Anti-Alzheimer's disease potential of coumarins from Angelica decursiva and Artemisia capillaris and structure-activity analysis
2016-11-29MdYousofAliSusomaJannatHyunAhJungRanJooChoiAnupomRoyJaeSueChoi
Md. Yousof Ali, Susoma Jannat, Hyun Ah Jung, Ran Joo Choi, Anupom Roy, Jae Sue Choi*
1Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea
2Department of Food Science and Human Nutrition, Chonbuk National University, Jeonju 561-756, Republic of Korea
3Angiogenesis& Chinese Medicine Laboratory, Department of Pharmacology, University of Cambridge, Cambridge, UK
Anti-Alzheimer's disease potential of coumarins from Angelica decursiva and Artemisia capillaris and structure-activity analysis
Md. Yousof Ali1, Susoma Jannat1, Hyun Ah Jung2*, Ran Joo Choi3, Anupom Roy1, Jae Sue Choi1*
1Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea
2Department of Food Science and Human Nutrition, Chonbuk National University, Jeonju 561-756, Republic of Korea
3Angiogenesis& Chinese Medicine Laboratory, Department of Pharmacology, University of Cambridge, Cambridge, UK
ARTICLE INFO
Article history:
Received 15 November 2015
Received in revised form 20 December 2015
Accepted 15 January 2016
Available online 20 February 2016
Umbelliferone 6-carboxylic acid
Esculetin
Daphnetin
Coumarins
Cholinesterase
BACE1
Objective: To use structure-activity analysis to study the anti-Alzheimer's disease (anti-AD) activity of natural coumarins isolated from Angelica decursiva and Artemisia capillaries, along with one purchased coumarin (daphnetin). Methods: Umbelliferone, umbelliferone 6-carboxylic acid, scopoletin, isoscopoletin, 7-methoxy coumarin, scoparone, scopolin, and esculetin have been previously isolated; however 2'-isopropyl psoralene was isolated from Angelica decursiva for the first time to evaluate their inhibitory effects against acetylcholinesterase (AChE), butyrylcholinesterase (BChE), and β-site amyloid precursor protein cleaving enzyme 1 (BACE1) enzyme activity. We scrutinized the potentials of coumarins as cholinesterase and BACE1 inhibitors via enzyme kinetics and molecular docking simulation. Results: Among the test compounds, umbelliferone 6-carboxylic acid, esculetin and daphnetin exhibited potent inhibitory activity against AChE, BChE and BACE1. Both esculetin and daphnetin have a catechol group and exhibit significant anti-AD activity against AChE and BChE. In contrast, presence of a sugar moiety and methoxylation markedly reduced the anti-AD activity of the coumarins investigated in this study. With respect to BACE1 inhibition, umbelliferone 6-carboxylic acid, esculetin and daphnetin contained carboxyl or catechol groups, which significantly contributed to their anti-AD activities. To further investigate these results, we generated a 3D structure of BACE1 using Autodock 4.2 and simulated binding of umbelliferone 6-carboxylic acid, esculetin and daphnetin. Docking simulations showed that different residues of BACE1 interacted with hydroxyl and carboxylic groups, and the binding energies of umbelliferone 6-carboxylic acid, esculetin and daphnetin were negative (-4.58, -6.25 and -6.37 kcal/mol respectively). Conclusions: Taken together, our results suggest that umbelliferone 6-carboxylic acid, esculetin and daphnetin have anti-AD effects by inhibiting AChE, BChE and BACE1, which might be useful against AD.
1. Introduction
Alzheimer’s disease (AD) is the major form of dementia and is a deadly neurodegenerative disease that is progressive in nature and develops through a multifactorial process. AD is the fourth leadingcause of death in developed countries, and predominates in Europe and USA following cardiovascular disease, cancer, and stroke[1]. The two most common hypotheses used to describe the pathology of AD are known as the ‘cholinergic hypothesis’ and ‘amyloid hypothesis’. The cholinergic hypothesis suggests that AD is caused by a deficiency in the brain levels of the cerebral neurotransmitter acetylcholine (ACh), which is hydrolyzed by acetylcholinesterase (AChE)[2]. Similarly, butyrylcholinesterase (BChE) activity is increased by 40%-90% during the progression of AD[3], and BChE inhibition is considered a potentially important aspect of treating AD. In addition to the AChE and BChE hypotheses, accumulation of amyloid-βpeptide (Aβ) in the brain is widely considered to be critically involved in the pathogenesis of AD[4]. Aβ plaquesemerge roughly 15 years before the symptoms of AD appear[5], and once AD develops the cognitive decline caused by neuronal damage cannot be reversed, even after Aβ levels in the brain are lowered by immunotherapy[6]. Thus, prevention of Aβ accumulation is considered an important part of preventing AD. Aβ is excised from amyloid-β precursor protein through sequential cleavage by aspartic protease β-secretase 1 (BACE1)[4]. Thus, because BACE1 initiates Aβ processing, inhibition of BACE1 activity may be an effective way to prevent Aβ accumulation[7].
Coumarins are an important class of natural compounds and are used as additives in both foods and cosmetics[8]. Coumarin has been reported to have antibacterial[9], anti-oxidant[10], anti-inflammatory and anticoagulant[11], and anti-AD activities[12]. Coumarins are characterized a fused benzene and α-pyrone ring that serves as the structural nucleus. Importantly, a number of studies have demonstrated the ability of coumarins to inhibit AChE[12-14], and the benzopyrone structural nucleus of coumarins is an essential aspect of hybrid molecules capable of simultaneously inhibiting AChE and AChE-induced β-amyloid aggregation[15]. Likewise, the possibility of numerous chemical substitutions afforded by the structural nucleus makes coumarins interesting molecules for drug discovery.
