Misconceptions in global reactions and formula writing Stig R.JOHANSSON*
2016-11-28TheSwedishSectionforDetonicsandCombustionSweden
The Swedish Section for Detonics and Combustion,Sweden
Available online 25 July 2016
Misconceptions in global reactions and formula writing Stig R.JOHANSSON*
The Swedish Section for Detonics and Combustion,Sweden
Available online 25 July 2016
The frequently used concept of“global reaction”is discussed and the reason for the confusion behind explained.The misconception is cleared by formula writing based on the donor–acceptor(donac)reaction concept and by applying the Grand Rule of Formula Writing that is based on it. ©2016 Production and hosting by Elsevier B.V.on behalf of China Ordnance Society.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Global reaction;Donac reactions;Formula writing;Pyrotechnics;Chlorate manufacture
1.Introduction
The concept of“global reaction”originates in formula writing and stoichiometry not being kept apart,which results in an erroneous mono-reaction formula and loss of degrees of freedom.The donor–acceptor(donac)method of formula writing[1]prevents the mistake from being made and does away with the misleading concept as such.The species being donated/accepted,Y,can be any molecule,atom or ion(e.g.,the protonforacid–basereactions,theelectronforredox reactions).
According to The Grand Rule of Formula Writing,a donor–acceptor reaction formula shall not contain more than two conjugated donor–acceptor pairs.One starts by specifying the reactants(chosen)in the initial state of the process to be studied as well as the products found(chemical analysis)in the final state.
An area where global reactions often appear is that of energetic materials.The donac method–how it works,what it means–will now be shown with a couple of examples.As illustrated by the chlorate example,the donac formula writing method takes us a little step further,viz.,from the empirical“know how”level to the phenomenological[1]“know why”level.
2.Air bag composition
A“typical air bag gas generator reaction”[2]is said to be
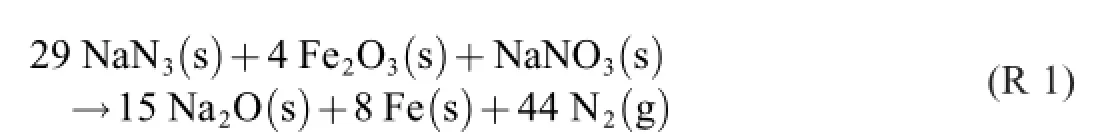
R 1 is a global-reaction formula of the process.The donac formula writing procedure preferably starts by applying“The Principle of States”,i.e.,by specifying the initial state containing the three reactants and the final state containing the three products–and residual reactants,if any–found
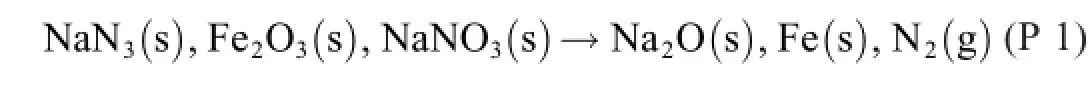
A suitable choice ofY is the oxygen atom,which is donated by Fe2O3and NaNO3,whereby Fe(+3)is reduced to Fe(0)and N(+5)to N(0).

The azide is the acceptor,where N(-1/3)is oxidized to N(0).

Combining these half-reactions so that the auxiliary“bartering item”,the oxygen atom,disappears,R 2+R 4 give the rule-abiding formula
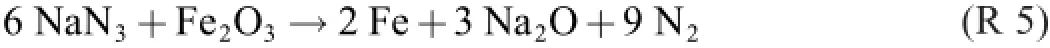
The molar nitrogen:azide ratio of R 5 is 9:6=1.50:1.Likewise,R 3+R 4 give and the ratio 8:5=1.60:1.Thus,the nitrate gives 7%more nitrogen gas per mole sodium azide than the iron oxide does,or 19%more gas per gram of composition.
http://dx.doi.org/10.1016/j.dt.2016.07.001
2214-9147/©2016 Production and hosting by Elsevier B.V.on behalf of China Ordnance Society.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).

