两个基于4-(1H-1,2,4-三氮唑)苯甲酸的4d-4f异核配位聚合物的合成、结构及荧光性质
2016-11-28方智利熊姗李康宇俞权锋徐文媛
方智利 熊姗 李康 宇俞 权锋 徐文媛
(华东交通大学理学院,南昌330013)
两个基于4-(1H-1,2,4-三氮唑)苯甲酸的4d-4f异核配位聚合物的合成、结构及荧光性质
方智利*熊姗李康宇俞权锋徐文媛
(华东交通大学理学院,南昌330013)
以4-(1H-1,2,4-三氮唑)苯甲酸(Htbc)为配体,采用水热法合成了两种新型4d-4f配位聚合物{[EuAg(tbc)3(H2O)]ClO4·H2O}n(1)和{[Eu2Ag3(tbc)6(NO3)2(H2O)2](ClO4)2·2H2O}n(2)。运用X射线单晶衍射法对该配位聚合物进行了结构测定,并对其进行了元素分析、红外光谱、PXRD及荧光光谱表征。单晶结构表明,配位聚合物1为正交晶系,空间群P212121,由一维三股螺旋链(其中2股右手螺旋和1股左手螺旋)通过Ag将其连成二维平面。配位聚合物2属于三斜晶系,空间群为P1,由内消旋一维两股螺旋链通过Ag将其连成二维平面。配位聚合物1和2的二维链结构均通过非共价键作用形成三维超分子,同时也表征了配位聚合物1和2的荧光性质。
4-(1H-1,2,4-三氮唑)苯甲酸;异核;晶体结构
0Introduction
Construction and characterization of metal-organic coordination polymers have attracted intense interest due to their potential applications in enantioselective separation[1-3],nonlinearoptical[4-5],andmagnetic properties[6-8].Heterometallic compounds formed from lanthanides and transition-metal ions are of interest not only because of their fascinating structural topology, but also for their potential applications as functional materials in luminescence[9-11],molecular adsorption[12], bimetallic catalysis[13-14]and magne-tism[15-17].There was also considerable progress to design and fabricate d-f heterometallic coordination polymers,from 1D chains, 2D lattices to 3D frameworks[10,18-20].Generally,two routes were used to prepare heterometallic coordination polymers:the first route is based on the use of the presynthesizedmetalcomplexeswhichcontain uncoordinated donors to combine with the second type of metal ions[21];the second route is based on the use of multidentate organic ligands to bind two types of metal ions in a one-pot reaction[19-20].Moreover,it is well known that lanthanide ions have a high affinity for binding to hard donors like the O atom,whereas most transition metal ions prefer to coordinate to soft donors like the N atom[10].As a consequence,it should be rational to select the polydentate compartmental ligand containing both O and N donors for the constructionofnewheterometalliccoordination polymers.These ligands such as pyazine carboxylic acid[22-23],imidazolecarboxylic acid[21,24]and pyridinecarboxylic acid[25]have been widely explored for preparing noveld-transitionandf-lanthanidehomometallic coordination polymers.Thus,4-(1H-1,2,4-triazol-1-yl) benzoic acid(Htbc),which contains three triazol nitrogen atoms and two carboxylate oxygen atoms, might provide the impetus for the synthesis of novel heterometalliccomplexes.Tothebestofour knowledge,there is no report that Htbc ligand is used to construct heterometallic coordination polymers.In the current work,Htbc with both N and O donor atoms was used to fabricate chiral Ln-Ag heterometallic coordination polymers.We herein report the synthesis, structure and characterization of two 2D heterometallic complexes based on Htbc ligands:one is a chiral 2D heterometallic Eu-Ag coordination polymer{[EuAg (tbc)3(H2O)]ClO4·H2O}n(1),containing triple-stranded helical(PPM)chains with[Eu-OCO]connectivity;the other is a novel achiral 2D heterometallic Eu-Ag coordination polymer{[Eu2Ag3(tbc)6(NO3)2(H2O)2](ClO4)2· 2H2O}n(2).
1 Experimental
1.1General procedures
Htbc was obtained from Jinan Henghua science and technology Ltd(Jinan,China).The other chemicals were commercially available reagents of analytical grade and used without further purification.Elemental (C,H,N)analyses were performed on Perkin-Elmer 240elementanalyzer.TheFT-IRspectrawere recorded from KBr pellets in the range of 4000~400 cm-1on a Nicolet 5DX spectrometer.Powder X-ray diffraction(PXRD)patterns of the samples were recorded using an X-ray diffractometer(BRUKER D8 ADVANCE)with Cu Kα radiation(λ=0.154 18 nm). Fluorescence spectra was recorded with an F-2500 FL Spectrophotometer analyzer.
Caution:Althoughwehaveexperiencedno problem with the compounds reported in this work, perchlorate salts of metal complexes with organic ligands are often explosive and should be handled with great caution.
