由羧酸配体和铁构筑的两个过渡金属配位聚合物的结构、表征和性质
2016-11-28黄艳菊杜刚郝向荣张铁赢
黄艳菊 杜刚 郝向荣 张铁赢
(1通化师范学院化学学院,通化134002) (2通化市第一中学校,通化134001)
由羧酸配体和铁构筑的两个过渡金属配位聚合物的结构、表征和性质
黄艳菊*,1杜刚2郝向荣1张铁赢1
(1通化师范学院化学学院,通化134002) (2通化市第一中学校,通化134001)
采用水热法合成了2个铁的过渡金属配位聚合物[Fe(Medpq)(BDC)H2O]n(1)和{[Fe(Medpq)(QUI)H2O]·2H2O}n(2)(Medpq=2-methyldipyrido[3,2-f:2′,3′-h]quinoxaline;H2BDC=terephthalic acid;H2QUI=2,3-pyridinedicarboxylic acid),并对其进行了元素分析、红外光谱和热重表征,并用X射线单晶衍射测定结构。2个配位聚合物中的中心铁(Ⅱ)离子,都呈现一个稍微扭曲的八面体几何构型。
配位聚合物;水热合成;羧酸配体
0Introduction
Atthisstage,owingtothepossibilityof structural variations and the controllability of the assembly process,the combination of anionic O-donor and N-donor ligands mixed ligands with metal cations indesigningcoordinationpolymersshouldbe considered as an attractive design strategy[1].
In particular,aromatic multicarboxylate ligands, forexample1,4-Benzenedicarboxylicacid,2,3-pyridinedicarboxylic acid(H2QUI)are well used in the construction of MOFs with interesting structures and special topologies due to their structural rigidity, chemical stability and appropriate connectivity[2-13]. On the other hand,there are a number of so-called“anomalies”of reactivity of N-heterocyclic complexesin aqueous solutions,such as bipyridine(bpy)and 1,10-phenanthroline(phen)complexes.So far,phen has been widely used to build supramolecular architectures because of its excellent coordinating ability and large conjugatedsystemthatcaneasilyformπ-π interactions[14-16].However,far less attention has been given to their derivatives.2-Methyldipyrido[3,2-f:2′,3′-h]quinoxaline(Medpq)as an important phen derivative possesses fruitful aromatic systems and is a good candidatefortheconstructionofmetal-organic supramolecular architectures.In this work,we designed and prepared the polymers using Medpq,namely [Fe(Medpq)(BDC)H2O]n(1)and{[Fe(Medpq)(QUI)H2O]· 2H2O}n(2).
1 Experimental
1.1Materials
The Medpq ligand was synthesized according tothe literaturemethod[17].Otherchemicals from commercial sources were of reagent grade and used without further purification.
1.2Instruments and measurements
Elemental analysis was carried out with a Perkin-Elmer240Canalyzer.TGmeasurementswere performed on a NETZSCH STA 449C analyzer.The Infrared(IR)spectrum was recorded from KBr pellets in the range of 4 000~400 cm-1on a Nicolet FTIR 170SX spectrometer.
1.3Syntheses and measurements
1.3.1Synthesis of[Fe(Medpq)(BDC)H2O]n(1)
Coordination polymer 1 was prepared from a mixture of FeSO4·7H2O(0.12 g),Medpq(0.10 g), H2BDC(0.08 g)and H2O(25 mL)while stirring at room temperature.When the pH value of the mixture was adjusted to about 6.5 with NaOH,the cloudy solution was put into a 30 mL Teflon-lined autoclave under autogenous pressure at 165℃for six days(Fig.1).After cooling to room temperature,light yellow block crystals of 1 were collected by filtration and washed with distilled water in 45%yield(based on Fe).Anal.Calcd. for C23H16FeN4O5(%):C,57.05;H,3.33;N,11.57. Found(%):C,57.08;H,3.35;N,11.49.IR(KBr,cm-1): 3 392m(ascribed to the stretching vibrations of H2O, which indicates the existence of water molecules in coordination polymer 1),1 634s,1 624s,1 580s,1 485 m,1355s,1368m,1258w,1133 m,827m,713m.