As part of our continuing efforts to identify compounds from natural resources that inhibit AChE, BChE, and BACE1, we recently found that the methanolic extract of Angelica decursiva (Umbelliferae) (A. decursiva) fulfills these criteria[16]. This plant is used traditionally as an anti-inflammatory, diuretic, expectorant, and diaphoretic, as well as a remedy for colds, influenza, hepatitis, arthritis, indigestion, coughs, chronic bronchitis, pleurisy, typhoid, headaches, flatulence, fever, colic, travel sickness, rheumatism, bacterial and fungal infections, and diseases of the urinary organs[17,18]. Furthermore, extensive investigations of different species of this genus have been carried out in the last decade, and as a result many classes of compounds have been isolated including different types of coumarin derivatives[19]. In addition, Artemisia capillaries (A. capillaries) is commonly distributed in sandy areas along the Korean coastline. This plant has been frequently used in the treatment of liver disease, including hepatitis, jaundice, fatty liver and bilious disorder. Infusions of the buds, stems and whole plant of A. capillaris have been used in traditional Chinese medicine primarily as a choleric, anti-inflammatory, antipyretic, and diuretic agent for treating epidemic hepatitis[20,21]. This study deals with the anti-AD activities and the structure-activity relationship of coumarins isolated from A. decursiva and A. capillaris. Specifically, because there is currently no detailed information regarding the molecular interactions between umbelliferone 6-carboxylic acid, esculetin, daphnetin and BACE1, we performed molecular docking analysis and detailed enzyme kinetic analysis in order to investigate the possibility of using compounds umbelliferone 6-carboxylic acid, esculetin and daphnetin as potent anti-AD drug candidates.
2. Materials and methods
2.1. General experimental procedures
The1H- and13C-NMR spectra were determined using a JEOL JNM ECP-400 spectrometer (Tokyo, Japan) at 400 MHz for1H and 100 MHz for13C in deuterated chloroform (CDCl3). Column chromatography was conducted using silica gel 60 (70-230 mesh, Merck, Darmstadt, Germany). All TLC analyses were conducted using pre-coated Merck Kieselgel 60 F254plates (20 cm×20 cm, 0.25 mm) and using 50 % H2SO4as the spray reagent.
2.2. Chemicals and reagents
Electric-eel acetylcholinesterase (AChE, EC3.1.1.7), horseserum butyrylcholinesterase (BChE, EC 3.1.1.8), acetyl thiocholine iodide (ACh), butyrylthiocholine chloride (BCh), 5,5’-dithiobis [2-nitrobenzoic acid] (DTNB), quercetin, daphnetin and berberine were purchased from E. Merck, Fluka, or Sigma- Aldrich unless otherwise stated. The BACE1 FRET assay kit (β-secretase) was purchased from Pan Vera Co. (Madison, WI, USA). All chemicals and solvents used for column chromatography were of reagent grade, purchased from commercial sources, and used as received.
2.3. Isolation of coumarins
Umbelliferone, umbelliferone 6-carboxylic acid, scopoletin, isoscopoletin, 7-methoxy coumarin, scoparone, scopolin and esculetin were isolated from A. decursiva and A. capillaris, according to the method described by Zhao et al[18] and Islam et al[21], respectively. 2’-Isopropyl psoralene was isolated from subfraction-2 of dichloromethane fraction from A. decursiva, and identified by spectroscopic evidence including1H and13C-NMR, as well as by comparison with spectral published data[22].
2.4. In vitro ChE enzyme assay
The inhibitory activities of the isolated coumarins towards ChE were measured using the spectrophotometric method developed by Ellman et al[23]. ACh and BCh were used as substrates to assay the inhibition of AChE and BChE, respectively. Each reaction mixture consisted of 140 μL sodium phosphate buffer (pH 8.0), 20 μL of test sample solution at a final concentration of 100 μM for all compounds, and 20 μL of either AChE or BChE solution, which were then combined and incubated for 15 min at room temperature. All test samples and positive control (berberine) were dissolved in 10% DMSO. Reactions were initiated upon addition of 10 μL of DTNB and 10 μL of either ACh or BCh, respectively. The enzymatic hydrolysis mediated by AChE or BChE was monitored according to the formation of the yellow 5-thio-2- nitrobenzoateanion at 412 nm for 15 min, which was due to the reaction of DTNB with thiocholine released from ACh or BCh, respectively. All reactions were performed in 96-well plates in triplicate and recorded using a microplate spectrophotometer (Molecular Devices).
2.5. In vitro BACE1 enzyme assay
The in vitro BACE1 enzyme assay was carried out according to the manufacturers recommended protocol with minor modifications. Briefly, a mixture of 10 μL of assay buffer (50 mM sodium acetate, pH 4.5), 10 μL of BACE1 (1.0 U/mL), 10 μL of the substrate (750 nM Rh-EVNLDAEFK-Quencher in 50 mM, ammonium bicarbonate) and 10 μL of the test samples were dissolved in 10% DMSO and incubated for 60 min at 25 ℃ in the dark. The proteolysis of two fluorophores (Rh- EVNLDAEFK-Quencher) by BACE1 was determined by monitoring formation of the fluorescent donor (Rh-EVNL) at wave lengths of 530-545 nm for excitation and 570-590 nm for emission. Fluorescence was measured with a microplate spectrofluorometer (Gemini EM, Molecular Devices, and Sunnyvale, CA, USA). Specifically, each reaction was excited at 545 nm and the emission intensity was recorded at 585 nm.