1.2Preparation of{[EuAg(tbc3)(H2O)]ClO4·H2O}n(1)
A mixture of Eu2O3(0.5 mmol),AgNO3(0.5 mmol), Htbc(0.5 mmol),HClO4(0.2 mL)and distilled water (10 mL)was sealed in a 20 mL Teflon-lined stainless steel reactor and then heated to 150℃for 72 h under autogenous pressure.Then the mixture was slowly cooled to room temperature at a rate of 5℃·h-1,and dark red crystals of 1 suitable for X-ray crystal analysis were obtained.Yield:50%.Anal.Calcd.for C27H22AgClEuN9O12(%):C 33.76,H 2.29,N 13.13; Found(%):C 33.92,H 2.11,N 13.35.IR(KBr,cm-1): 3 456vs,3 132 m,2 385 m,1 606 s,1 544 s,1 413 s, 1 282 m,1 107 m,1 080 m,781 m,501.
2.3Preparation of{[Eu2Ag3(tbc)6(NO3)2(H2O)2]·
(ClO4)2(H2O)2}n(2)
A mixture of Eu2O3(0.5 mmol),AgNO3(0.5 mmol), Htbc(0.5 mmol),HClO4(0.1 mL)and distilled water (10 mL)was sealed in a 20 mL Teflon-lined stainless steel reactor and then heated to 170℃for 72 h under autogenous pressure.Then the mixture was slowly cooled to room temperature at a rate of 5℃·h-1,and colorless crystals of 2 suitable for X-ray crystal analysis were obtained.Yield:10%.Anal.Calcd.for C54H45Ag3Cl2Eu2N20O30(%):C 30.10,H 2.09,N 13.01; Found(%):C 30.47,H 2.14,N 13.35.IR(KBr,cm-1): 3 128 vs,2 916 m,2 788 m,2 510 m,1 913 m,1 691 s, 1 605 s,1 524 s,1 445 m,1 412 s,1 261m,1275 s, 1 221m,1 156 m,975 s,861 m,774 m.
1.3X-ray crystallographic study
All the diffraction data for complexes 1 and 2 were collected on a Bruker SMART APEXⅡCCD diffractometer equipped with graphite-monochromated Mo Kα radiation(λ=0.071 073 nm)using the ω-scan technique.Multi-scanabsorptioncorrectionswere applied with the SADABS program[26].The structures were solved by direct methods using the SHELXS-97 program and all the non-hydrogen atoms were refined anisotropically with the full-matrix least-squares on F2using the SHELXL-97 program[27].The hydrogen atoms of water molecules were located in the difference Fourier maps and the other hydrogen atoms were generated geometrically and refined as riding atoms with isotropic thermal factors.Crystallographic data and structure determination summaries for 1 and 2 are given in Table 1.Selected bond lengths and angles are listed in Table 2.
CCDC:1018336,1;1024087,2.
2 Results and discussion

Table 1 Crystal data for compounds 1 and 2
2.1Description of the crystal structure of 1
Single-crystal X-ray diffraction analysis of1 reveals that the complex crystallizes in the orthorhombic chiral space group P212121and possesses a 2Dheterometallic coordination framework.The asymmetric unit of 1 contains one perchlorate(ClO4-),three tbc anion,one Euion,one Agion,one coordinated watermoleculeandoneuncoordinatedwater molecule.As shown in Fig.1,the Eucenter is coordinated with seven oxygen atoms,among which six oxygen atoms are derived from six different tbc ions and one water molecule completing the coordination sphere of the Euion with O7donor set. The Eu-O bond lengths(0.231 4(4)~0.240 3(5)nm) are in the normal range[8].The bond angles around the central Euatom vary from 68.76(18)°to 177.2(2)°, similar to those reported for other seven-coordinated Eucoordinationpolymerswithoxygendonorligands[10].As far as the Agion in the coordination frameworkisconcerned,itexhibitsalinear configuration,being coordinated by two N atoms from two bridging tbc ligands.The Ag-N bond lengths are in the range of 0.213 5(6)to 0.214 5(7)nm,and the N-Ag-N bond angle is 174.5(3)°.In complex 1,the Htbc ligands exhibit two types of distinctly different coordination modes:one acts as a bridging ligand to coordinate one Agion and two Euions(Scheme 1,ModeⅠ)and the other acts a terminal ligand to coordinate two Euions(Scheme 1,ModeⅡ).