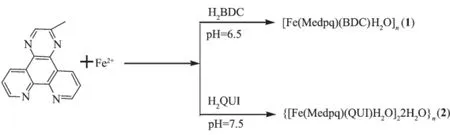
Fig.1Synthetic routes of the coordination polymers
1.3.2Synthesis of{[Fe(Medpq)(QUI)H2O]·2H2O}n(2)
Coordination polymer 2 was synthesized by a method similar to that of 1,except that the pH value of the reaction was adjusted to 7.5 with NaOH and using H2QUI(0.1 g)instead of H2BDC.Yellow block of 2 were collected by filtration and washed with distilled water in 40%yield(based on Fe).Anal. Calcd.(%)for C22H19FeN5O7:C,50.69;H,3.67;N, 13.44.Found(%):C,50.76;H,3.48;N,13.45.IR (KBr,cm-1):3 395m(ascribed to the stretching vibrations ofH2O,whichindicatestheexistenceofwater molecules),1634s,1610s,1580s,1523m,1355s,1368 m,1258w,1133m,827m,713m.
1.4Crystal structure determination and physical measurements
Crystallographic data of the two coordination polymers were collected at 293 K on a Bruker SMART 1000 CCD X-ray diffractometer with a graphitemonochromatic Mo Kα radiation(λ=0.071 073 nm)by using ω scan mode in the 2°range of 6.0°~50.8°(1) and 6.3°~50.0°(2).The structures of coordination polymers 1 and 2 were solved by direct methods withSHELXS-97 program[18]and refined by SHELXL-97[19]using full-matrix least-squares techniques on F2.All the hydrogen atoms were placed in the calculated sites and included in the final refinement in the riding model approximation with displacement parameters derived from the parent atoms to which they were bonded.Allnon-hydrogenatomswererefined anisotropically.AllHatomswerepositioned geometrically(C-H 0.093 nm for CH or 0.096 nm for CH3)and refined as a riding mode,with Uiso(H)= 1.2Ueq(C).The H atoms of one uncoordination water molecule in coordination polymer 2 could not be positioned reliably.Other H atoms of water molecules were located from difference Fourier maps.Further crystallographic data and experimental details for structural analyses of both the two complexes are summarized in Table 1.
CCDC:1436495,1;1436494,2.

Table 1Crystallographic data for the coordination polymers 1 and 2
2 Results and discussion
2.1Description of crystal structures
2.1.1Crystal structure of[Fe(Medpq)(BDC)H2O]n(1) Single-crystal X-ray diffraction analysis reveals that coordination polymer 1 crystallizes in P21/n space group and consists of a one-dimensional structure. There are one Feion,one Medpq ligand,two half BDC ligands and one coordinate water molecule in the symmetric unit(Fig.2).The Feion is hexa
coordinated with six atoms(N1,N2,O1,O2,O3,O5) from one Medpq ligand,two different BDC ligands andonecoordinatewatermolecule,assuminga slightlydistortedoctahedralgeometry.Thebond lengths are 0.217 0(3)and 0.216 8(3)nm for Fe-N, 0.205 9(2)~0.224 8(3)nm for Fe-Ocarboxylate,and 0.212 7(3) nm for Fe-Owater,respectively.The N(O)-Fe-O(N)angles range from 59.46(10)°to 166.00(11)°.The selected bond parameters are given in Table 2.