2.6. Kinetic parameters of different coumarins towards AChE, BChE and BACE1 inhibition
In order to determine the mechanism of inhibition of the different coumarins, AChE and BChE inhibition was evaluated by monitoring the effects of different concentrations of substrate (0.6 to 0.1 mM for different samples). Specifically, AChE and BChE inhibition assays were performed as described above, but the substrate concentration was varied. Reactions were initiated upon addition of 10 μL of DTNB and 10 μL of either Ach or Bch at the indicated concentrations. In addition, to determine the kinetic mechanisms of the different coumarins towards BACE1, we employed two complementary kinetic methods, namely Lineweaver-Burk and Dixon plots[24,25]. Specifically, Dixon plots for inhibition of BACE1 by umbelliferone 6-carboxylic acid, esculetin and daphnetinwere obtained in the presence of different concentrations of substrate: 375 nM (▼); 250 nM (○) and 150 nM (●). Likewise, the test concentrations of umbelliferone 6-carboxylic acid, esculetin and daphnetin for the BACE1 inhibition kinetic analysis were 0.1 μM (▼), 1 μM (○), and 10 μM (●) for umbelliferone 6-carboxylic acid; 2.5 μM (▼), 12.5 μM (○), and 62.5 μM (●) for esculetin; and 2.5 μM (▼), 25 μM (○), and 100 μM (●) for daphnetin. Inhibition constants (Ki) were determined by interpretation of Dixon plots, where the value of the x-axis intercept was taken as -Ki[25,26].
2.7. BACE1 molecular docking simulations
To estimate the structure of the enzyme-inhibitor complex and to ensure accuracy, repeatability, and reliability of docking results, we employed Autodock 4.2 software. In our study, the test concentrations of coumarins for AChE were 100 μM, 50 μM, and 10 μM for umbelliferone 6-carboxylic acid, 100 μM, 20 μM, and 4 μM for esculetin; and 100 μM, 50 μM, and 25 μM for daphnetin. Likewise, the test concentrations for BChE were 100 μM, 50 μM and 25 μM for umbelliferone 6-carboxylic acid; 100 μM, 20 μM, and 1 μM for esculetin; and 50 μM, 25 μM, and 10 μM for daphnetin. Specifically, we used Autodock 4.2 to dock different coumarins into the binding site of the BACE1 crystallographic structure, which was defined as all residues 5-6 Å from the inhibitor in the original complex. Autodock 4.2 uses a semiempirical free energy force field to predict binding free energies of protein-ligand complexes of known structures and binding energy for both bound and unbound states. Twelve ligand structures were constructed and minimized using Chemdraw and LigandScout software for 2D and 3D conformations, respectively. For docking studies, the crystal structure of the BACE1 protein target was obtained from the protein sequence alignment [Protein Data Bank (PDB ID 2wjo)]. The 3D structures of umbelliferone 6-carboxylic acid, esculetin and daphnetin were generated and minimized using Chemdraw and LigandScout, for 2D and 3D conformation, respectively.
2.8. Statistical analysis
All results are expressed as the mean±SEM of triplicate samples. Results were analyzed using one-way ANOVA and Student’s t-test where appropriate (Systat Inc., Evaston, IL,USA). Values of P<0.05 were considered statistically significant.
3. Results
3.1. Inhibitory activity of coumarins against AChE, BChE and BACE1
In order to evaluate the anti-AD activity of the isolated coumarins, we first evaluated their abilities to inhibit AChE, BChE, and BACE1. A total of 10 coumarin derivatives were screened for their in vitro AChE, BChE and BACE1 inhibitory capacities (Table 1). Our results showed that esculetin and daphnetin exhibited good AChE inhibitory activity with IC50values of 6.13 and 11.57 μM, while umbelliferone 6-carboxylic acid, umbelliferone, scopoletin, isoscopoletin, 2’-isopropyl psoralene, as well as 7-methoxy coumarin showed moderate inhibitory activities with IC50values of 104.12, 145.19, 150.28, 153.77, 173.89, and 186.47 μM, respectively. Next we investigated the BChE inhibitory activities of the isolated coumarins (Table 1). The coumarins daphnetin, esculetin, and umbelliferone 6-carboxylic acid exhibited promising BChE inhibitory activitywith IC50values of 8.66, 9.29, and 27.19 μM, which compared favorably to the positive control berberine (IC50of 7.01 μM). On the other hand, umbelliferone, isoscopoletin, 7-methoxy coumarin, scopoletin, and 2’-isopropyl psoralene possessed only moderate inhibitory activity towards BChE with IC50values of 105.28, 110.30, 119.39, 159.30, and 179.22 μM, respectively. Finally, with respect to BACE1 inhibitory activity, umbelliferone 6-carboxylic acid, esculetin and daphnetin exhibited strong inhibitory activity with IC50values of 0.34, 7.67, and 11.19 μM compared to the positive control quercetin 13.98 μM. On the other hand, isoscopoletin, umbelliferone, and 7-methoxy coumarin exhibited moderate inhibitory activities with IC50values of 146.43, 143.1, and 172.26 μM respectively. All of the other compounds were inactive at the concentrations tested.
3.2. Kinetic parameters of coumarins on AChE and BChE inhibition
In order to determine the manner of AChE and BChE inhibition by the different isolated coumarins, we performed kinetics analyses using different concentrations of substrate (0.2 mM to 0.6 mM) and inhibitors, and evaluated the results using Dixon plots, which are a graphical method for determining the type of enzyme inhibition. Dixon plots are generated by plotting the reciprocal enzyme velocity (1/V) against inhibitor concentration (I), and can be used to determine the dissociation or Kifor the enzyme-inhibitor complex, in which the value of the x-axis intercept is the -Ki[26]. As shown in Figures 1A-C and Table 2, the manner of inhibition of umbelliferone 6-carboxylic acid and esculetin was noncompetitive with Kivalues of 80.99 μM and 25.09 μM, while compound daphnetin exhibited mixed type inhibition with a Kivalue of 19.98 μM for AChE. Similarly, Figures 1D-F and Table 2, indicated that the type of inhibition of umbelliferone 6-carboxylic acid was noncompetitive with a Kivalue of 49.78 μM, whereas esculetin and daphnetin exhibited mixed type inhibition with Kivalues of 16.11 μM and 7.18 μM, respectively.

Figure 1. Dixon plot of the inhibition of AChE and BChE by umbelliferone 6-carboxylic acid, esculetin and daphnetin in the presence of different substrate and inhibitor concentrations.

Figure 2. Dixon plots of BACE1 inhibition by coumarins.