Table 2Selected bond distances(nm)and angles(°)for 1 and 2
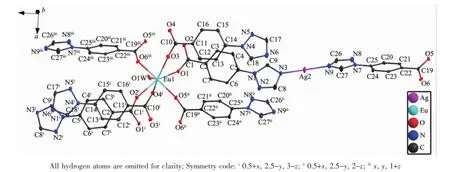
Fig.1Coordination environment of the Eu3+and Ag+ions in complex 1 with 30%thermal ellipsoids
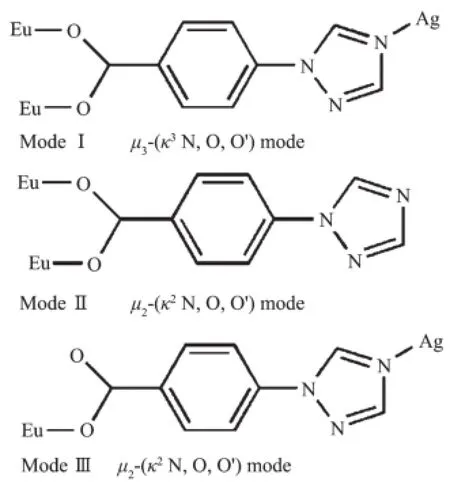
Scheme 1Coordination modes of the tbc anions in compounds 1 and 2
In the structure of 1,the chiral unit can be considered as a right-handed helix.A pair of Euions are interconnected through three carboxylate groups of three ligands tbc-with modesⅠandⅡto give a[Eu(μ3-OCO)2(μ2-OCO)]unit with Eu1…Eu1 distance of 0.499 1 nm,which may be viewed as a secondary building unit(SBU)to further extend into 1D triple strand right-right-left-handed helical(PPM) chain along the a axis(Fig.2).Although all the components appearing in the system are achiral,1 crystallizes in the chiral space group P212121.A direct vision of the topology of the 1D chain structure is illustrated in Fig.2.As you can see from Fig.2,the net handedness of the chain is right-handed.
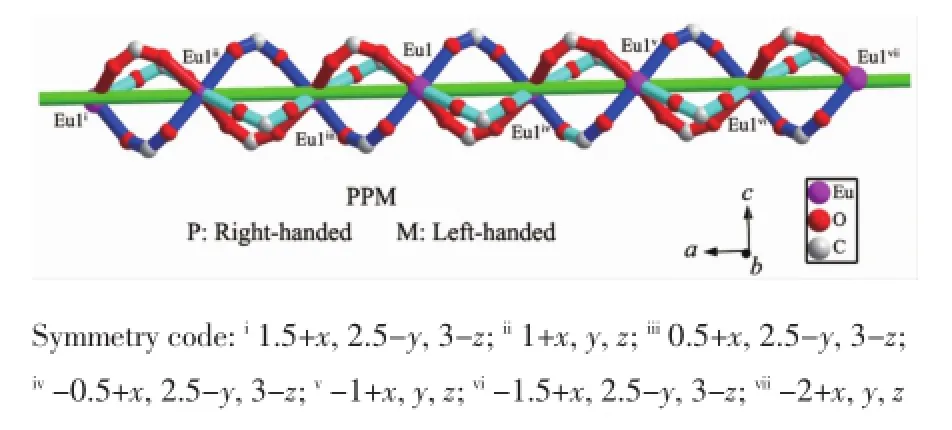
Fig.21D three-strand helical chains[Eu(μ3-OCO)2(μ2-OCO)](PPM)in compound 1 along the a axis
Theseparallelright-right-left(PPM)-handed triple stranded helical chains are further linked by the [AgN2]unit constructed from two triazole nitrogen atoms of the μ3-(κ3N,O,O′)-tbc-ligands,leading to the formation of a wave-like 2D 4d-4f heterometallic coordinationpolymerintheacplane(Fig.3). Furthermore,theadjacent2Dlayersarelinked throughhydrogenbonds(Table3)involving carboxylateoxygenatoms,coordinatedwater, uncoordinated water molecules,ClO4-and triazol nitrogen atoms,thereby producing a 3D supramolecular network(Fig.4).
