Experimental research on electrochemical machining of titanium alloy Ti60 for a blisk
2016-11-21ChenXuezhenXuZhengyangZhuDongFangZhongdongZhuDi
Chen Xuezhen,Xu Zhengyang,Zhu Dong,Fang Zhongdong,Zhu Di
College of Mechanical and Electrical Engineering,Nanjing University of Aeronautics&Astronautics,Nanjing 210016,China
1.Introduction
Electrochemical machining(ECM)is a nontraditional machining process that is used to machine extremely hard materials that are dif ficult to cut with conventional machining methods.1,2ECM generates no burr and no stress,and provides a long tool life,with a damage-free machined surface,a high material removal rate,and good surface quality.3–5TheECM process was originally developed for manufacturing complex-shaped components in the defense and aerospace industries,and has been extended to many other industries such as manufacturing of automotive and surgical components,forging of dies,and,more recently,miniature manufacturing.6,7ECM of new composite materials has recently been investigated.8–10
In ECM of titanium alloy,defects such as pitting and poor surface roughness often appear.In addition,a passivation film iseasilyproducedduringECM,11–15leadingtoahighdecomposition voltage.Furthermore,the electrolytic products from ECM mostly form an insoluble floc,which easily adheres to the surface of the anode,resulting in differences in dissolution behavior between the substrate and anode surfaces.Therefore,it is dif ficult to obtain good surface quality in ECM of titanium alloy.Clifton et al.investigated the surface characteristics and integrity of γ-TiAl subjected to ECM using perchlorate and chloride electrolytes.Large areas of grain boundaries appeared on the surface,which were generally of high integrity with no evidence of structural defects.16Dhobe Shirish et al.conducted experiments on ECM of commercially pure titanium using a sodium bromide electrolyte(20 g/L)at a tool feed rate of 0.1 mm/min.The roughness of the oxide-layered machined surfacewasintherangeof2.4–2.93 μm,suitableforuseintitanium implants without the need for further surface preparation.17Qu etal.reportedtheuseofwireECMwithaxialelectrolyte flushing to machine titanium alloy(TC1).The machining parameters were optimized by Taguchi experiments using sodium chloride and sodium nitrate electrolytes.18Zaytsev et al.proposed optimal conditions for ECM of Ti–6Al–4V titanium alloy with a microsecond pulsed current,under which there appeared to be neithersurface-layerhydrogennorpitting.19Klockeetal.found slightly more rapid dissolution of the α-phase in a detailed cross section,with a higher magni fication showing a slight waviness–but without any rim zone – of the surface for Ti–6Al–4V.20
It is clear from the above results that different processing parameters are often applied to different titanium alloys.There have been very few studies of ECM of the new titanium alloy Ti60(Ti–5.6Al–4.8Sn–2Zr–1Mo–0.35Si–0.7Nd),which is of particular interest for aerospace applications.Therefore,this study investigates the effects of electrolyte concentration,applied voltage,pulse frequency,duty cycle,electrolyte temperature,and feed rate on surface roughness,and optimizes the process parameters.Finally,blisk blades with good surface finish are fabricated.
2.Experimental procedure
In order to obtain good surface finish,an ECM system and a machining fixture are required.In addition,the appropriate levels and factors of the orthogonal experiment are very important.
2.1.ECM system
The ECM system is very complex.To guarantee the reliability of each test,the stability of the machining process parameters,such as electrolyte temperature,electrolyte flow rate,concentration,and spindle motion accuracy,must be ensured.