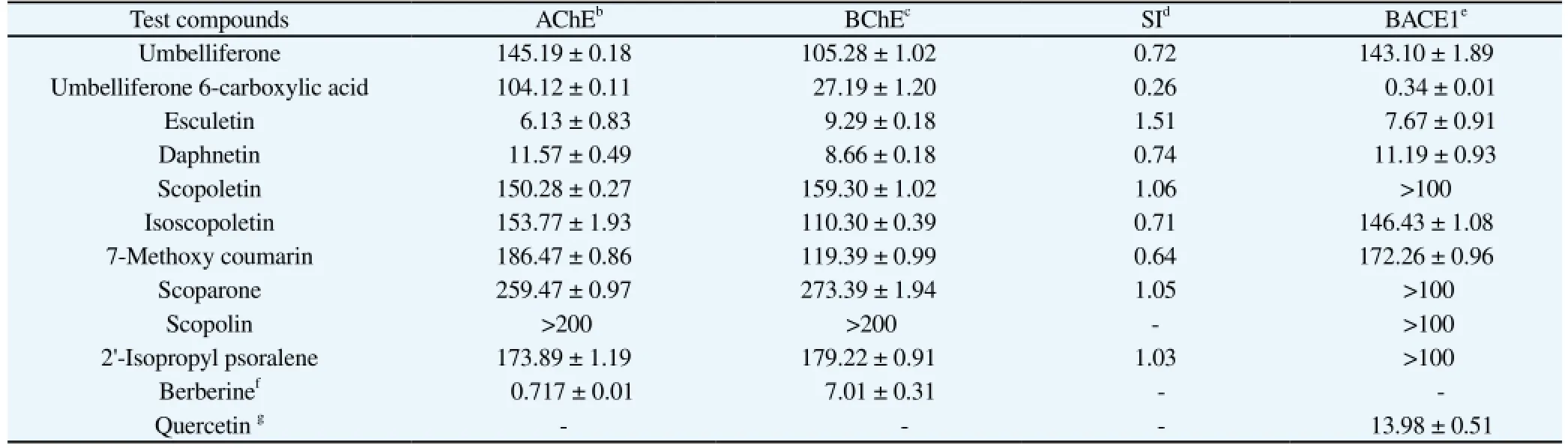
Table 1ChEs and BACE1 inhibitory activities of coumarins isolated from A. decursiva and A. capillaries (IC50)(mean±SEM).
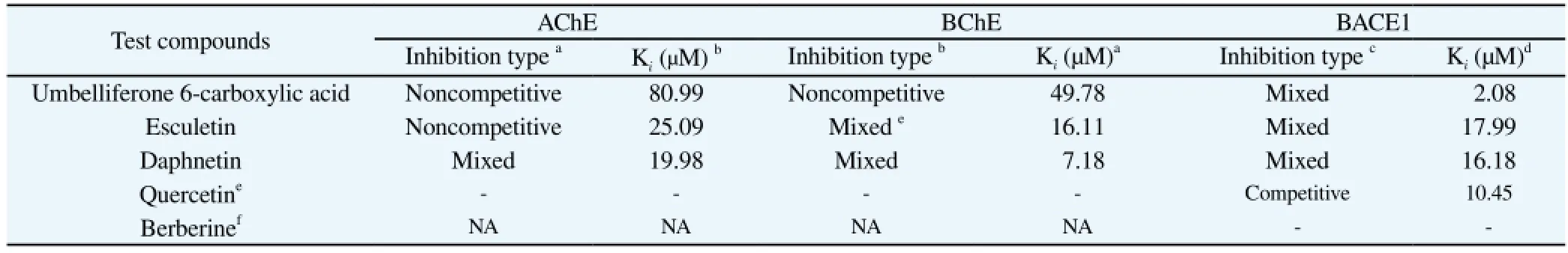
Table 2Mode of inhibition and dissociation constants (Ki) of coumarins for AChE, BChE and BACE1 based on enzyme kinetic plot.
3.3. Kinetic parameters of coumarins against BACE1 inhibition
To determine the mode of enzyme BACE1 inhibition by the different coumarins we performed kinetics analysis using Lineweaver-Burk plots and Dixon plots[24-26] employing different concentrations of substrate (150 nM, 250 nM, and 375 nM) and inhibitors. Kivalue were determined by interpretation of Dixon plots as described above. The test concentrations of the different coumarins were as follows: 10 μM, 1 μM, and 0.1 μM for umbelliferone 6-carboxylic acid; 62.5 μM, 12.5 μM and 2.5 μM for esculetin; and 100 μM, 25 μM, and 2.5 μM for daphnetin. As shown in Figures 2A-F and Table 2, the manner of BACE1 inhibition of umbelliferone 6-carboxylic acid, esculetin and daphnetin was mixed, with Kivalues of 2.08 μM, 17.99 μM, and 16.18 μM, respectively. A lower Kivalue is indicative of tighter enzyme binding and thus a more effective inhibitor. Thus, these results suggested that umbelliferone 6-carboxylic acid, esculetin and daphnetin might be good BACE1 inhibitors.