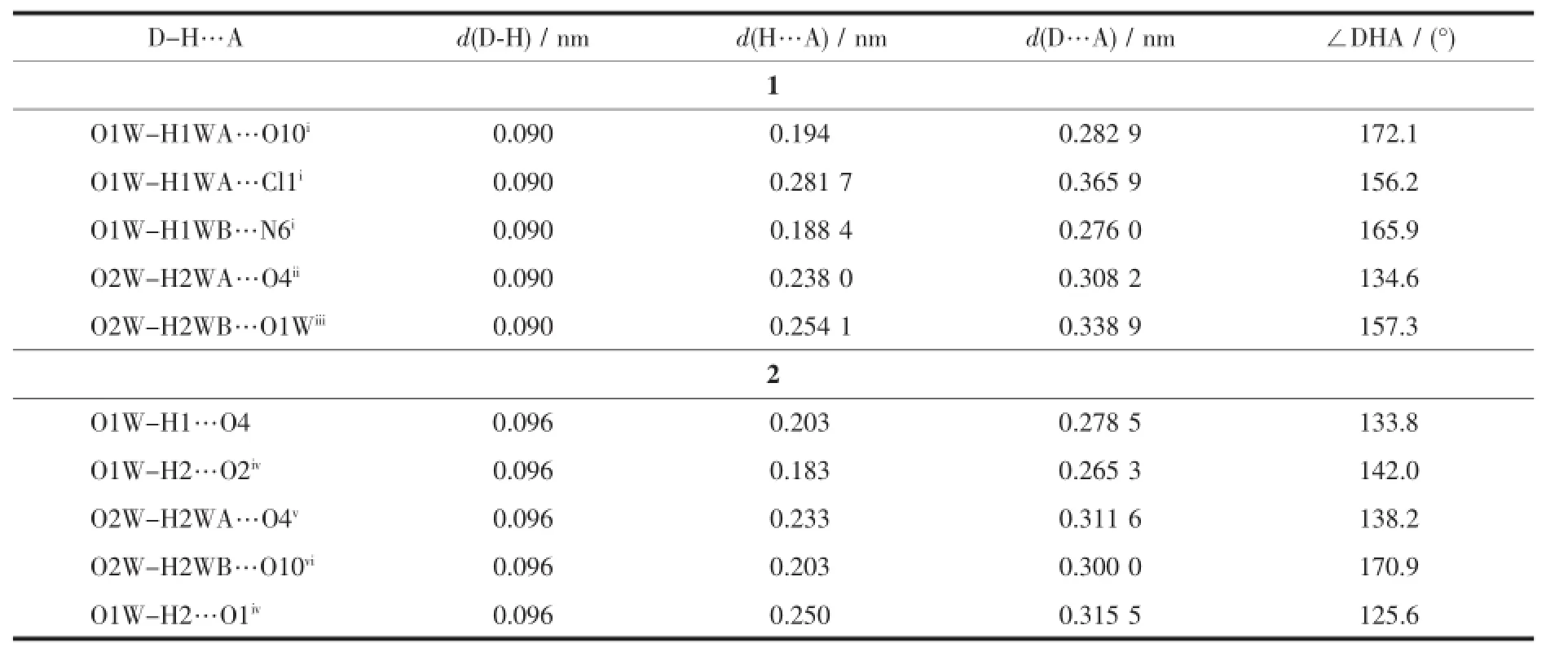
Table 3Hydrogen bonding parameters for complex 1 and 2
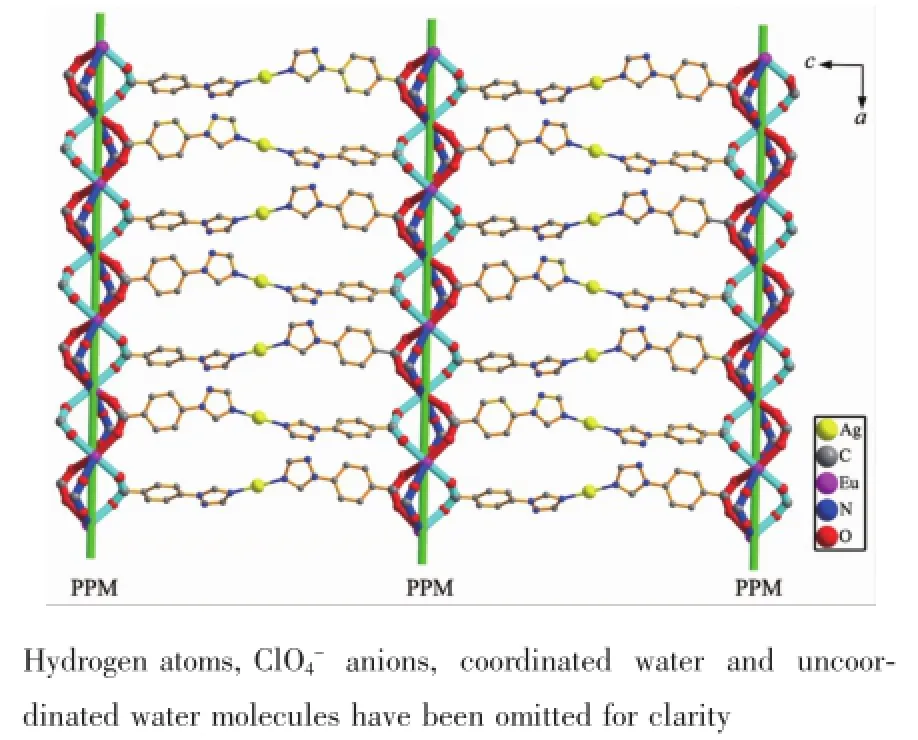
Fig.32D chiral heterometallic layer in 1 fabricated by the coordination link of ligand tbc-in modeⅠbetweentwoadjacentright-right-left(PPM)-handed helical chains in the ac plane,in which the ligand tbc-has coordination modeⅡ
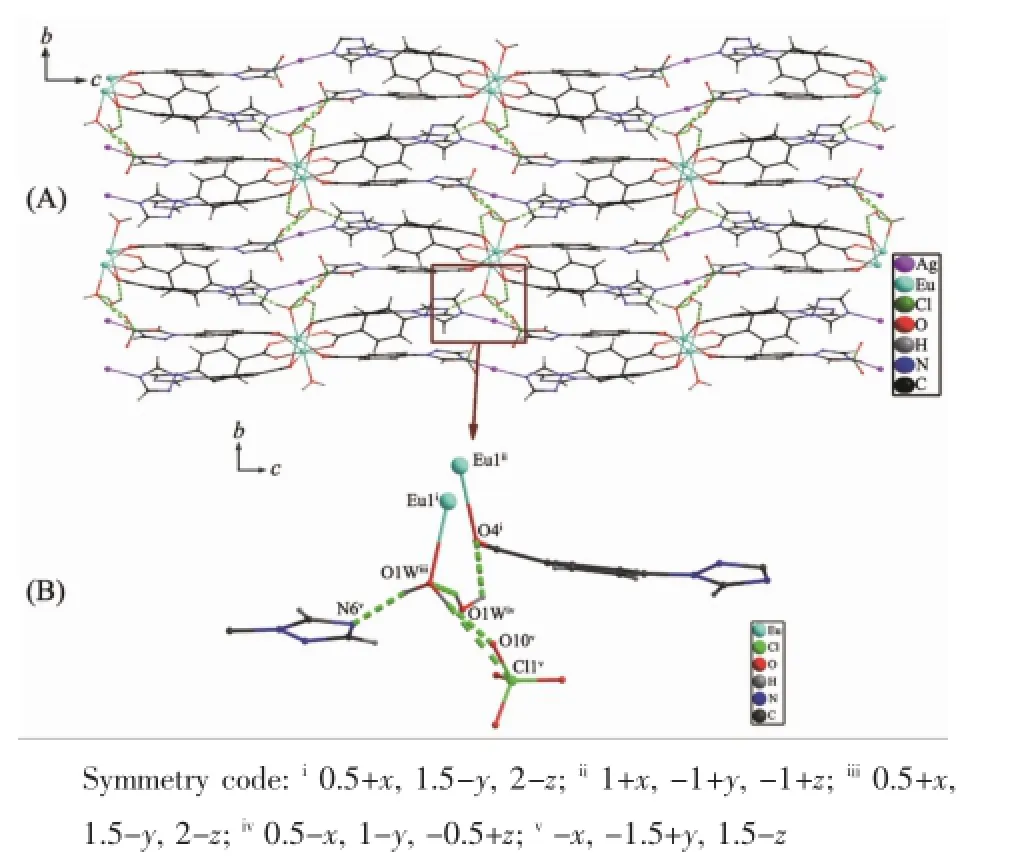
Fig.4Packing structure constructed through hydrogen bonds(dot line)with an-ABAB-sequence along the b axis
2.2Description of the crystal structures of 2
Single crystal X-ray diffraction analyses reveal that complex 2 crystallizes in the triclinic space group P1 and feature interesting 2D 4d-4f heterometallic coordination polymers.As depicted in Fig.5,the symmetric unit of 2 consists of one crystallographically independent Eucation,two Agcation(The crystallographicallyoccupancyofthetwoAgcations is 1 and 0.5 respectively),three unique tbcanions,one coordinated nitrate ion,one perchlorate),one coordinated water molecule,as well as onelatticewatermolecule.Thecoordination polyhedron around the central Euatom can be visualizedasslightlydistortedbicappedtrigonal prismatic