Therefore,in this system,the temperature deviation of the clean electrolyte is controlled within ±0.5 °C using a heat exchanger.In ECM,the flow rate of the clean electrolyte is controlled by a programmable logic controller.The large amount of electrolytic products from ECM are stored in a second electrolyte tank.To ensure a clean in flow of electrolyte,the electrolyte in this tank is filtered into the clean electrolyte tank through a frame filter every few minutes.If the volume of the electrolytic products is signi ficant after a few months,the frame filter press will be run,in order to compress the electrolytic products into pieces.The pulsed power supply and the movement of the spindle are controlled by an independent system.The ECM device used in these studies is shown in Fig.1.
2.2.Machining fixture
During ECM,a workpiece moves toward the cathode,which is stationary.The cathode and the connecting rod for the anode are made out of stainless steel.A schematic of the machining fixture is shown in Fig.2.The workpiece is Ti60 with heat treatment of 1050 °C/2 h air cooled followed by 700 °C/2 h air cooled.The workpiece is cylindrical with a diameter of 20 mm.Before the ECM test,to remove the oxide film on the surface of the workpiece,its end surface is polished.The workpiece is shown in Fig.3.
The machined surface roughness is measured using a surface roughness tester with an accuracy of 0.01 μm and a resolution of 0.001 μm(Perthometer Mahr1,Germany).The main components on the surface after ECM are detected using a scanning electron microscope(SEM,Hitachi S3400N,Japan).
2.3.Orthogonal experiment design
In ECM,an oxide film is easily produced on the surface of the titanium alloy.18As the thickness of the oxide film increases,the surface roughness becomes worse.However,the oxide film thickness can be reduced by using a halide electrolyte,which has a high activation ability.The ranking of activation ability for some commonly used ions is:Br->Cl->I->ClO3->NO3->SO42-.At the same time,the equipment corrodes easily with NaBr or KBr electrolyte.Therefore,NaCl is the most suitable electrolyte.In these experiments,a concentration in the range of 4wt%–16wt%is applied for NaCl electrolyte.
Because a high electrode potential is used in ECM of titanium alloy,the process parameters are relatively large.A voltage range of 20–40 V and a temperature range of 23–55 °C are used in the experiments.In addition,the pulsed power supply enables timely removal of the product during the intermittent machining time in ECM.Therefore,a pulsed power supply is necessary,and it must have suf ficient time to remove the floc product.The frequency and duty cycle of the pulsed power supply are 0.2–1.0 kHz and 0.2–0.6,respectively.Feed rates in the range of 0.2–0.6 mm/min are used after trial-and-error determination.
In this study,an appropriate L25(56)orthogonal array is applied.The six machining parameters and five levels are shown in Table 1.Twenty- five experiments are performed.
3.Presentation of results
The experimental parameters and results are shown in Table 2.Additionally,each experimental result is tested twice to increase the reliability of the tests.
The range analysis in Table 3 shows that the preferred scheme isA2B1C2D2E1F4,and a veri fication test is conducted.The speci fic parameters and the experimental results are shown in Table 4.Fig.4 shows the in fluences of machining parameters on surface roughness.The impact of each factor on the surface roughness willbe described in the following subsections.
3.1.Effect of electrolyte concentration on surface roughness

