Na2Ti3O7纳米片原位制备与钠离子电池负极材料应用
2016-11-18陈程成张宁刘永畅王一菁陈
陈程成张 宁刘永畅王一菁陈 军,2,*
(1南开大学化学学院,先进能源材料教育部重点实验室,天津 300071;2南开大学,天津化学化工协同创新中心,天津 300071)
Na2Ti3O7纳米片原位制备与钠离子电池负极材料应用
陈程成1张 宁1刘永畅1王一菁1陈 军1,2,*
(1南开大学化学学院,先进能源材料教育部重点实验室,天津 300071;2南开大学,天津化学化工协同创新中心,天津 300071)
报道了Na2Ti3O7纳米片的原位生长和钠离子电池负极材料的应用。通过简单的腐蚀市售的钛片制备出相互连接的微纳结构的Na2Ti3O7纳米片。此外,腐蚀后的钛片在不用添加导电剂或粘结剂的情况下,可以直接作为电极材料使用。这种电极材料表现出优越的电化学性能,在50 mA·g–1的电流密度下具有175 mAh·g–1的可逆容量,在2000 mA·g–1的电流密度下循环3000周后,其容量仍保持120 mAh·g–1,容量保持率为96.5%。Na2Ti3O7纳米片电极的优越电化学性能归因于二维结构具有较短的离子/电子扩散路径以及无粘结剂结构能有效的增加电极的电子传导能力。结果表明,这种微纳结构能够有效地克服Na2Ti3O7作为电极材料离子/电子导电性差的缺点。因此,这种无粘结剂结构的Na2Ti3O7纳米片负极材料是一种很有潜力的钠离子负极材料。
钛酸钠;纳米片;无粘结剂;负极材料;钠离子电池
1 Introduction
Rechargeable sodium ion batteries (SIBs) have attracted interest owing to the broad distribution, abundance, and low cost of sodium resources1–3. Unfortunately, the anode materials such as carbon species, alloys, and conversion-type transition metal oxides are facing the problems of inactivity, poor cycling life, and low rate performance mainly due to the sluggish reaction caused by larger size than those of Li+and heavy molecular mass of Na ion4–6. Therefore, it remains a great challenge to exploit the anode with low cost, long cycling life, and high power density for SIBs7–9.
Ti-base oxides have been considered as potential electrode materials for their small structural expansion and applicable operating voltage10. As a typical insertion Ti-base oxides, Na2Ti3O7(NTO) has been regarded as a promising anode material owing to its low cost, good cycling stability, and proper voltage plateau centered at 0.3 V (vs Na/Na+)11. Nevertheless, the poor ion conductivity and electron conductivity of NTO seriously impact its rate performance12,13. To solve the problems, typical methods including carbon coating and morphological control have been utilized. Na2Ti3O7/C composites were obtained by rheological phase method with capacity of 111.8 mAh·g–1at 177 mA·g–1after 100 cycles14. In addition, the micro-spheric NTO consisting of nanotubes was synthesized by hydrothermal method, displaying a capacity of 90 mAh·g–1at high current density of 1770 mA·g–1with 81% capacity retention after 100 cycles15. On the basis of such work, how to further improve rate capability of NTO electrode to satisfy the demand of high-power devices is still an urgent task16–18.
Recently, two-dimension (2D) nanosheets with short ion transport length, large contact surface areas, and stable structure have attrated interest in enhancing the ion conductivity19–21. Specifically, Wan′s group19has successfully fabricated 2D Li4Ti5O12nanosheets for lithium ion batteries (LIBs) with excellent rate performance. However, report on NTO nanosheets as anode of SIBs is limited22. Therefore, it is worth to prepare NTO nanosheets with facile method and design the tailored structure for overcoming poor ion/electron conductivity.