The coordination polymer adopt zigzag chain structuresbridgedbytheBDCligandsinthe bisbidentate and bis-monodentate modes with Fe-Fe distance of about 1.142 3 nm(Fig.3).Hydrogen bonding interactions are usually important in the synthesisofsupramoleculararchitectures.There arepersistentstrongO-H…Ohydrogenbondinginteractions between BDC ligands and coordinate water molecules and weak C-H…O hydrogen bonding interactions between BDC ligands and Medpq ligands (O5…O2i0.280 3(4)nm,O5…O4 0.260 2(4)nm,C1…O4i0.314 9(5)nm,C2…O4i0.315 9(5)nm,C3…O1ii0.320 1(5)nm;O5-H5A…O2 165(4)°,O5-H5B…O4 158(5)°,C1-H1…O4 124°,C2-H2…O4 121°,C3-H3…O1 133°;Symmetry codes:i-x,1-y,-z;ii1/2-x,1/2+y,1/2-z),which play an important role in stabilizing the network structure and controlling the orientation of ligands.At the same time,the aromatic ring of ligands(BDC ligand and Medpq ligand)and symmetry of the two adjacent equivalent of aromatic ring(Symmetry codes:i1/2-x,-1/2+y,1/2-z;ii-x,1-y,1-z;iii1/2-x,1/2+y,1/2-z;iv1/2+x,1/2-y,1/2+z;v-1/2+x,1/2-y,-1/2+z)have π-π interactions(Cg(4)→Cg(5)ii0.361 6(2)nm,Cg(5)→Cg(6)ii0.358 5(2)nm, Cg(5)→Cg(8)iii0.363 6(2)nm,Cg(5)→Cg(8)iv0.363 6(2)nm,Cg(8)→Cg(5)i0.363 6(2)nm,Cg(8)→Cg(5)v0.363 6(2)nm,Cg(6)→Cg(5)ii0.358 5(2)nm,Cg(6)→Cg(6)ii0.359 3(2)nm,Cg(6)→Cg(8)iv0.372 4(2)nm, Cg(6)→Cg(8)iii0.372 4(2)nm,Cg(8)→Cg(6)i0.372 4(2) nm,Cg(8)→Cg(6)v0.372 4(2)nm)for defined rings: Cg(4):N2→C12→C11→C10→C9→C14,Cg(5):N3→C5→C8→N4→C7→C6,Cg(6):C4→C5→C8→C9→ C14→C13andCg(8):C21→C22→C23→C21A→C22A→C23A.Through hydrogen bonding interactions and π-π interactions between the adjacent aromatic ring of Medpq ligands and BDC ligands,coordination polymer 1 formed two-dimensional layer structure (Fig.4).

Fig.2 ORTEP drawing of 1 showing the local coordination environment of Fe

Fig.3 View of 1D zigzag chain structure of the coordination polymer 1

Fig.4 View of 2D layer structure of the coordination polymer 1
2.1.2Crystal structure of{[Fe(Medpq)(QUI)H2O]· 2H2O}n(2)
Single-crystal X-ray diffraction analysis reveals that coordination polymer 2 crystallizes in C2/c space group.The local coordination environment of Feconsists of one Medpq ligand,one coordinate water molecule,two half QUI ligands and two free water (Fig.5).The Feion is six-coordination with O1,N1, N2,N5A,O5AandO2fromcoordinatewater molecule,Medpqligand,QUIligand,andits symmetrical QUI ligand,respectively,assuming a slightlydistortedoctahedralgeometry.Thebond distances are Fe-N 0.215 8(6)~0.221 2(6)nm,Fe-Ocarboxylate0.209 3(5)nm~0.210 1(5)nm and Fe-Owater0.217 3(5)nm,respectively.The N(O)-Fe-O(N)bond angles range from 75.6(2)°to 169.6(2)°.The selected important bond parameters are given in Table 2.The interesting feature of coordination polymer 2 is that each QUI ligand links two symmetry-related iron atoms into an infinite 1D chain(Fig.6).
