the live birth rates were significantly higher than those in group A (P<0.05), which also proved that NAC was responsible for the prevention and treatment of infection-associated preterm labor. There are researches finding that AP-1/MCP-1 signal pathways in the uterus tissue of patients with uterine cavity amniotic infection showed high expression of the state[8,25,26]. It is speculated that the abnormality of AP-1/MCP-1 signal pathways may be an important link of the incidence of infection-associated preterm labor[27]. The results of this study showed that the protein expressions of the AP-1 and MCP-1 in the uterus tissues of mice in groups B and C at T1, T2, T3and T4were all significantly lower than those in group A (P<0.05), which indicated that the abnormality of AP-1/MCP-1 signal pathways took part in the incidence of infection-associated preterm labor. Moreover, NAC could decrease the preterm birth rate by interdicting the activating pathway of AP-1 and relieving the inflammation damage for those pregnant mice.
The findings of this study suggest that NAC can lower the incidence rate of infection-associated preterm labor by prohibiting the activation of the protein AP-1/MCP-1 and decreasing the expressions of NF-κBp65 and TNF-α in the pregnant tissues of preterm mice to reduce the inflammatory reactions.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Morwood CJ, Lappas M. The citrus flavone nobiletin reduces proinflammatory and pro-labour mediators in fetal membranes and myometrium: implications for preterm birth. PloS One 2014; 9(9): e108390.
[2] Wu Q, Yu JP, Liu HY. The influence of premature rupture of membrane combined with lower reproductive tract infections on the fetuses and pregnant women. Chin J Clin Obstet Gynecol 2014; 15(2): 159-161.
[3] Fan MS, Jiang ZY, Zou YF, Qu L, Zhou X, Sun LZ. Effect of transforming growth factor β1 on the expression of matrix metalloproteinase 9, tissue inhibitor of metalloproteinase 1 and nuclear factor kappa B signalling pathway in the human amniotic cells WISH. Zhonghua Fu Chan Ke Za Zhi 2013; 48(1): 29-33.
[4] Lorenz J, Seebach E, Hackmayer G, Greth C, Bauer RJ, Kleinschmidt K, et al. Melanocortin 1 receptor-signaling deficiency results in an articular cartilage phenotype and accelerates pathogenesis of surgically induced murine osteoarthritis. PLoS One 2014; 9(9): e105858.
[5] Ma X, Becker Buscaglia LE, Barker JR, Li Y. MicroRNAs in NF-kappaB signaling. J Mol Cell Biol 2011; 3(3): 159-166.
[6] Chandiramani M, Seed PT, Orsi NM, Ekbote UV, Bennett PR, Shennan AH, et al. Limited relationship between cervico-vaginal fluid cytokine profiles and cervical shortening in women at high risk of spontaneous preterm birth. PLoS One 2012; 7(12): e52412.
[7] Uchide N, Ohyama K, Bessho T, Takeichi M, Toyoda H. Possible roles of proinflammatory and chemoattractive cytokines produced by human fetal membrane cells in the pathology of adverse pregnancy outcomesassociated with influenza virus infection. Mediators Inflamm 2012; 2012: 270670.
[8] Castro-Leyva V, Espejel-Nuñez A, Barroso G, Zaga-Clavellina V, Flores-Pliego A, Morales-Mendez I, et al. Preserved ex vivo inflammatory status in decidual cells from women with preterm labor and subclinical intrauterine infection. PLoS One 2012; 7(8): e43605.
[9] Zhao FX, Xing ZW, Liu RH. Study on the expression of TLR4, NF-κ B and TNF-α and the intervention of NAC by the placenta of preterm birth in infected Balb/c mice. Chin J Immunol 2009; 25(4): 328-332.
[10] Saini R, Saini S, Saini SR. Periodontitis: A risk for delivery of premature labor and low-birth-weight infants. J Nat Sci Biol Med 2010; 1(1): 40-42.
[11] Kumar D, Schatz F, Moore RM, Mercer BM, Rangaswamy N, Mansour JM, et al. The effects of thrombin and cytokines upon the biomechanics and remodeling of isolated amnion membrane, in vitro. Placenta 2011; 32(3): 206-213.
[12] Hsu TY, Lin H, Lan KC, Ou CY, Tsai CC, Cheng BH, et al. High interleukin-16 concentrations in the early second trimester amniotic fluid: an independent predictive marker for preterm birth. J Matern Fetal Neonatal Med 2013; 26(3): 285-289.
[13] Thomakos N, Daskalakis G, Papapanagiotou A, Papantoniou N, Mesogitis S, Antsaklis A. Amniotic fluid interleukin-6 and tumor necrosis factor-α at mid- trimester genetic amniocentesis: relationship to intra-amniotic microbial invasion and preterm delivery. Eur J Obstet Gynecol Reprod Biol 2010; 148(2): 147-151.