Here, we report on a facile preparation of NTO nanosheets and their application as high-performance anode for SIBs. NTO nanosheets were prepared by in-situ engraving the titanium foils. Meantime, synthesis conditions were optimized to obtain nanosheets with proper size and crystallinity. Furthermore, NTO nanosheets with interconnected micro-nano architecture can directly grow on the current collector. The unique structure not only shortens the ion pathway, but also ensures rapid electron transfer. The electrode of NTO nanosheets shows a stable reversible capacity of 175 mAh·g–1at 50 mA·g–1and 107 mAh·g–1at 4000 mA·g–1. Even at a high current density of 2000 mA·g–1, the reversible capacity still reaches 120 mAh·g–1after 3000 cycles with a capacity retention of 96.5%. This is the highest value reported for NTO. Meantime, the flexible full sodium-ion battery has been successfully assembled using the binder-free NTO as anode, displaying good electrochemical performance.
2 Experimental
2.1 Material synthesis
The 30 μm thick Ti foils (99.9% purity) with excellent flexibility and toughness were chosen as Ti base. In a typical preparation, the whole Ti foil was punched into several wafers with 1 cm in diameter. Then, the Ti wafers were ultrasonically cleaned in acetone, deionized water, and ethanol for few minutes, respectively. Five Ti wafers were taken into 50 mL Teflon-lined stainless autoclave, which was filled with 10 mL 1mol·L–1sodium hydroxide solution (99.8% purity). Then, the Teflon-lined stainless autoclaves were sealed and placed in an oven at 180 °C for 24 h. After cooling down to room temperature, the corroded Ti wafers were collected and washed with deionized water and ethanol, then dried at 80 °C for 12 h. The precursor Ti wafers were calcined in air at 400, 600, and 800 °C for 5 h to obtain the Na2Ti3O7electrodes (denoted as NTO-400, NTO-600, and NTO-800). In the process of optimization, different concentrations of sodium hydroxide solution with 0, 0.1, 0.5, 1, 2 mol·L–1were chosen to react with Ti wafers (detail information in Fig.S1–Fig.S4, Supporting Information).
2.2 Material characterization
The crystal structure of samples were determined by powder X-ray diffraction (XRD, Rigaku D/Max-2500, Cu-Kαradiation). Raman spectra were recorded using a confocal Raman microscope (DXR, Thermo-Fisher Scientific). The contents of Ti and Na in the solution were measured by using an ICP-9000 (N + M) USA Thermo Jarrell-Ash Corp instrument. The morphology and structure of Na2Ti3O7were characterized using scanning electron microscope (SEM, JEOL JSM-6700F Field Emission, operating at 5 kV) and high-resolution transmission electron microscope (HRTEM, JEOL JEM-2010 FEF TEM, operating at 200 kV). The electronic states of samples were investigated by X-ray photoelectron spectroscopy (XPS, PHI 5000Versa Probe).
2.3 Electrochemical measurement
The electrochemical performance was measured using a two electrode coin-type cell (CR2032) and assembled in an argonfilled glove box. One side of the as-prepared Na2Ti3O7wafers was scraped off by blade to expose the Ti bases as current collector. Then, the wafers were directly used as anode for sodium ion battery without further operation. The pure sodium foils were used as the counter electrode. The glass fibers were used as separators. The electrolyte was 1 mol·L–1NaPF6in propylene carbonate (PC) solution with 0.7 mmol fluoroethylene carbonate (FEC). Mass of active material was calculated from the increase amount of Ti foils. The loading density of anode is about 0.74 mg·cm–2. The charge-discharge cycle tests and galvanostatic intermittent titration technique (GITT) were run under different current densities between cut off voltages of 0.01–2.5 V (vs Na/Na+) on a CT2001A cell test instrument(LAND Electronic Co.) at room temperature. Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS)were measured on a CHI660E electrochemical work station.