Fig.1 ECM system for titanium alloy.

Fig.2 Machining fixture.
As shown in Fig.4(a),the surface roughness improves(i.e.,decreases)as the concentration increases in the range of 4wt%–13wt%.This is because the higher the electrolyte concentration is,the stronger the activation is.Then,the oxide film will have very little effect on ECM as the concentration increases.The dissolution of the elements in the alloy will be uniform,and the surface roughness is better.However,when the concentration is more than 13wt%,the value of the roughness hardly changes.The activation ability of the electrolyte is good enough when the concentration is more than 13wt%,and the surface roughness does not signi ficantly improve thereafter.As the concentration of the electrolyte increasing further,the conductivity of the electrolyte improves,leading to more electrolytic products and increasing of the machining gap.The relatively larger machining gap results in lower flow velocity.Then,the electrolytic product on the anode surface is difficult to remove,causing unevenly dissolution of the surface.Therefore,the surface roughness will not be further improved when the concentration is over 13wt%.

Fig.3 Experimental workpiece.
3.2.Effect of frequency on surface roughness
As shown in Fig.4(b),when the frequency is less than 0.4 kHz,the surface has a poor roughness.For frequencies above 0.4 kHz,the surface roughness is approximately 1.2 μm.
The main advantage of the pulsed power supply is intermittent corrosion during ECM.In ECM of titanium alloy,the electrolytic product is mostly insoluble hydroxide.If the frequency of the pulsed supply is too low,there is insuf ficient time for removal of the product,which therefore accumulates in the processing area,resulting in poor roughness.When the frequency exceeds 0.4 kHz,the effect of the pulsed ECM is fully embodied.When the power frequency is increased,the power cycle becomes shorter,and the time of a single pulse is shorter,leading to less electrolytic product at each power cycle which is washed away more easily at the pulse interval.Then,the machining process will be more stable and the surface will have a better surface roughness.When the power frequency is more than 0.4 kHz,although the pulse interval for removing of the product is shorter,the quantity of the electrolytic product generated at each power cycle is also smaller.Then the product of electrochemical machining,such as hydroxide precipitate,heat,and gas bubble,can be excluded from the machining gap suf ficiently and the electrolyte conductivity can be kept uniform.Thus,the machining surface roughness is still good.
3.3.Effect of duty cycle on surface roughness
In addition to frequency,the duty cycle is also a main parameter of the pulsed power supply.At a duty cycle of 0.3,the surface has its best surface roughness,as demonstrated in Fig.4(c).Under the condition of a low duty cycle(in the range of 0.3–0.4),the processing time is relatively short in each power supply period,so there is enough time for removal of the electrolytic product.Electrochemical dissolution during processing is much easier,and the surface roughness is improved.However,if the duty cycle is extremely low(0.2),the averagevoltage becomes too low and the machining gap becomes very small while the other processing parameters remain the same.The ECM products will pile up in the machining gap and can hardly be taken away.Therefore,a duty cycle of 0.3 is the best choice.
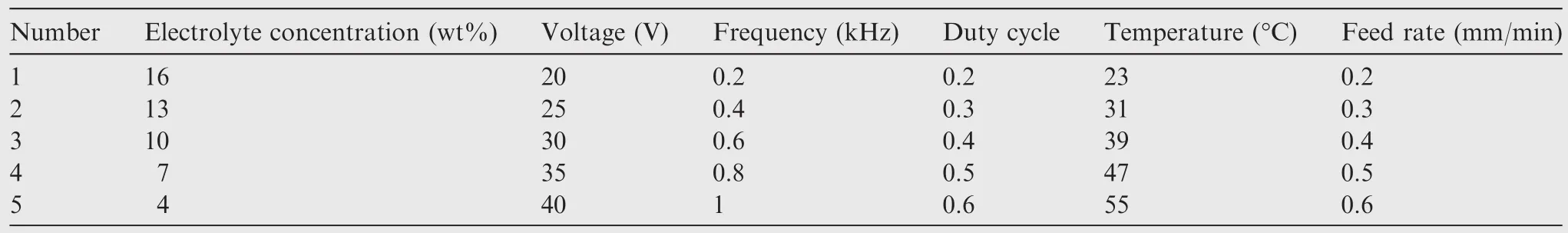
Table 1 Experimental machining parameters.