In coordination polymer 2,strong O-H…O hydrogen bonds are observed between QUI ligands, water molecules and hydroxyl ions(O1…O3 0.263 8(9) nm,O1…O4i0.278 7(8)nm,O6…O7 0.273 1(18)nm, O6…N4ii0.302 4(15)nm;O1-H1B…O3 147.8°,O1-H1A…O4 138.2°,O6-H6D…O7 129.4°,O6-H6A…N4 124.2°).The weak C-H…O hydrogen bonds are observed between QUI ligands and Medpq ligands(C3…O2iii0.335 2(11)nm,C11…O3i0.302 0(13)nm, C20…O4iv0.318 2(10)nm;C3-H3…O2 144.4°,C11-H11…O3 132.5°,C20-H20…O4 151.5°;Symmetry codes:i1/2-x,-1/2+y,1/2-z;ii-x,1+y,1/2-z;iii-x,y,1/2-z;ivx,1-y,1/2+z).
In coordination polymer 2,intermolecular π-π stacking interactions are found between the aryl ring ofMedpqligandsanditsequivalentsymmetry (Symmetry codes:i-x,y,1/2-z;ii-x,-y,-z)in an offset fashion.The distances for these intermolecular π-π stacking interactions are 0.362 7(5)nm for Cg(4)→Cg(4)i,0.370 5(7)nm for Cg(5)→Cg(6)i,0.375 3(7) nm for Cg(5)→Cg(6)ii,0.370 5(7)nm for Cg(6)→Cg(5)i, 0.375 2(7)nm for Cg(6)→Cg(5)ii,0.391 3(7)nm for Cg(6)→Cg(8)i,0.391 3(7)nm for Cg(8)→Cg(6)iand 0.364 6(6)nm for Cg(8)→Cg(8)i(Cg(4):N(1)→C(1)→C(2)→C(3)→C(4)→C(13),Cg(5):N(2)→C(12)→C(11)→C(10)→C(9)→C(14),Cg(6):N(3)→C(5)→C(8)→N (4)→C(7)→C(6)and Cg(8):C(4)→C(5)→C(8)→C(9)→C(14)→C(13)).
With the help of above two kinds of interactions, thetitlecoordinationpolymerformed2Dlayer structure(Fig.7).

Fig.5 ORTEP drawing of 2 showing the local coordination environment of Fe

Fig.6 View of one-dimensional infinite chains of coordination polymer 2
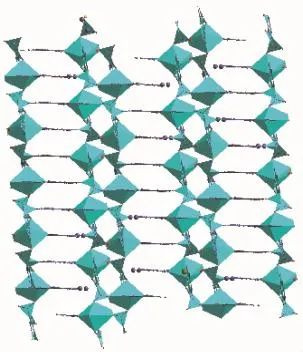
Fig.7 View of 2D layer structure of the coordination polymer 2
2.2Thermal analyses
The stability of 1 and 2 was investigated by thermal gravimetric analysis(Fig.8).The first weight loss of 3.9%(1)and 10.4%(2)are in the ranges of 139.8~347.6℃and 111.8~226.3℃,respectively, corresponding to the removal of water molecules (Calcd.3.7%for 1 and 10.4%for 2).The second weight loss of 31.8%(1)and 26.6%(2)are in the ranges of 347.6~408.6℃and 226.3~424.9℃, respectively,correspondingtotheremovalof carboxylic acid ligand(Calcd.31.3%for 1 and 28.1%for 2).The last weight loss of 49.9%(1)and 48.1%(2)are in the ranges of 408.6~599.9℃and 424.9~1197.9℃,respectively,corresponding to the removal of Medpq ligand(Calcd.50.2%for 1 and 47.6%for 2).After 599.9 and 1197.9℃,no weight lossisobserved,indicatingthecomplete decomposition of 1 and 2.The residual weight of 14.4%(1)and 14.9%(2)(Calcd.14.8%for 1 and 13.9%for 2)correspond to the metal oxide FeO.