3 Results and discussion
3.1 Structure and morphology analysis
Fig.1(a) shows XRD pattern of NTO on Ti substrate. The main peaks of NTO-400 and NTO-600 are in good agreement with the characteristic peaks of NTO (JCPDS No. 31-1329)14,15. However, NTO-800 exists TiO2phase, which may be due to the oxidation of part Ti substrate at such a high temperature. Therefore, 400 and 600 °C should be the appropriate temperature to obtain pure NTO. Raman spectra of NTO-400 and NTO-600were also used to prove their further structure (Fig.S5a). The Raman bands at about 280, 450, 640, 902 cm–1should be assigned to the feature of NTO nanostructure23. Bands at 280 and 450 cm–1are attributed to the Na―O―Ti stretching. The peaks close to 140 and 902 cm–1represent symmetric stretch of short Ti―O bonds involving non-bridging oxygen coordinated with sodium ions. The broad peaks located at 640 and 703 cm–1belong to Ti―O―Ti stretching in edge shared TiO612. Meanwhile, SEM with energy dispersive X-ray spectrum (EDX) (Fig.S5b)exhibits that three elements of Na, Ti, and O are uniformly distributed on the nanosheets. The mole ratio of Na : Ti : O (14.6 : 31.9 : 53.5) is in good agreement with Na2Ti3O7. The crystal structure of Na2Ti3O7along the b-axis is illustrated in Fig.1b. The layered structure is composed of three abreast TiO6octahedra, which build up the extended zigzag (Ti3O7)2–layers. Within the layers, there are enough spaces for sodium ions insertion/extraction during electrochemical process. Fig.1(c, d) shows the SEM images of NTO-600. The morphology of NTO-600 is homogeneous nanosheets. Nanosheets have extremely smooth surface with about 35 nm in thickness. The NTO-400 shows the similar sheet-like morphology(Fig.S6(a, b). Compared the cross-section SEM of NTO-600(Fig.1(e) with NTO-400 (Fig.S6c), the active material layer of NTO-600 is more thick and porous than that of NTO-400. Moreover, NTO nanosheets firmly grow on the Ti substrate, which can provide better electronic conductivity and superior stability than that of traditional electrodes24,25. Fig.1(f) shows the TEM image of the NTO-600. It can be clearly seen that the nanosheets interconnected with each other build up a firm micron cluster. Fig.1(g) shows the TEM image of a single nanosheet with 100 nm in width. The corresponding energy dispersive spectrometer (EDS) mapping demonstrates the uniform distribution of Na, Ti, and O elements. Fig.1(h) displays the HRTEM image of NTO-600, exhibiting obvious lattice fringe with high crystallinity26,27. The lattice fringes of 0.84 nm matches well with the (001) faces. However, it is hard to find clear lattice fringe in NTO-400 (Fig.S7(a, b)). This suggests that proper heating temperature produces high crystallinity of NTO.
3.2 Electrochemical performance

Fig.1 Structural and morphological analyses of NTO nanosheets
Fig.2(a) shows the CV of NTO-600 in the potential range of 0.1–2.5 V (vs Na/Na+) at a scan rate of 0.2 mV·s–1. In the first cathodic scan, a weak peak around 1.1 V could be attributed to contribution from pseudo-capacitive process. The large peak ranged from 0.7 to 0.13 V corresponds to the insertion of Na+and the formation of solid electrolyte interface (SEI) layer. In this process, the Na+ions insert into Na2Ti3O7forming theNa2+xTi3O7, meantime, part Ti4+ions are reduced to Ti3+ions to keep charge balance (detailed valence variation in Fig.S8). During the initial anodic process, the prominent peak at about 0.5 V is related to the extraction of Na+and oxidation process of Ti3+to Ti4+. In particular, a broad peak located at around 1.5 V, vanishing in following cycles, is attributed to the structure change to accommodate the electrochemical reaction, which corresponds to incomplete oxidation of Ti3+after charging to 2.0 V. Furthermore, the curves of the second and the third cycles are almost coincident, implying the excellent reversibility. Fig.2(b)shows the corresponding charge-discharge curves. The initial discharge capacity and charge capacity are 296 and 145 mAh·g–1, respectively, with the coulombic efficiency of 49%. The large capacity loss in the following discharge stages is mainly due to the irreversible formation of SEI layer, which is consistent with the CV analysis. Subsequently, the coulombic efficiency rapidly increases to above 90% and the reversible capacity becomes stable around 188 mAh·g–1. Notably, the average Na+insertion potential is relatively low, which is suitable for the high energy density anode material.