arrangement with a[EuO8]coordination sphere:five carboxylate oxygen atoms come from five individual tbc-anions;one oxygen comes from one coordinated water molecule;other two oxygen atoms come from one nitrate anion.The Eu-O bond lengths are 0.230 4(5)and 0.258 6(6)nm.The bond angles around the central Euatom vary from 24.75(17)°to 165.22(18)°,similar to those reported for other eightcoordinated Eucoordination polymers with oxygen donor ligands[26].In complex 2,the Htbc ligandsexhibit two bridging modes(Scheme 1,ModeⅠandⅢ):one acts as a μ2bridge between one Euion and one Agion;the other one acts as a μ3bridge between two Euions and one Agion.Concerning two Agions,both Ag1 and Ag2 ions are two coordinated with two nitrogen atoms belonging to two different tbc-anions,forming the linear configuration. The Ag-N bond lengths are in the range of 0.211 3(6) to 0.214 3(6)nm,and the N-Ag-N bond angles are 180.0(6)°and 174.1(2)°.The main difference is that Ag1 ion is coordinated by two N atoms from two triazole rings with μ3-(κ3N,O,O′)coordination mode, while Ag2 is coordinated by two N atoms from two triazole groups with μ3-(κ3N,O,O′)and μ2-(κ2N,O) coordination modes.