3.4. Molecular docking study of the inhibitory activity of the coumarins against BACE1
To follow up our results for the most active BACE1 inhibitory compounds we next performed molecular docking simulations. Specifically, we used Autodock 4.2 to predict and investigate the interactions between umbelliferone 6-carboxylic acid, esculetin, daphnetin and BACE1. Molecular docking models of these three coumarins and 2-Amino-3-{(1r)-1-cyclohexyl-2-[(cyclohexylcarbonyl) amino] ethyl}-6-phenoxyquinazolin-3-Ium (QUD) are illustrated in Figures 3-5. QUD is the most potent nonpeptic BACE1 inhibitor reported to date according to protein data bank. Ligand-enzyme complexes with umbelliferone 6-carboxylic acid, esculetin, daphnetin and QUD were stably posed in the same pocket of BACE1 by Autodock 4.2. As illustrated in Figures 3-5, the corresponding ligand interactions of umbelliferone 6-carboxylic acid in the active site of BACE1 were facilitated by hydrogen-bonding interactions between the Arg235 residue and one carboxylic group interaction between each of the Tyr71, Arg235 and Thr72 residues with one hydroxyl group at C-7 and one carboxylic group at C-6. Similarly, the corresponding ligand interaction for esculetin in the active site of BACE1 consisted of two hydrogenbonding interactions with the Asn37, Ser36, and Ile126 residues of BACE1 and two hydroxyl groups of the compound, while binding of daphnetin in the active site of BACE1 was mediated by two hydrogen-bonding interactions with Asp259 and Phe257 residues of the enzyme and two hydroxyl groups of the compound. The binding energies of these three compounds were -4.58, -6.25 and -6.37 kcal/ mol, respectively, which compared favorably with that of QUD, which had a binding energy of -10.99 kcal/mol. Taken together, these results indicated that additional hydrogen bonding and carboxylic groups might stabilize the open form of BACE1 and facilitate tighter binding to the active site resulting in more effective enzyme inhibition. Importantly, in vitro evaluation and in silico molecular docking data both strongly suggest that umbelliferone 6-carboxylic acid, esculetin and daphnetin may inhibit and/or prevent AD by targeting the formation of β-amyloid.

Figure 3. (A) Molecular docking model of BACE1 with umbelliferone 6-carboxylic acid (red color) and QUD (blue color). (B) Diagram of the ligand interaction of umbelliferone 6-carboxylic acid in the active site of BACE1.

Figure 4. (A) Molecular docking model of BACE1 with esculetin (magenta color) and QUD (white color). (B) Diagram of the ligand interaction of esculetin in the active site of BACE1.

Figure 5. (A) Molecular docking model of BACE1 with daphnetin (magenta color) and QUD (white color). (B) Diagram of the ligand interaction of daphnetin in the active site of BACE1.
4. Discussion
Recognition of the ability of natural products to positively impact health and well-being continues to grow both culturally and scientifically. As a result, there is a growing interest in demonstrating relationships between consumption of natural products and risk reduction and/or prevention of various disease and health conditions. As such, efforts to study and identify addedvalue natural products with health-promoting properties have also prompted the development of new drugs. For example, the benefits of natural products have been successfully demonstrated in AD[27]. Although its etiology remains unknown, the neuropathological profile of AD is associated with memory loss and is consistent with the presence of numerous plaques and cholinergic deficiency due to the degeneration or atrophy of cholinergic neurons in the basal forebrain[28]. With respect to the regulation of cognitive functions, the central cholinergic system is considered to be the most important neurotransmitter[29]. Indeed, cholinesterases such as AChE and BChE are considered key enzymes that play significant roles in cholinergic transmission by hydrolyzing the neurotransmitter Ach[30]. Loss of basal forebrain cholinergic cells results in an important reduction of ACh, which is believed to play a defining role in the cognitive impairment associated with AD, senile dementia, ataxia, and myasthenia gravis[29]. Moreover, at the molecular level, patients affected by AD exhibit abnormal deposits of Aβ and abnormal spiral filaments in neurons, increased oxidative stress, and low levels of Ach[31].
The death of neurons during the progression of AD usually affects the levels of brain neurotransmitters. In addition to ACh, other neurotransmitters such as glutamate and serotonin are also affected during the later stages of AD[32]. There is unfortunately no cure for AD, and treatment strategies are primarily symptomatic. Thus, current efforts to develop new therapeutics are focused on the cholinergic hypothesis, which aim to target AChE inhibition to improve cholinergic neurotransmission in the brain[33]. Interestingly, recent studies have shown that AChE has additional non-cholinergic function by binding to A[34]. Several of the cholinesterase inhibitors currently in use, namely donepezil, tacrine, rivastigmine, and galantamine, have adverse side-effects such as gastrointestinal disturbances, nausea, vomiting, and diarrhea and also have bioavailability issues[35]. For these reasons, there is growing scientific interest in identifying natural sources of AChE, BChE and BACE1 inhibitors with safer profiles.
To evaluate the potential of coumarins as anti-AD agents, we investigated their ability to inhibit AChE, BChE and BACE1 using a modified version of the assay described by Ellman et al[23]. A total of 10 coumarin derivatives were screened for their ability to inhibit AChE, BChE and BACE1 in vitro. The results of the screening showed that esculetin and daphnetin had good AChE and BChE inhibitory activities with IC50values of 6.13 and 11.57 μM for AChE, respectively, as well as good BChE inhibitory activities with IC50values of 8.66 and 9.29 μM, respectively. With respect to BACE1 inhibition, umbelliferone 6-carboxylic acid, esculetin and daphnetin had strong inhibitory activity with IC50values of 0.34, 7.67, and 11.19 μM, respectively.
In order to establish a structure-activity relationship between the coumarins and the target enzyme inhibition, the inhibitory effects of all coumarins against AChE, BChE and BACE1 were investigated. Among the molecules investigated, both esculetin and daphnetin, which have free hydroxyl groups at the 6, 7, or 8 positions, exhibited the highest inhibitory potential against AChE. A comparison of the inhibitory potential of different coumarin derivatives indicated that the presence of an o-dihydroxyl (catechol) group markedly increased inhibitory activity. Umbelliferone has a free hydroxyl group at the 7 position whereas umbelliferone 6-carboxylic acid contains both a free hydroxyl group at the 7 position and a carboxylic group at position 6. Interestingly, despite this difference in structure, the latter compound retained inhibitory activity, although it was reduced compared to esculetin and daphnetin. Substitution with a methoxyl or glycosyl group diminished the inhibitory potential as evidenced by the activity of scopoletin, isoscopoletin, scopolin, scoparone, and 7-methoxy coumarin. 2’-Isopropyl psoralene contains two methyl groups, which appeared to reduce its inhibitory activity towards AChE. Based on the above structure-activity relationship, the AChE inhibitory activity appears to be largely dependent on the presence of the hydroxyl groups at the 6, 7, or 8 positions, with methoxylation and glycosylation at these positions greatly diminishing inhibitor potency.