Fig.2 (a) Cyclic voltammograms of the initial three cycles for NTO-600 range from 0.1 to 2.5 V at a rate of 0.2 mV·s-1; (b) charge-discharge curves of the initial three cycles; (c) cycling performance of the binder-free NTO and traditional NTO electrodes (using the CMC and PVdF as binder); (d) rate performance of the binder-free NTO electrodes; (e) a long-term cycling performance of NTO-600 electrode started from the fourth cycle at 2000 mA·g-1(after the activation of three cycles at 50 mA·g-1)
Fig.2(c) displays the cycling performance in the voltage range of 0.1–2.5 V (vs Na/Na+) at current density of 50 mA·g–1. For comparison, the electrodes of bulk NTO (NTO-B) were prepared using sodium carboxy-methyl cellulose (CMC) and polyvinylidene fluoride (PVdF) as binder, respectively. Clearly, the discharge capacities of binder-free NTO nanosheets electrodes are generally higher than that of traditional bulk NTO electrodes. This may be due to the insufficient utilization of bulk NTO during the electrochemical process. Moreover, the discharge capacities of binder-free NTO electrodes keep stable after the activation of a few cycles. However, the traditional electrodes have serious capacity fade, especially in the initial 20 cycles. This could be ascribed to the poor electronic conductivity and easy exfoliation of traditional bulk NTO electrodes. More importantly, the NTO-600 electrode exhibits preferable electrochemical performance with the discharge capacity of 175 mAh·g–1, nearly its theoretical capacity (177 mAh·g–1). The capacity retention is 94.7% after 100 cycles implying the excellent cyclic property. This is because the binder-free nanosheets in-situ generate on the Ti foil with tight adhesion and superior electronic conductivity. In addition, comparing the coulombic efficiency of two binder-free electrodes (Fig.S9), the coulombic efficiency of NTO-600 is above 90% after activated for 5 cycles. But the coulombic efficiency of NTO-400 is always below 90% and quite unstable even after 40 cycles. It indicates that NTO-600 are more stable than NTO-400 during the Na+insertion/extraction process. It could be attributed to the more porous architecture and fine crystallinity of NTO-600. Fig.2(d)shows the rate performance of binder-free electrodes at different current densities from 50 to 4000 mA·g–1. It can be observed that NTO-600 electrodes have the best rate performance. The discharge capacities of NTO-600 electrode is 186 (50 mA·g–1), 166 (100 mA·g–1), 141 (500 mA·g–1), 120 (1000 mA·g–1), 114 (2000 mA·g–1), and 107 mAh·g–1(4000 mA·g–1)shown in Fig.S10, respectively. More importantly, when the current density returns to 50 mA·g–1, the capacities revert to 175 mAh·g–1with 94% capacity retention implying the outstanding adaptability. The negligible potential polarization and high stability of NTO-600 at such a high current density demonstrate the excellent reaction kinetics.
Fig.2(e) shows the long-term cycling performance of NTO-600 at a high current density of 2000 mA·g–1. Under such rapid sodium ion insertion/extraction conditions, NTO-600 electrode still keeps considerable average capacities of 120 mAh·g–1with capacity retention of 96.5% even after 3000 cycles. Meanwhile, the coulombic efficiency is close to 100% all the time, indicating the excellent reversibility. Furthermore, the corresponding charge-discharge curves (Fig.S11) show that the charge/discharge time consumption is only 250 s at 2000 mA·g–1, which meets the demands of efficient and rapid charging/discharging for high performance SIBs.28As expected, compared with other various Na2Ti3O7anode materials reported recently (Fig.S12), the binder-free NTO nanosheet electrode exhibits the best electrochemical capability, especially in high rate performance. To satisfy the commercial demands for wide temperature range, the charge-discharge curves operated at –15, 25, 50, and 80 °C are exhibited in Fig.S13. With the increase of temperature, the discharge capacities have great improvements. This is due to the increase of the activity of Na+at high temperature25. More inspiringly, the discharge capacity remains 138 mAh·g–1at –15 °C with the coulombic efficiency of 92.3%, implying the favorable temperature adaptability29.