Two of the tbc-ligands in complex 2 link to two Eu1 atoms in a μ3-(κ3N,O,O′)mode(Scheme 1a).As illustrated in Fig.6,every two Eu1 ions connect to four μ3-tbc-ligands and every two μ3-tbc-ligands link two Eu1 ions,giving rise to an infinite 1D mesomeric chain consisting of a right-left-handedhelical(PM) chains along the a axis(Fig.6a),where the adjacent two nonbonding Eu(Ⅲ)…Eu(Ⅲ)distances are equal to 0.4935 3(6)nm and 0.527 72(6)nm,respectively. These 1D chains are further linked by the[N-Ag1-N] unit from two triazole nitrogen atoms of the μ3-tbc-ligands and the[N-Ag2-N]unit from two triazole nitrogen atoms of the μ3-(κ3N,O,O′)-tbc-and μ2-(κ2N,O)-tbc-ligands,leading to the formation of a n interesting2D4d-4fheterometalliccoordination polymer in the ab plane(Fig.7).Furthermore,the 2D networks are stacked via interdigitation along the c axis(Fig.8).The alternate 2D layers are linked by van der Waals interaction and hydrogen bonds(Table 3) involving oxygen atoms of the ligands,perchlorate oxygen atoms and uncoordinated water molecules, thereby producing a 3D supramolecular network(Fig.8).

Fig.5 Coordination environment of the Eu3+and Ag+ions in complex 2 with 30%thermal ellipsoids
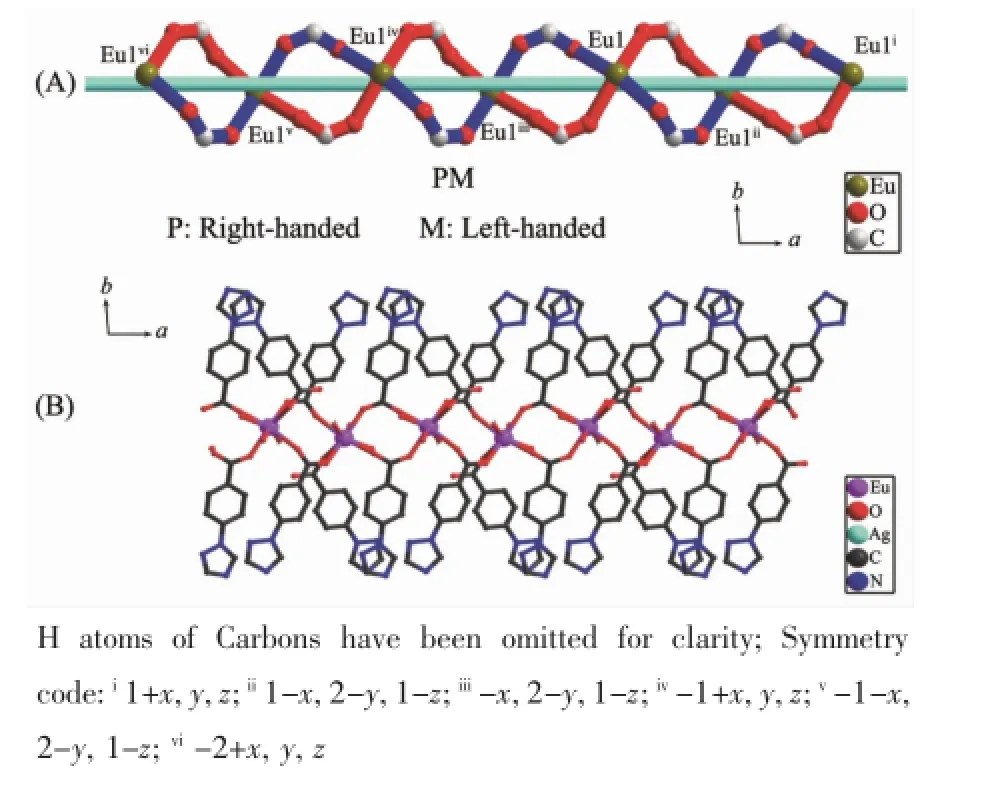
Fig.6 (a)1D meso-chain[Eu(μ3-OCO)2](PM)in compound 2 along the a axis;(b)A fragment of the 1D meso-chain of complex 2
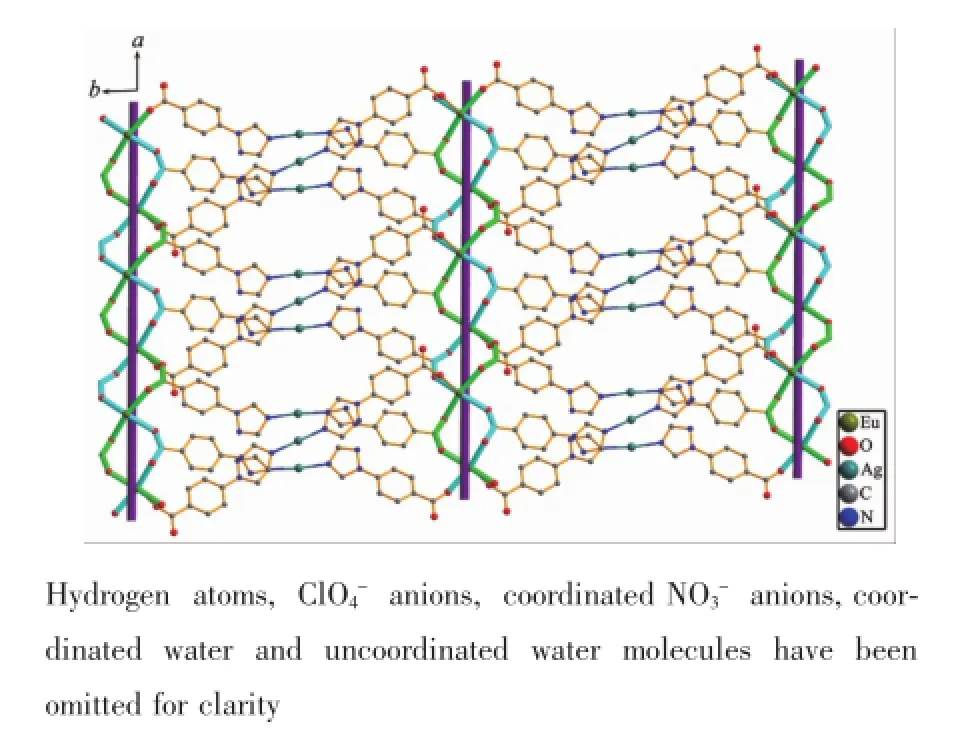
Fig.72D heterometallic layer in 2 fabricated by the coordination link of ligand tbc-in modesⅠandⅢbetween two adjacent meso-chains in the ab plane

Fig.83D supramolecular framework organized by the H-bonding(dot line)in complex 2
2.3PXRD pattern and photoluminescent properties