Table 2 L25(56)orthogonal array layout and test results.
3.4.Effect of temperature on surface roughness
As shown in Fig.4(d),the surface roughness becomes poorer with increasing temperature.In addition,when the electrolyte temperature is higher than 50°C,the quality of the surface deteriorates.
As already mentioned,in ECM of titanium alloy,the electrolytic products are mostly insoluble hydroxides and hydrated oxide,and the higher the temperature is,the stronger the oxidation reaction is.With a sodium chloride electrolyte,the products are mostly Ti(OH)3,Ti(OH)4,Ti3+,and Ti4+.However,when the temperature is too high,some of the trivalent titanium ions(Ti3+)in the anode continue to react to give tetravalent ions(Ti4+),as shown in the following equation:
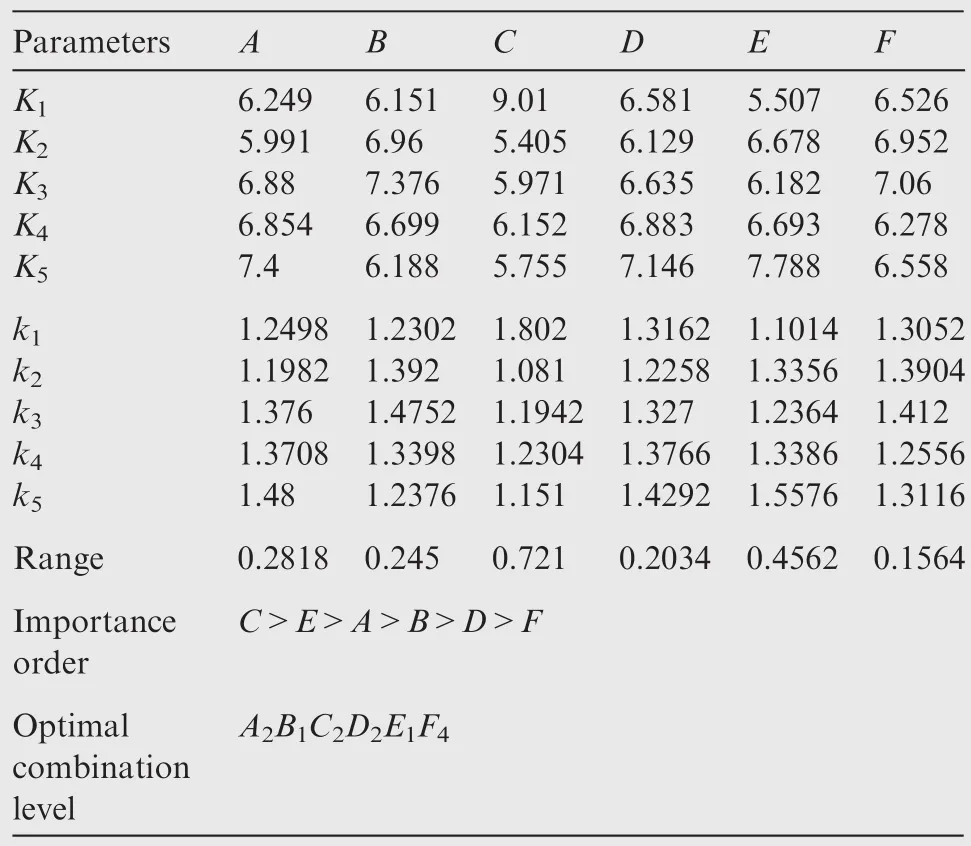
Table 3 Experimental results and analysis.

Table 4 Preferable scheme according to Table 3.

Fig.4 In fluences of machining parameters on surface roughness.

Another part of Ti3+forms insoluble hydroxide,such as in the following equation:

Ti(OH)3is oxidized by oxygen into H2TiO3,as shown in the following equation:
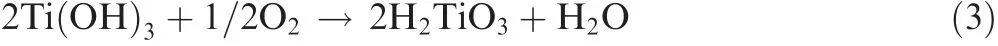
Tetravalent Ti ions(Ti4+)form Ti(OH)4,as shown in the following equation:

Higher temperatures promote reactions(1)–(4).Ti(OH)3,H2TiO3,and Ti(OH)4are all insoluble in water and can easily adhere to the surface.Therefore,the surface roughness deteriorates as the temperature increases in the range of 23–55 °C.Eqs.(1)–(4)can be represented in Fig.5.The components on the surface after ECM are determined using an SEM(Fig.6 and Table 5),which are mainly titanium oxides.The peaks due to Al,Sn,and Zr come from the composition of the substrate,and the one due to Na represents residual sodium chloride solution.
3.5.Effect of voltage on surface roughness
As shown in Fig.4(e),the surface roughness becomes worse with increasing voltage in the range of 20–30 V.However,the surface roughness is better when the voltage rises above 30 V.
As the electrolytic reaction becomes more intense with increasing voltage,a large amount of electrolytic product will be produced.At the same time,the velocity of the electrolyte basically remains unchanged,the amount of the electrolytic products removed by the electrolyte is relatively small,and more products gather in the processing zone.As a result,the electrical conductivity becomes uneven in the machining gap,leading to the production of an oxide film and to uneven surface dissolution.This is the reason for worse roughness with increasing voltage between 20 and 30 V.However,as the voltage increases further,the resulted enhancement of electrolytic activity is suf ficient to overcome the increase in productformation,thereby leading to a decreasein the thickness of the oxide film.In addition,the dissolution of the workpiece surface proceeds more uniformly.Therefore,the surface roughness improves as the voltage increases in the range of 30–40 V.
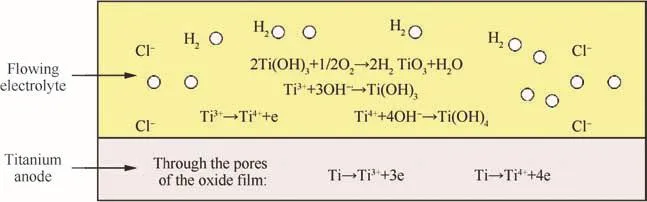
Fig.5 Schematic of the formation of the oxide layer on the anode surface.