[14] Sorokin Y, Romero R, Mele L, Wapner RJ, Iams JD, Dudley DJ, et al. Maternal serum interleukin-6, C-reactive protein, and matrix metalloproteinase-9 concentrations as risk factors for preterm birth < 32 weeks and adverse neonatal outcomes. Am J Perinatol 2010; 27(8): 631-640.
[15] Gulati S, Agrawal S, Raghunandan C, Bhattacharya J, Saili A, Agarwal S, et al. Maternal serum interleukin-6 and its association with clinicopathological infectious morbidity in preterm premature rupture of membranes: a prospective cohort study. J Matern Fetal Neonatal Med 2012; 25(8): 1428-1432.
[16] Taylor BD, Holzman CB, Fichorova RN, Tian Y, Jones NM, Fu W, et al. Inflammation biomarkers in vaginal fluid and preterm delivery. Hum Reprod 2013; 28(4): 942-952.
[17] Deeks ED. 17 α-Hydroxyprogesterone caproate (Makena™): in the prevention of preterm birth. Paediatr Drugs 2011; 13(5): 337-345.
[18] Kobayashi H. The entry of fetal and amniotic fluid components into the uterine vessel circulation leads to sterile inflammatory processes during parturition. Front Immunol 2012; 3: 321.
[19] Puchner K, Iavazzo C, Gourgiotis D, Boutsikou M, Baka S, Hassiakos D, et al. Mid-trimester amniotic fluid interleukins (IL-1β, IL-10 and IL-18) as possible predictors of preterm delivery. In Vivo 2011; 25(1): 141-148.
[20] Harper M, Zheng SL, Thom E, Klebanoff MA, Thorp J Jr, Sorokin Y, et al. Cytokine gene polymorphisms and length of gestation. Obstet Gynecol 2011; 117(1): 125-130.
[21] Gomez-Lopez N, Vadillo-Perez L, Hernandez-Carbajal A, Godines-Enriquez M, Olson DM, Vadillo-Ortega F. Specific inflammatory microenvironments in the zones of the fetal membranes at term delivery. Am J Obstet Gynecol 2011; 205(3): 235.e15-e24.
[22] Zhao Y, Koga K, Osuga Y, Izumi G, Takamura M, Harada M, et al. Cyclic stretch augments production of neutrophil chemokines and matrix metalloproteinase-1 in human uterine smooth muscle cells. Am J Reprod Immunol 2013; 69(3): 240-247.
[23] Ding XY, Ni X, Gu H. Study on the proinflammatory cytokine in spontaneous preterm birth. Chin J Birth Health Heredity 2014; 22(4): 134-136.
[24] Xing ZW, Zhao FX, Liu RH, Mu YQ. Effects of NAC on expression of TLR4 and NF-κB in mouse placenta of premature birth associated with infection. Chin J Family Planning 2009; 17(5): 267-271.
[25] Hua R, Pease JE, Sooranna SR, Viney JM, Nelson SM, Myatt L, et al. Stretch and inflammatory cytokines drive myometrial chemokine expression via NF-κB activation. Endocrinology 2012; 153(1): 481-491.
[26] Li XL, Li HS. Inflammatory mediums and preterm birth. Chin J Pract Gynecol Obstet 2014; 30(6): 484-488.
[27] Xu ZH, Li L, Zeng WY, Yang X. Effect of curcumin on infection associated preterm labor in mice. Sichuan Med J 2011; 32(7): 994-997.
ent heading
10.1016/j.apjtm.2016.01.012
*Corresponding author: MQian Yan, Associate chief physician, Yantaishan Hospital Affiliated to Taishan Medical University, Yantai, Shandong 264000, China.
Tel: 13705355287
E-mail: jiangl1in973@163.com
Foundation project: It is supported by the special plan of maternal and child health molecular genetic medicine of Maternal and Child Health Care Center in Chinese Center for Disease Control and Prevention (Grant No. FY-ZX-ZD-0059).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Influence of lactulose on interventional therapy for HCC patients with hepatocirrhosis and hypersplenism
- Immunological evidence of Zika virus transmission in Thailand
- Anti-Alzheimer's disease potential of coumarins from Angelica decursiva and Artemisia capillaris and structure-activity analysis
- Dengue virus non-structural 1 protein interacts with heterogeneous nuclear ribonucleoprotein H in human monocytic cells
- Effect of roselle calyx extract on in vitro viability and biofilm formation ability of oral pathogenic bacteria
- Evaluation of anti-tubercular activity of linolenic acid and conjugatedlinoleic acid as effective inhibitors against Mycobacterium tuberculosis