The product development task for this life-saving pyrotechnic product must be to get as much gas as possible in shortest possible time.Correct formula writing has now led us to a question that must have a rational answer,viz.,why is the iron oxide there?A possible answer may be that it acts as a catalyst;“in shortest possible time”is also an important characteristic of the composition.If so,one should perhaps start looking for more effective alternatives that in addition to promoting faster gas production also“cost”less yield reduction(a lucky strike would be to find a catalyst increasing the gas production!).
The correct description of the state change–which turned out to be a poly-reaction process–is R 5 and R 6 in parallel. Adding them can be done in any ratio.If this be 1:1,for example,we get(the quotation marks indicate“false”)
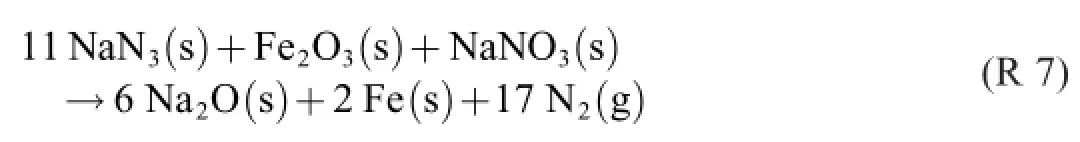
and the molar nitrogen:azide ratio 17:11=1.55:1.The ratio of the Laib–Conkling formula R 1,which presumably is the optimal one,is 44:29=1.52:1.This formula is the addition result of 4·(R 5)+1·(R 6).
With a global formula,a degree of freedom is lost and the“chemistry”becomes obscure–and the questions promoting creativity and thus the development work do not present themselves.
How is the overall stoichiometry of a global“experimental”formula like R 1 and R 7 found if not by empirical x·(R 5)+y·(R 6)addition?The auxiliary merit of a global formula is that the molar NaN3:Fe2O3:NaNO3ratios are perspicuously given by the chosen stoichiometric numbers,e.g.,29:4:1 for R 1 and 11:1:1 for R 7.For finding the optimal ratios in the laboratory,various mixes have to be prepared and tested.The choice of NaN3:Fe2O3:NaNO3ratios may be facilitated by understanding that it in fact is a choice of(R 5):(R 6)ratios(i.e., of x and y).
3.Pyrotechnic nitrate composition
The following“main reaction formula”appeared in a conference paper[3].
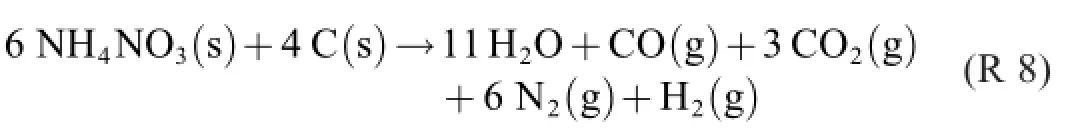
We find that it contains five donac pairs,viz.

where ammonium nitrogen is oxidized from N(-3)to N(0), nitrate nitrogen reduced from N(+5)to N(0),hydrogen reduced from H(+1)to H(0)and carbon oxidized from C(0)to C(+2) and C(+4).The nitrogen atoms in ammonium nitrate are stoichiometrically coupled to the formal valence of N(+1).With Y1=O andY2=H,we get one oxygen donor and three oxygen acceptors
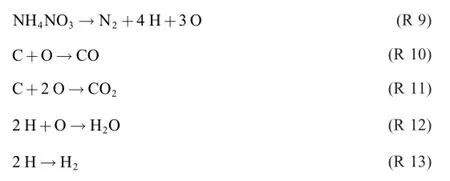
Cancelling the bartering objects by the following operations

R 14 through R 17 can be added to a global reaction in any relation by multiplying them by a,b,c,d,respectively.As far as R 8 is concerned,the stoichiometric relations between them are a+b+c+2d=6(for NH4NO3and N2),a+b+3c+d=4(C), 2a+b+4d=11(H2O),a+3c=1(CO),b+d=3(CO2)and b+2c=1(H2).Despite the fact that the equation system is redundant,solving for the 4 unknowns is not possible due to interrelations.Selecting the fourth,fifth and sixth equations only,we get the relations d=3-b,a=1-3c and b=1-2c. Choosing a=1,we finally get b=1,c=0 and d=2,giving R 8.Thus,R 14 and R 15 account for 25%each of the process, R 17 for 50%.
Even if R 8 happens to reflect the correct product distribution according to a certain set of quantitative analysis data,it should be obvious–and good to know–that the product distribution depends on the prevailing conditions of the study. Degrees of freedom have been lost–obviously without the writer being aware of it.
With correct formula writing,we are faced with an interesting,practical question:What determines the reaction ratios,i.e., the multiplication factors a,b,c and d?
4.Electrochemical chlorate formation[4,5]
The overall reaction formula of the electrochemical process
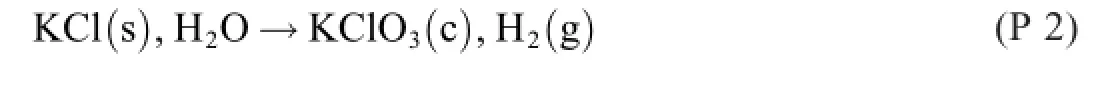