The PXRD pattern of 1 is in good agreement with the ones simulated from single-crystal structural data,which confirmed the purity of the bulk sample (Fig.9).
Because of the excellent luminescent properties of Euions,the luminescences of 1 and 2 were investigated.The emission spectrum of 1(Fig.10a)at room temperature upon excitation at 395 nm displays redluminescenceandexhibitsthecharacteristic transition of5D0→7FJ(J=0~4)of Euions.The emission spectrum is dominated by the characteristic5D0→7F2electron dipole transition at 615 nm.In addition,two weak peaks at 651 nm and 705 nm can be attributed to the5D0→7F3and5D0→7F4transition, respectively[28].Similarly,complex 2 exhibits the characteristic emission of Euions(Fig.10b).

Fig.9PXRD pattern of 1

Fig.10 Solid-state emission spectra of complexes 1(a) and 2(b)
3 Conclusions
In conclusion,we have successfully synthesized twonovel2DEu-Agheterometalliccoordination polymers.Compound 1 is chiral and its 2D lattices are constructed from the parallel right-right-left(PPM)-handed triple stranded helical chains[Eu(OCO)3]nwhich are further linked by Agand the fragments of tbc-ligands.In complex 2,the 1D chains of [Eu(OCO)2]nare linked by the[N-Ag1-N]unit from two triazole nitrogen atoms of the μ3-tbc-ligands andthe[N-Ag2-N]unit from two triazole nitrogen atoms of the μ3-(κ3N,O,O′)-tbc-and μ2-(κ2N,O)-tbc-ligands, leading to the formation of an 2D 4d-4f heterometallic coordination polymer.The complexes 1 and 2 exhibit characteristic lanthanide luminescence of rare earth ion Eu3+.The result obtained here can enrich the structurechemistryoftransition-lanthanideswith chiral helix Ln-OCO-Ln cluster cores.