Table 5 Components on the surface of the machined Ti60.
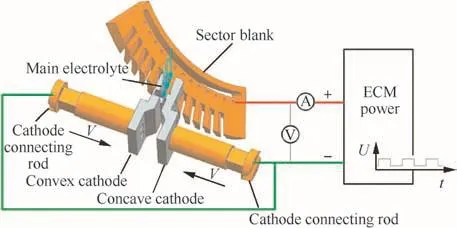
Fig.7 Method for ECM of blades in a sector blank.
3.6.Effect of feed rate on surface roughness
Table 3 and Fig.4(f)demonstrate that the in fluence of the feed rate on the surface roughness is smaller than those of the other parameters.As shown in Fig.4(f),at a feed rate of 0.2–0.6 mm/min,the surfaceroughnessis approximately 1.25–1.41 μm,and the surface has the best surface roughness at a feed rate of 0.5 mm/min.
Because there are multitudinous electrochemical products generated during the process which will in fluence the distribution of electrolyte conductivity,thus the electrolytic products must be excluded timely and effectively.In order to exclude the products easily,the machining gap needs to be generally larger which can be obtained by a lower feed rate.As shown in Fig.4(f),when the feed rate is higher than 0.5 mm/min,the surface roughness is poorer.The main reason is that the machining gap becomes extremely smaller as the feed rate increasing,the product is easily piled up,and the electrical conductivity of the electrolyte in the processing zone is uneven,leading to nonuniform dissolution of the surface elements and poorer surface roughness.When the feed rate becomes lower,as below 0.4 mm/min,the machining gap is too large,resulting in an obvious decrease of current density.Then the ability of the electrolyte activation on the surface is also decreased and the dissolution of the surface is uneven,leading the surface roughness worse.At a feed rate of 0.5 mm/min,the surface has the best surface roughness.The reason is that the electrolyte with an appropriate flow rate takes away the electrolytic products timely.At this time,the machining gap is wide enough,and the current density is also high.
4.Manufacturing experiment
4.1.Experimental method

Fig.6 Workpiece after ECM and its spectrum from a SEM.

Fig.8 System image for blades ECM.

Fig.9 Workblank for the blades ECM of a Ti60 blisk.
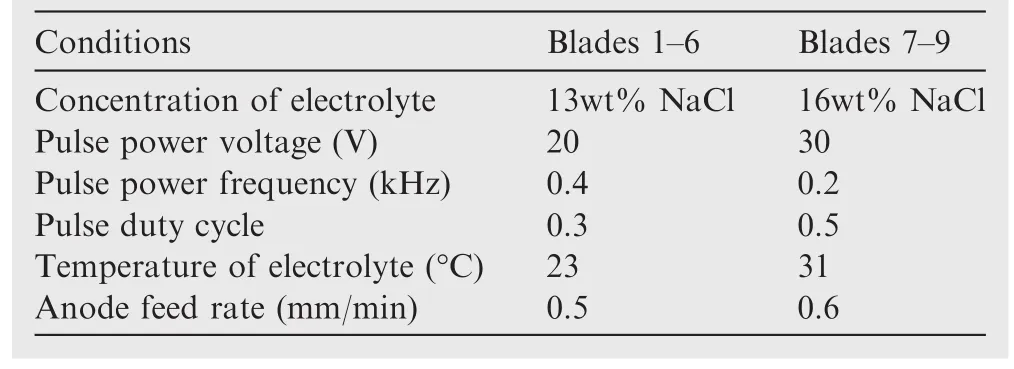
Table 6 Parameters for the blades of a Ti60 blisk.
In ECM,a blisk is fixed to the rotary table at a certain angle.Because of the narrowness of the channels between the blades,both a convex and a concave cathode are used.The sheet cathodes are designed to feed into the channels between the blades and machine the blades through the motions of the cathode connecting rods.The main electrolyte in the processing zone is provided through the method of lateral flow.The processing method and the way of the flow field are shown in Fig.7.Vis the feed speed of the cathodes.
4.2.Machine tool and machined workpiece
Because of the novel nature of this machining procedure,a precision machine tool with motion in six axes had to be specially developed,with an automatic motion control system from Siemens PLC.The machine tool is shown in Fig.8(a).In order to ensure suf ficient electrolyte of the processing area and the right flow field,a reasonable fixture is necessary.This fixture should not only have good strength,but also be not conductive.Therefore,the material of the fixture is special epoxy resin.The fixture for the blades of a Ti60 blisk in ECM is shown in Fig.8(b).Cathodes are made with the material of stainless steel through computer simulation and the principle of ECM,and are shown in Fig.8(c).The workblank is also obtained through ECM,as shown in Fig.9.Meanwhile,the primary allowance of a blade before surface machining is about 3 mm.
ECM of a blade is more complicated than the machining of a simple cylindrical workpiece described in Sections 2 and 3,owing to the complex flow field and the variable machining gap.