The electrolysis part is(note that Cl2is a solute)
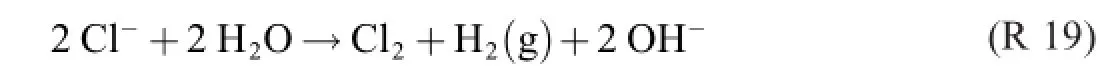
preferably followed in a separate reactor by
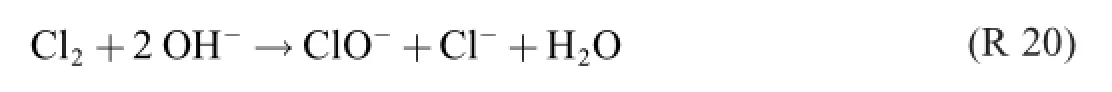
Here the last main-road process,the Balard autoxidation reactions,occurs([5],p.2)
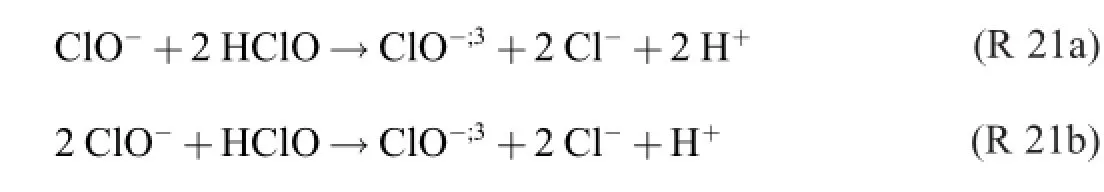
If R 20 were allowed to occur in the electrolyte,the following“one and only”yield-decreasing by-reaction–as maintained in the literature–takes place at the anode.