References:
[1]Xie S M,Zhang Z J,Wang Z Y,et al.J.Am.Chem.Soc., 2011,133:11892-11895
[2]Nuzhdin A L,Dybtsev D N,Bryliakov K P,et al.J.Am. Chem.Soc.,2007,129:12958-12959
[3]Seo J S,Whang D,Lee H,et al.Nature,2000,404:982-986
[4]Qu Z R,Zhao H,Wang Y P,et al.Chem.Eur.J.,2004,10: 53-60
[5]Ye H T,Ren C Y,Hou G F,et al.Cryst.Growth Des.,2014, 14:3309-3318
[6]Train C,Gheorghe R,Krstic V,et al.Nat.Mater.,2008,7: 729-734
[7]Barron L D.Nat.Mater.,2008,7:691-692
[8]Zhang W,Xiong R G.Chem.Rev.,2012,112:1163-1195
[9]YU Gang(禹钢),BIAN Zu-Qiang(卞祖强),LIU Zhi-Wei(刘志伟),et al.Sci.China Ser.B:Chem.(中国科学B:化学), 2014,4(2):267-276
[10]Gu X,Xue D.Inorg.Chem.,2006,45:9257-9261
[11]Lestari W W,Lnnecke P,Streit H C,et al.Eur.J.Inorg. Chem.,2014:1775-1782
[12]Li J R,Tao Y,Yu Q,et al.Chem.Eur.J.,2008,14:2771-2776
[13]YAO Ke-Min(姚克敏),LI Ning(李宁),HUANG Qiao-Hong (黄巧虹),et al.Sci.China Ser.B:Chem.(中国科学B:化学),1998,28(6):517-523
[14]Liu Q Y,Xiong W L,Liu C M,et al.Inorg.Chem.,2013, 52:6773-6775
[15]Tanase S,Andruh M,Müller A,et al.Chem.Commun., 2001:1084-1085
[16]GU Jin-Zhong(顾金忠),JIANG Long(姜隆),LU Tong-Bu(鲁统部),et al.Chinese J.Inorg.Chem.(无机化学学报),2008, 24:1743-1747
[17]Lampropoulos C,Koo C,Hill S O,et al.Inorg.Chem.,2008, 47:11180-11190
[18]Mukherjee S,Lan Y,Novitchi G,et al.Polyhedron,2009, 28:1782-1787
[19]Li Z Y,Huang H Q,Xu L,et al.Cryst.Growth Des.,2013, 13:918-925
[20]Sun Y G,Wu Y L,Xiong G,et al.Dalton Trans.,2010,39: 11383-11395
[21]Chen R L,Chen X Y,Zheng S R,et al.Cryst.Growth Des., 2013,13:4428-4434
[22]Andrews M B,Cahill C L.CrystEngComm,2011,13:7068-7078
[23]Cai B,Yang P,Dai J W,et al.CrystEngComm,2011,13: 985-991
[24]Cai S L,Zheng S R,Wen Z Z,et al.Cryst.Growth.Des., 2012,12:2355-2361
[25]Zhou Y,Li X,Zhang L,et al.Inorg.Chem.,2014,53:3362-3370
[26]Sheldrick G M.SADABS,Program for Empirical Absorption Correction,University of Göttingen,Germany,1996.
[27]Sheldrick G M.SHELXS-97 and SHELXL-97,Program for the Solution of Crystal Structure,University of Göttingen, Germany,1997.
[28]Quici S,Cavazzini M,Marzanni G,et al.Inorg.Chem., 2005,44:529-537
Two Heterometallic 4d-4f Coordination Polymers Based on 4-(1H-1,2,4-Triazol-1-yl)benzoic Acid:Syntheses,Structures,and Fluorescence Properties
FANG Zhi-Li*XIONG ShanLI Kang-YuYU Quan-FengXU Wen-Yuan
(School of Science,East China Jiaotong University,Nanchang 330013,China)
Two 2D heterometallic 4d-4f coordination polymers,{[EuAg(tbc)3(H2O)]ClO4·H2O}n(1)and{[Eu2Ag3(tbc)6(NO3)2(H2O)2](ClO4)2·2H2O}n(2)(Htbc=4-(1H-1,2,4-triazol-1-yl)benzoic acid)were synthesized under hydrothermal condition using one-step approach and structurally characterized by elemental analysis,FT-IR,powder X-ray diffraction and single-crystal X-ray diffraction.The single-crystal X-ray diffraction analysis reveals that the two complexes present two different types of two dimensional(2D)structures.Complex 1 crystallized in an orthorhombic manner having a P212121space group,consisting of 1D triple-stranded helical(PPM)[Eu(μ3-OCO)2(μ2-OCO)]motif as chain which are further linked by the fragments of tbc-ligands and Ag.Polymer 2 is crystallized in a triclinic fashion having aspace group,consisting of 1D mesomeric chains[Eu(μ3-OCO)2] which were further linked by the two different[N-Ag-N]units to form an interesting 2D 4d-4f heterometallic coordination polymer in the ab plane.The solid-state luminescent behaviors of compounds 1 and 2 were investigated at room temperature.CCDC:1018336,1;1024087 2.
4-(1H-1,2,4-triazol-1-yl)benzoic acid ligand;heterometallic;crystal structure
O614.122;O614.33+8
A
1001-4861(2016)02-0280-09
10.11862/CJIC.2016.046
2015-06-01。收修改稿日期:2015-12-04。
国家自然科学基金(No.21365012)资助项目。
*通信联系人。E-mail:fangzhili1972@126.com;会员登记号:。