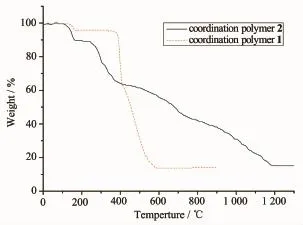
Fig.8TG curves of the coordination polymers
References:
[1]Yang L Y,Wu L Z,Liu L,et al.Dyes Pigm.,2014,101:196-202
[2]Xu J,Su Z,Chen M S,et al.Inorg.Chim.Acta,2009,362: 4002-4008
[3]Sun R,Li Y Z,Bai J F,et al.Cryst.Growth Des.,2007,7: 890-894
[4]Wang Y F,Li S H,Ma L F,et al.Inorg.Chem.Commun., 2015,62:42-46
[5]Wang S,Bai J F,Xing H,et al.Cryst.Growth Des.,2007,7: 747-754
[6]Zhai Q G,Lu C Z,Wu X Y,et al.Cryst.Growth Des., 2007,7:2332-2342
[7]Lan Y Q,Wang X L,Li S L,et al.Chem.Commun.,2007, 46:4863-4865
[8]Zhang X T,Fan L M,Fan W L,et al.Z.Anorg.Allg.Chem., 2015,10:1808-1812
[9]Li D S,Zhang P,Zhao J,et al.Cryst.Growth Des.,2012, 12:1697-1702
[10]Zhao Y,Chang X H,Liu G Z,et al.Cryst.Growth Des., 2015,15:966-974
[11]Ye B H,Tong M L,Chen X M.Coord.Chem.Rev.,2005,5: 545-565
[12]Hao X R,Su Z M,Zhao Y H,et al.Acta Crystallogr.Sect. C.,2005,61:m469-m471
[13]Choi H S,Suh M P,Angew.Chem.Int.Ed.,2009,48:6865 -6869
[14]Margerum D W.J.Am.Chem.Soc.,1957,79:2728-2733
[15]Tao J Q,Gu Y L,Zhou X H,et al.Chin.J.Chem.,2009,27: 1280-1284
[16]Liu C B,Gao L,Wang J,et al.J.Coord.Chem.,2012,65: 4156-4167
[17]Huang Y J,Yan Y S,Pan Y R,et al.J Cluster Sci., 2015,26:925-936
[18]Sheldrick G M.SHELXS 97,Program for the Solution of Crystal Structure,University of Göttingen,Germany,1997.
[19]Sheldrick G M.SHELXL 97,Program for the Refinement of Crystal Structure,University of Göttingen,Germany,1997.
Two Coordination Polymers Based on Carboxylate Ligands and Fe:Preparation, Structural Characterization and Properties
HUANG Yan-Ju*,1DU Gang2HAO Xiang-Rong1ZHANG Tie-Ying1
(1Department of Chemistry,Tonghua Normal University,Tonghua,Jilin 134002,China) (2Tonghuas No.1 Middle School,Tonghua,Jilin 134001,China)
Two ironcoordination polymers[Fe(Medpq)(BDC)H2O]n(1)and{[Fe(Medpq)(QUI)H2O]·2H2O}n(2) (H2BDC=terephthalic acid,H2QUI=2,3-pyridinedicarboxylic acid and Medpq=2-methyldipyrido[3,2-f:2′,3′-h] quinoxaline)were prepared by hydrothermal method.They were characterized by elemental analysis,IR and thermogravimetric analysis,and their structures were determined by single-crystal X-ray diffraction.The Feions of the two coordination polymers all assumed a slightly distorted octahedral geometry.CCDC:1436495,1; 1436494,2.
coordination polymer;hydrothermal processing;carboxylate ligand
O614.81+1
A
1001-4861(2016)02-0313-07
10.11862/CJIC.2016.049
2015-04-16。收修改稿日期:2015-12-28。
吉林省教育厅“十二五”科学技术研究项目(No.2013396)和吉林省科技厅自然科学基金(No.20130102002JC)资助。
*通信联系人。E-mail:huangyanju2007@163.com