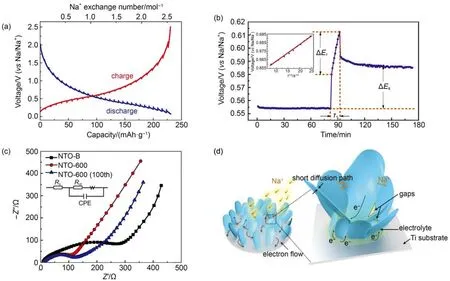
Fig.3 (a) GITT profiles of NTO-600 electrode at a current density of 20 mA·g-1; (b) a selected pulse of GITT profile during the stage of charge process (insert image: the linear relationship between Eτwith τ1/2); (c) Nyquist plots of the intial state of NTO-B, NTO-600 and the charged-state(100th) of NTO-600 (inset: the corresponding the equivalent circuit); (d) schematic diagram of electron/ion transport in the tailored binder-free 2D nanosheets architecture
3.3 Flexible SIBs
In order to further study the relationship between structure and electrochemical property, the GITT and EIS have been employed to analyze ion/electron conductivity of binder-free nanosheet NTO electrode. Fig.3(a) displays the GITT mode corresponding to kinetic characteristic of the insertion of Na+into Na2Ti3O7. The quasi-equilibrium redox potential curve of the NTO-600 is very similar to the above charge-discharge curve (Fig.2(b)). It demonstrates that the NTO-600 has good ionic conductivity30. Moreover, sodium ion diffusion coefficient (DNa+) of NTO can be calculated from the GITT curves18,31. Fig.3(b) shows the selected pulse at stable charging process(0.55–0.62 V). For the linear relationship between Eτ(cell voltage) and τ1/2(radication of the time period of the current pulse) shown in the insert image, the DNa+ can be obtained from the equation below (related physical quantities in Supporting Information: GITT discussion)

The value of sodium chemical diffusion coefficient during the extraction Na+process is estimated to be approximately 4.37 × 10–11cm2·s–1. The result is considerably higher than that of reported NTO and other electrode materials for sodium ion battery (Table S3). It firmly evidences that the tailored 2D nanostructure greatly increases the ion diffusion during electrochemical process.
Subsequently, EIS plots are performed to corroborate the electronic conductivity of binder-free NTO nanosheets electrode. Fig.3(c) shows that the charge-transfer resistance (Rct, the value of which corresponds to the diameter of the semicirclediameter) of binder-free NTO-600 electrode (132 Ω, fitted by Zview) is much smaller than that of traditional NTO-B electrode (354 Ω). This result should be attributed to good electronic conductivity of binder-free structure. Moreover, Rctof NTO-600 remains 134 Ω after 100 cycles, which is very close to the initial state implying the excellent electronic conductivity during the electrochemical process.

Fig.4 (a) Schematic illustration of flexible full SIB; (b) a green LED lighted up by the flexible sodium ion full battery under the bended status;(c) the charge-discharge profiles of flexible full SIB at 50 mA·g-1
To clarify the reason for the excellent rate performance and super-long life, the unique architecture and morphology should also be paid more attention. Fig.S14 shows the SEM images of NTO-600 after 100 and 500 cycles. The nanosheets remain in the original morphology after 100 cycles. Even after 500 cycles, the porous architecture still can be observed. Furthermore, the structure and morphology of NTO-600 electrodes after 3000 cycles have been measured in Fig.S15. The results show that pulverization and agglomeration occur on the electrode surface, leading to the slight capacity loss. However, the NTO-600 maintains the Na2Ti3O7phase with well crystallinity.