Regarding BChE inhibitory activity, umbelliferone 6-carboxylic acid, esculetin and daphnetin showed significant inhibitory activity. In particular, daphnetin, esculetin, and umbelliferone 6-carboxylic acid were as good as the positive control berberine. Indeed, both daphnetin and esculetin, which have a free hydroxyl group at the 6, 7, or 8 positions, displayed the highest inhibitory potential against BChE. On the other hand, umbelliferone and umbelliferone 6-carboxylic acid, which both have a free hydroxyl group at the 7 position, retained inhibitory activity but it was reduced compared to esculetin and daphnetin. Similar to the results described above, methoxylation at the 6, 7, or 8 positions (scopoletin, scopolin, scoparone and 7-methoxy coumarin) drastically abolished inhibitory activity, while glycosylation at the 7 position (isoscopoletin) resulted in complete inactivity at the concentrations tested. Based on these observations, it was clear that the presence of a free hydroxyl group is very important for BChE inhibitory activity. Importantly, these results were similar to those described in previous AD[12].
In order to elucidate the relationship between structure and activity, we investigated the ability of the different coumarins to inhibit BACE1. Umbelliferone 6-carboxylic acid, esculetin, and daphnetin exhibited promising inhibitory activity against BACE1 compared to the positive control quercetin. Umbelliferone 6-carboxylic acid has a free carboxyl group at the C-6 position while esculetin and daphnetin have free hydroxyl groups at the C-6, 7, or 8 positions. Umbelliferone, isoscopoletin, and 7-methoxy coumarin had moderate inhibitory activity against BACE1. Thus, based on our results, presence of a free carboxyl and free hydroxyl group at the 6, 7 or 8 positions of coumarin appears to be important for BACE1 inhibitory activity.
Various active compounds and extracts obtained from medicinal plants have been assessed for their efficacy against AD[36]. However,synergistic interactions are possible from plant extracts due to the simultaneous presence of dozens of bioactive compounds. Indeed, use of a single molecule for disease treatment and research is often preferred in order to better understand the mechanism of action. Therefore, it is very important to elucidate the active components in plant extracts. In the present study, we screened several pure coumarin derivatives for their ability to inhibit cholinesterase and BACE1 in vitro. There have been relatively few reports concerning the anticholinesterase capacity of coumarins. Previously, Kang et al[37] showed that the methanolic extract of Angelica gigas (A. gigas) roots (Umbelliferae) has significant anti-AChE activity, which ultimately led to the isolation of twelve coumarin derivatives. Orhan et al[38] has been reported that coumarins have anticholinesterase activity. Relevant to the present study, another furanocoumarin derivative called nodakenin was isolated from A. gigas of Korean origin and examined for its effect on learning and memory impairment induced by scopolamine[39]. Decursinol, a coumarintype compound from A. gigas roots, was also shown to have good anti-AChE effects[12]. In another study that employed a structurebased pharmacophore model, the two coumarins scopoletin and scopolin were investigated for their ability to inhibit AChE using a bioautography thin-layer chromatography assay[40], the results of which were consistent with our findings. Specifically, both scopoletin and scopolin had moderate but nevertheless remarkable, dosedependent, and long-lasting inhibitory effects against AChE. On the other hand, the CH2Cl2extract of Peucedanum ostruthium roots was shown to have significant inhibitory effects on AChE[41], and through bioactivity-guided fractionation four coumarin derivatives (ostruthin, imperatorin, ostruthol, and oxypeucedanin hydrate) were identified as the active components.
In an attempt to clarify the manner of AChE inhibition by the active coumarins identified in this study, we performed kinetics analyses using different substrate concentrations (0.6, 0.4, and 0.2 mM for umbelliferone 6-carboxylic acid and esculetin; 0.6, 0.3 and 0.1 mM for daphnetin) and analyzed the data using Dixon plots. As shown in our study, the manner of inhibition of umbelliferone 6-carboxylic acid and esculetin was noncompetitive with Kivalues of 80.99 and 25.09 μM, respectively, while daphnetin showed mixed type inhibition with a Kivalue of 19.98 μM. Likewise, to determine the type of BChE inhibition, we performed kinetics analysis of umbelliferone 6-carboxylic acid, esculetin and daphnetin by monitoring the effects of different concentrations of substrate (0.6 to 0.1 mM) and analyzed the data by Dixon plots. As shown in Figures 1D-F, umbelliferone 6-carboxylic acid exhibited noncompetitive type inhibition with a Kivalue of 49.78 μM, whereas esculetin and daphnetin showed mixed type inhibition with Kivalues of 16.11 and 7.18 μM, respectively. Lastly, to clarify the mode of enzyme BACE1 inhibition by the most potent coumarins, kinetics analysis was performed and the results were evaluated using Lineweaver-Burk plots and Dixon plots[24-26] at different substrate (150, 250, and 375 nM) and inhibitor concentrations. In analyzing the data, lower Kivalues are indicative of tighter binding of inhibitors to the enzyme and thus a more effective inhibitor. Thus, the Kivalues indicated that umbelliferone 6-carboxylic acid, esculetin and daphnetin may be excellent candidates as BACE1 inhibitors.