Fig.10 Blades in a Ti60 blisk produced using ECM.

Fig.11 Surface roughness of Ti60 blades.

Table 7 Surface roughness of blades.
Many veri fication tests have been conducted with the parameters indicated in Table 6.Blades of a segment of the Ti60 blisk have been successfully fabricated(see Fig.10).The average of the surface roughness in blades 1–6 is 1.019 μm,and the average of the surface roughness in blades 7–9 is 2.014 μm.Fig.11 shows the roughness of the surface with the tester of Perthometer Mahr1.The results indicate that the optimized parameters are reasonable and the process is stable.In addition,the main reason for the poor roughness of blades 7–9 is the low frequency of power supply.As already mentioned,if the frequency is relatively low,the excessive product does not have enough time to be removed,and then gathers in the processing area,resulting in poor roughness.The roughness of blades 1–6 is a little worse than 0.912 μm(see Table 4).One possible reason is the large processing area and the complex flow field,which result in the electrolyte product hardly being removed(see Table 7).
5.Conclusions
(1)Thefrequencyofthepulsedpowersupplyandthetemperature of the electrolyte are the most important factors in fluencing surface roughness during ECM of Ti60 titanium alloy.The advantages of pulsed ECM can be most effectively realized at a relatively high frequency,with a better surface finish being achieved at frequencies above 400 Hz.The roughness becomes worse with increasing temperature.In addition,when the electrolyte temperatureishigherthan50°C,thereisadeteriorationinthesurface quality of the titanium alloy surface.
(2)The optimized parameters for ECM of Ti60 titanium alloy are NaCl concentration 13wt%,voltage 20 V,frequency 0.4 kHz,duty cycle 0.3,temperature 23°C,and feed rate 0.5 mm/min,and the best surface roughness obtained is 0.912 μm.
(3)Blades of a Ti60 alloy blisk have been successfully manufactured with the optimized parameters,with a surface roughness of approximately 1.019 μm.
Acknowledgements
This study was co-supported by the Natural Science Foundation of China(No.51205199),the Program for New Century Excellent Talents in University(NCET-12-0627)of China,the Fundamental Research Funds for the Central Universities(NE2014104)of China,the Funding of Jiangsu Innovation Program for Graduate Education(No.CXLX13_141)of China,and the Fundamental Research Funds for the Central Universities of China.
1.Rao RV,Pawar PJ,Shankar R.Multi-objective optimization of electrochemical machining process parameters using a particle swarm optimization algorithm.JEngManuf2008;222(8):949–58.
2.Burger M,Koll L,Werner EA,Platz A.Electrochemical machining characteristics and resulting surface quality of the nickel-base single-crystalline material LEK94.J Manuf Processes2012;14(1):62–70.
3.Qu NS,Hu Y,Zhu D,Xu ZY.Electrochemical machining of blisk channels with progressive-pressure electrolyte flow.Mater Manuf Processes2014;29(5):572–8.
4.Lu YH,Liu K,Zhao DB.Electrochemical machining analysis on grid cathode composed of square cells.Chin J Mech Eng2013;26(4):668–74.