This formula of electrochemical chlorate formation was given by the German chemists Foerster and Müller in 1902[6]. Forty-three years later and in another country,the Spaniards Rius and Llopis published their“stoichiometry”[7].
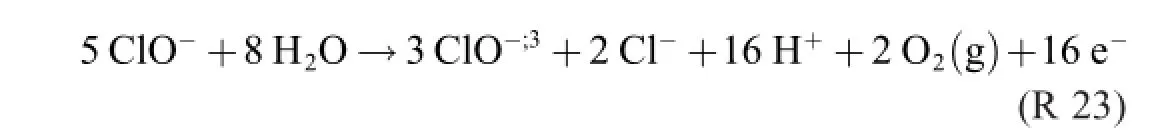
The chlorate yield of R 22 is 66.7%(only 6 O atoms out of 9 give chlorate,3 are lost as O2),the corresponding yield of R 22 is 69.2%(9 O out of 13)[8].
In a textbook on electrochemistry,this disturbing disagreement caused the footnote comment that if the Spaniards’result is confirmed by further experiments,“then the theoretical data for the current yield,as given by Foerster and Müller,must be corrected”.
There is nothing to correct as far as R 22 as such is concerned.
Both R 22 and R 23 contain three donac pairs,which is two too many.As a consequence,three electron reaction formulas are required for describing the process.
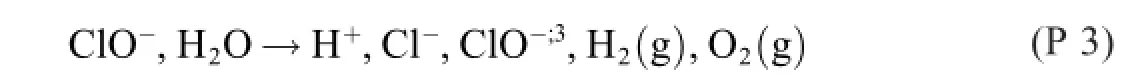
viz.
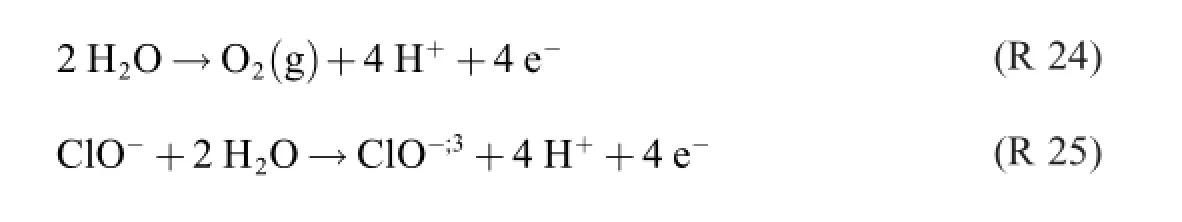
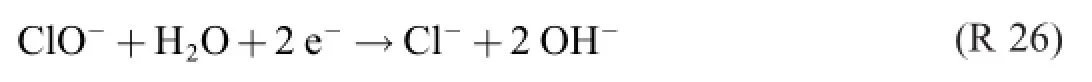
In the reaction formulas from 1902 and 1945 these electron (but not necessarily electrode)reactions have been inconsiderably added.With The Grand Rule in mind,it goes without saying that the product distribution varies with the test conditions,and that it is very unlikely that they should have been the same in Spain 1945 as they were in Germany 1902–or in any other study,for that matter.
A question that now presents itself is:what is the yieldreducing process,then?Noting that R 26 is a cathode reaction, if any,and not a chlorate producing reaction makes R 25 alone look like a possible electrochemical chlorate formation candidate.It is to be noted that the yield of this reaction is 100%! Remains R 24 to account for the reduction;the percentage could–at least in principle–be anything.
Among the yield-reducing processes of R 18 is,of course, Cl2→Cl2(g).
5.Conclusion
Erroneously written and thought-hampering“global reactions”,as now discussed,is just one example of the need to“increase our knowledge”,as the Scottish philosopher W.A. Sinclair puts it.He writes([9]):“To increase our knowledge is to alter for the better our ways of‘selecting’and‘grouping’[10];to notice it in ways which are new to us,and probably strange”.And further:“we move from one to another without noticing it”,which is exactly what led to“global reaction”.
References
[1]Johansson SR.Elementary chemical mathematics(chemistry in a broader setting).AuthorHouse;2016.p.7,9–15,278.
[2]Laib GR,Conkling J.Trends and directions in civilian pyrotechnics technology:an American perspective.Plenary lecture presented at the 16th international pyrotechnics seminar,Jönköping,Sweden,1991.
[3]Yuliang Y,Fengjia Y,Shuzen X.Mechanism and kinetic parameters of the reaction of pyrotechnic mixture of NH4NO3,C and NH4Cl. Proceedings of the international symposium on pyrotechnics and explosives,Beijing,1987,p.155.
[4]WranglénG.Kloratelektrolysensteori(thetheoryofchlorate electrolysis).Teknisk Tidskrift 1967;10:177.
[5]Hammar L,Wranglén G.Cathodic and anodic efficiency losses in chlorate electrolysis.Electrochim Acta 1964;9:1.
[6]Foerster F,Müller E.Ztschr Elektrochem 1902;8:515–665.
[7]Rius A,Llopis J.Anal fis y chim 1945;41:1030.
[8]Angel G.Klorat och perklorat(chlorate and perchlorate).Handbok i kemisk teknologi,vol.2.Stockholm:1948.p.406.
[9]Sinclair WA.An introduction to philosophy.2nd ed.Oxford:Oxford University Press;1945.
[10]Einstein A.Induction and deduction in physics.Berliner Tageblatt;1919.
Peer review under responsibility of China Ordnance Society.
.Tel.:+4636 163734.
E-mail address:stru.johansson@telia.com(S.R.JOHANSSON).
17 May 2016;revised 12 July 2016;accepted 18 July 2016
杂志排行
Defence Technology的其它文章
- Laser cut hole matrices in novel armour plate steel for appliqué battlefield vehicle protection
- Theoretical analysis of the surface temperature regulation of an infrared false target subjected to periodical ambient conditions
- An inexpensive underwater mine countermeasures simulator with real-time 3D after action review
- Metallurgical analysis of a failed maraging steel shear screw used in the band separation system of a satellite launch vehicle
- Selective maintenance problem for series–parallel system under economic dependence
- An overview on importance,synthetic strategies and studies of 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane(HNIW)