Based on our enzyme kinetic results regarding type of inhibition and Kivalue of umbelliferone 6-carboxylic acid, esculetin and daphnetin towards BACE1, we next analyzed the molecular structure of BACE1/inhibitor complexes using Autodock 4.2[42,43]. Specifically, we used this software to simulate binding between BACE1 and inhibitors and evaluate the binding site-directed inhibition of BACE1. The docking results for umbelliferone 6-carboxylic acid, esculetin and daphnetin returned negative binding energies of -4.58, -6.25 and -6.37 kcal/mol, respectively, suggesting that all three coumarins are high affinity enzyme-inhibitors that are able to tightly bind to the active site of BACE1. Autodock 4.2 is used to simulate inhibitors into the binding sites of enzymes that are located at a distance of 5-6 Å, and in this way molecular docking studies a powerful method to predict substructures that fit into binding pockets of enzyme in order to study inhibition and activation. The ligand-enzyme complexes with these three compounds and QUD were stably posed in the same pocket of BACE1 by Autodock 4.2. The corresponding ligand interactions of umbelliferone 6-carboxylic acid in the active site of BACE1 consisted of one hydrogen-bond between Arg235 and one carboxylic group interaction between the Tyr71, Arg235 and Thr72 residues of BACE1 with one hydroxyl group at C-7 and one carboxylic group. The corresponding ligand interactions of esculetin in the active site of BACE1 comprised two hydrogen-bonding interactions between the enzyme at Asn37, Ser36, and Ile126 with two hydroxyl groups of esculetin, while the binding of daphnetin in the active site of BACE1 consisted of two hydrogen-bonding interactions between enzyme at Asp259 and Phe257 and two hydroxyl groups. Taken together, the in vitro results and molecular docking data indicated that umbelliferone 6-carboxylic acid, esculetin and daphnetin have a strong potential to inhibit and prevent AD by targeting β-amyloid formation through BACE1.
In summary, our isolated coumarins have a significant ability to inhibit BACE1, BChE, and AChE. Based on structure-activity relationships of these coumarins, we speculated that the presence of a free hydroxyl group (catechol) at the C-6, 7, and 8 positions plays a predominant role in AChE and BChE inhibition, while the presence of a carboxyl and catechol group plays a crucial role in BACE1 inhibition. Taken together with molecular docking data, the results of the present study suggest that umbelliferone 6-carboxylic acid, esculetin and daphnetin may be good candidates for development as therapeutic agents for the treatment and prevention of AD by targeting β-amyloid formation. Further in vivo and cellular based studies are needed to help clarify the detailed mechanism of action of these compounds in the brain membrane and other organs.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This research was supported by the Basic Science ResearchProgram through the National Research Foundation of Korea and funded by the Ministry of Science, ICT & Future Planning (grant No. 2014R1A1A3051684 and 2012R1A6A1028677).
[1] Orhan G, Orhan I, Sener B. Recent developments in natural and synthetic drug research for Alzheimer’s disease. Lett Drug Design Disc 2006; 3(4): 268-274.
[2] Schliebs R, Arendt T. The cholinergic system in aging and neuronal degeneration. Behav Brain Res 2011; 221(2): 555-563.
[3] Geula C, Darvesh S. Butyrylcholinesterase, cholinergic neurotransmission and the pathology of Alzheimer’s disease. Drugs Today 2004; 40(8): 711-721.
[4] Querfurth H, Laferla F. Mechanisms of disease: Alzheimer’s disease. N Engl J Med 2010; 362: 329-344.
[5] Selkoe D. Preventing Alzheimer’s disease. Science 2012; 337(6101): 1488-1492.
[6] Gandy S. Perspective: prevention is better than cure. Nature 2011; 475(7355): S15.
[7] Vassar R, Kandalepas P. The β-secretase enzyme BACE1 as a therapeutic target for Alzheimer’s disease. Alzheimers Res Ther 2011; 3(3): 20-26.
[8] O’Kennedy R, Thornes RD. Coumarins: biology, applications and mode of action. Chichester: Wiley & Sons;1997.
[9] Ukhov SV, Kon’shin ME, Odegova TF. Synthesis and antimicrobial activity of 2 iminocoumarin-3-carboxylic acid amides. Pharm Chem J 2001; 35(7): 364-365.
[10] Vukovic N, Sukdolak S, Solujic S, Niciforovic N. An efficient synthesis and antioxidant properties of novel imino and amino derivatives of 4-hydroxy coumarins. Arch Pharm Res 2010; 33(1): 5-15.
[11] Hamdi N, Dixneuf PH. Synthesis of triazole and coumarin compounds and their physiological activity. Top Heterocycl Chem 2007; 10: 123-153.
[12] Kang SY, Lee KY, Sung SH, Park MJ, Kim YC. Coumarins isolated from Angelica gigas inhibit acetylcholinesterase: structure-activity relationships. J Nat Prod 2001; 64(5): 683-685.
[13] Bruhlmann C, Ooms F, Carrupt PA, Testa B, Catto M, Leonetti F. Coumarins derivatives as dual inhibitors of acetylcholinesterase and monoamine oxidase. J Med Chem 2001; 44(19): 3195-3198.
[14] Shen Q, Peng Q, Shao J, Liu X, Huang Z, Pu X. Synthesis and biological evaluation of functionalized coumarins as acetylcholinesterase inhibitors. Eur J Med Chem 2005; 40(12): 1307-1315.
[15] Piazzi L, Cavalli A, Colizzi F, Belluti F, Bartolini M, Mancini F. Multitarget directed coumarin derivatives: hAChE and BACE1 inhibitors as potential anti-Alzheimer compounds. Bioorg Med Chem Lett 2008; 18(1): 423-426.
[16] Ali MY, Jung HA, Choi JS. Anti-diabetic and anti-Alzheimer’s disease activities of Angelica decursiva. Arch Pharm Res 2015; DOI 10.1007/ s12272-015-0629-0.
[17] Sarkar SD, Nahar L. Natural medicine: the genus Angelica. Curr Med Chem 2004; 11(11): 1479- 1500.
[18] Zhao D, Islam MN, Ahn BR, Jung HA, Kim BW, Choi JS. In vitro antioxidant and anti-inflammatory activities of Angelica decursiva. Arch Pharm Res 2012; 35(1): 179-192.
[19] Sarkhail P. Traditional uses phytochemistry and pharmacological properties of the genus Peucedanum: A review. J Ethnopharmacol 2014; 156: 235-270.
[20] Tang W, Eisenbrand G. Chinese drugs of plant origin, chemistry, phamacology and use in traditional and modern medicine. New york: Springer Verlag; 1992.