5.Deconinck D,Van DS,Albu C,Hotoiu L,Deconinck J.Study of the effects of heat removal on the copying accuracy of the electrochemical machining process.Electrochim Acta2011;56(16):5642–9.
6.Rajurkar KP,Zhu D,Mcgeough JA,Kozak J,Silva AD.New developments in electro-chemical machining.CIRP Ann–Manuf Technol1999;48(2):567–79.
7.Ronnie M,Murali MS.Modeling and fabrication of micro tools by pulsed electrochemical machining.J Mater Process Technol2012;212(7):1567–72.
8.Rao SR,Padmanabhan G.Multi-response optimization of electrochemical machining of Al-Si/B4C composites using RSM.Int J Manuf Mater Mech Eng2013;3(3):42–56.
9.Lohrengel MM,Rataj KP,Schubert N.Electrochemical machining of hard metals WC/Co as example.Powder Metall2014;57(1):21–30.
10.Senthilkumar C,Ganesan G,Karthikeyan R.Study of electrochemical machining characteristics of Al/SiCpcomposites.Int J Adv Manuf Technol2009;43(3–4):256–63.
11.Ishizakia H,Ito S.Fabrication of titanium oxide film with high crystallinity by the new electrochemical techniques.Mater Res Soc Symp Proc2012;1394:120–6.
12.Gilbert JL,Buckley CA,Lautenschlager EP.Titanium oxide film fracture and repassivation:the effect of potential,pH and aeration.ASTM Spec Tech Publ1996;1272:199–215.
13.Huanga YZ,Blackwood DJ.Characterisation of titanium oxide film grown in 0.9%NaCl at different sweep rates.Electrochim Acta2005;51(6):1099–107.
14.Liu JH,Wu L,Li SM,Yu M,Yi JL.Preparation and characterization of anodic oxide film on titanium alloy Ti-10V-2Fe-3Al in sodium oxalate electrolyte.Acta Aeronaut et Astronaut Sin2010;31(4):852–6[Chinese].
15.De SK.The in fluence of fluoride on the physicochemical properties of anodic oxide films formed on titanium surfaces.Langmuir2008;24(10):5324–31.
16.Clifton D,Mount AR,Jardine DJ,Roth R.Electrochemical machining of gamma titanium aluminide intermetallics.J Mater Process Technol2001;108(3):338–48.
17.Dhobe Shirish D,DoloiB,Bhattacharyya B.Surface characteristics of ECMed titanium work samples for biomedical applications.Int J Adv Manuf Technol2011;55(1-4):177–88.
18.Qu NS,Fang XL,Li W,Zeng YB,Zhu D.Wire electrochemical machining with axial electrolyte flushing for titanium alloy.Chin J Aeronaut2013;26(1):224–9.
19.Zaytsev AN,Idrisov TR,Zhitnikov VP,Gimaev NZ.About some problems of the surface accuracy and quality increase of Ti-6Al-4V alloys at pulse ECM.Proceedings of the ASME Int Mech Eng Congress and Exposition;Anaheim,California:Springer;2004.p.181–7.
20.Klocke F,Klink A,Veselovac D,Aspinwall DK,Soo SL,Schmidt M,et al.Turbo machinery component manufacture by application of electrochemical.Electro-physical and photonic processes.CIRP Ann–Manuf Technol2014;63:703–26.
杂志排行
CHINESE JOURNAL OF AERONAUTICS的其它文章
- Plastic wrinkling prediction in thin-walled part forming process:A review
- Progress of continuously rotating detonation engines
- Microstructure control techniques in primary hot working of titanium alloy bars:A review
- A hybrid original approach for prediction of the aerodynamic coefficients of an ATR-42 scaled wing model
- Dynamic modeling and analysis of vortex filament motion using a novel curve- fitting method
- Boundary-layer transition prediction using a simpli fied correlation-based model