[21] Islam MN, Jung HA, Sohn HS, Kim HM, Choi JS. Potent α -glucosidase and protein tyrosine phosphatase 1B inhibitors from Artemisia capillaris. Arch Pharm Res 2013; 36: 542-552.
[22] Anaya AL, Rubalcava MM, Cruz-Ortega R, Santana GC, Nchez-Monterrubio PN, Bautista BEH, Mata R. Allelochemicals from Stauranthus perforatus, a Rutaceous tree of the Yucatan Peninsula, Mexico. Phytochem 2005; 66: 487-494.
[23] Ellman GL, Courtney KD, Andres VJ, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961; 7(2): 88-95.
[24] Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc 1934; 56(3): 658-666.
[25] Dixon M. The determination of enzyme inhibitor constant. Biochem J 1953; 55(1): 170-171.
[26] Cornish-Bowden A. A simple graphical method for determining the inhibition constants of mixed, uncompetitive and non-competitive inhibitors. Biochem J 1974; 137(1): 143-144.
[27] Perry EK, Pickering AT, Wang WW, Houghton PJ, Perry NSL. Medicinal plants and Alzheimer’s disease: from ethnobotany to phytotherapy. J Pharm Pharmacol 1999; 51(5): 527-534.
[28] Roberson MR, Harrell LE. Cholinergic activity and amyloid precursor protein metabolism. Brain Res Rev 1997; 25(1): 50-69.
[29] Mukherjee PK, Kumar V, Mal M, Houghton PJ. Acetylcholinesterase inhibitors from plants. Phytomed 2007; 14(4): 289-300.
[30] Bruhlmann C, Marston A, Hostettmann K, Carrupt PA, Testa B. Screening of non-alkaloidal natural compounds as acetylcholinesterase inhibitors. Chem Biodivers 2004; 1(6): 819-829.
[31] Scarpini E, Scheltens P, Feldman H. Treatment of Alzheimer’s disease; current status and new perspectives. Lancet Neurol 2003; 2(9): 539-547.
[32] Holmes C, Wilkinson D. Molecular biology of Alzheimer’s disease. Adv Psychiatr Treat 2000; 6(3): 193-200.
[33] Tariot PN, Federoff H. Current treatment for Alzheimer disease and future prospects. J Alzheimer Dis Assoc Disord 2003; 17: 105-113.
[34] Dinamarca MC, Weinstein D, Monasterio O, Inestrosa NC. The synaptic protein neuroligin-1 interacts with the amyloid β-peptide. Is there a role in Alzheimer’s disease? Biochem 2011; 50(38): 8127-8137.
[35] Schulz V. Ginkgo extract or cholinesterase inhibitors in patients with dementia: what clinical trial and guidelines fail to consider. Phytomed 2003; 10(4): 74-79.
[36] Mantle D, Pickering AT, Perry EK. Medicinal plant extracts for the treatment of dementia. CNS Drugs 2000; 13(3): 201-213.
[37] Kang SY, Lee KY, Park MJ, Kim YC, Markelonis GJ, Oh TH, et al. Decursin from Angelica gigas mitigates amnesia induced by scopolamine in mice. Neurobiol Learn Memory 2003; 79(1): 11-18.
[38] Orhan I, Tosun F, Sener B. Coumarin, anthroquinone and stilbene derivatives with anticholinesterase activity. Z Naturforsch 2008; 63(5-6): 366-370.
[39] Kim DH, Kim DY, Kim YC, Jung JW, Lee S, Yoon BH, et al. Nodakenin, a coumarin compound, ameliorates scopolamine-induced memory disruption in mice. Life Sci 2007; 80(21): 1944-1950.
[40] Rollinger JM, Hornick A, Lange T, Stuppner H, Prast H. Acetylcholinesterase inhibitory activity of scopolin and scopoletin discovered by virtual screening of natural products. J Med Chem 2004; 47(25): 6248-6254.
[41] Urbain A, Marston A, Hostettman K. Coumarins from Peucedanum ostruthiumas inhibitors of acetylcholinesterase. Pharm Biol 2005; 43(8): 647-650.
[42] Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and AutoDock-Tools4: automated docking with selective receptor flexibility. J Comput Chem 2009; 30(16): 2785-2791.
[43] Bustanji Y, Al-Masri IM, Qasem A, Al-Bakri AG, Taha MO. In silico screening for non-nucleoside HIV-1 reverse transcriptase inhibitors using physicochemical filters and high-throughput docking followed by in vitro evaluation. Chem Biol Drug Des 2009; 74(3): 258-265.
ent heading
10.1016/j.apjtm.2016.01.014
Jae Sue Choi, Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea.
Tel.: +82-51-629-5845
Fax: +82 51 629 5842
E-mail: choijs@pknu.ac.kr
Hyun Ah Jung, Department of Food Science and Human Nutrition, Chonbuk National University, Jeonju 561-756, Republic of Korea.
Tel.: 82-63-270-4882
Fax: 82-63-270-3854
E-mail: jungha@jbnu.ac.kr
Foundation project: This research was supported by the Basic Science Research Program through the National Research Foundation of Korea and funded by the Ministry of Science, ICT & Future Planning (grant No. 2014R1A1A3051684 and 2012R1A6A1028677).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Preventive and therapeutic effect of N-Acetyl-L-cysteine on infection-associated preterm labor in mice
- First histopathological study in kidneys of rodents naturally infected with Leptospira pathogenic species from Yucatan, Mexico
- Dengue virus non-structural 1 protein interacts with heterogeneous nuclear ribonucleoprotein H in human monocytic cells
- Effect of roselle calyx extract on in vitro viability and biofilm formation ability of oral pathogenic bacteria
- Evaluation of anti-tubercular activity of linolenic acid and conjugatedlinoleic acid as effective inhibitors against Mycobacterium tuberculosis
- Recognition of a multiple antigen peptide containing sequence from mimotope of the dengue type 3 virus NS4B protein by human antibodies