According to above analysis, the reasonable schematic of electron/ion transport has been exhibited in Fig.3(d). Firstly, the unique 2D nanosheets possess significantly short diffusion paths of Na+and large contact surface areas. The Na+can freely diffuse between the adjacent sheets. Furthermore, the gaps and holes among the nanosheets contribute to the diffusion of electrolyte into the inner area of active materials. Second, the insitu generated nanosheets offer good electronic contact between the active materials and the Ti current collector. For the electrochemical reaction starting from the bottom to the whole electrode, it immensely accelerates the electron flowing between the Ti substrate and NTO nanosheets, which avoids the destruction of the morphological structure24. On the other hand, without the inactive and insulated polymeric binder, Na+flux could continuously and efficiently pass the surface of NTO into the lattice structure32. Thus, both the ion diffusion capability and the electron conductivity of NTO have been considerably improved by the ingenious structural design.
We further assembled a flexible SIB to show its application for such bendable anode in flexible devices. Fig.4(a) shows the structural schematic of the flexible full SIB. In this cell, the anode is made of 2 cm × 5 cm NTO nanosheet electrode, the cathode is NaVPO4F33loaded on Al foil, and the separator is flexible glass fiber. The mass ratio of anode and cathode is carefully calculated as 1 : 1.77 described in Fig.S16. The real flexible full SIB is shown in Fig.4(b), which is very thin and light. It can easily light up a green LED. More importantly, no matter the battery is flat or bended, the light is always very bright, and the working voltage only has negligible change (Fig.S17), which declares that the bending almost has no bad effect on ions/electrons transport. This should be attributed to the good flexibility and resilience of the binder-free NTO electrode. Thus, the anode can be freely bended, then recover (insert image of Fig.4(b). Furthermore, Fig.4(c) shows the charge-discharge profiles of flexible full SIB at 50 mA·g–1within the voltage window of 1.5–4.0 V. The cell displays good electrochemical performance with stable reversible capacity of 82 mAh·g–1for 30 cycles (Fig.S18). In particular, the in-situ NTO nanosheet anode as a novel flexible electrode makes it possible to apply to various special devices, such as bendable portable energy storage equipments34.
4 Conclusions
In summary, Na2Ti3O7electrode with the interconnected nanosheets delicately generated on the Ti current collector is prepared by facile etch reaction. The unique structure of Na2Ti3O7nanosheets offers the short ion/electron diffusion pathway, large contact surface areas, and stable porous architecture, which greatly improved the ion/electron conductivity of Na2Ti3O7. When evaluated as anode for SIBs, NTO-600 electrode delivers a reversible capacity of 175 mAh·g–1at 50 mA·g–1with 94.7% retention after 100 cycles. Moreover, the capacity of 107 mAh·g–1even at 4000 mA·g–1and 120 mAh·g–1over 3000 cycles at 2000 mA·g–1with high capacityretention of 96.5% are obtained, showing long-term cycling life and high rate capability. The easily prepared binder-free Na2Ti3O7nanosheets provide a new insight into designing the advanced anode materials for SIBs.
Supporting Information: available free of charge via the internet at http://www.whxb.pku.edu.cn.
(1)Li, W. J.; Chou, S. L.; Wang, J. Z.; Liu, H. K.; Dou, S. X. Nano Lett. 2013, 13, 5480. doi: 10.1021/nl403053v
(2)Duan, W. C.; Zhu, Z. Q.; Li, H.; Hu, Z.; Zhang, K.; Cheng, F. Y.;Chen, J. J. Mater. Chem. A 2014, 2, 8668.
(3)Zheng, J. Y.; Wang, R.; Li, H. Acta Phys. -Chim. Sin. 2014, 30, 1855. [郑杰允, 汪 锐, 李 泓. 物理化学学报, 2014, 30, 1855.] doi: 10.3866/PKU.WHXB201407151
(4)Tang, Y.; Zhang, Y.; Deng, J.; Wei, J.; Tam, H. L.; Chandran, B. K.; Dong, Z.; Chen, Z.; Chen, X. D. Adv. Mater. 2014, 26, 6111. doi: 10.1002/adma.201402000
(5)Huang, Z. L.; Wang, L. P.; Mou, C. X.; Li, J. Z. Acta Phys. -Chim. Sin. 2014, 30 (10), 1787. [黄宗令, 王丽平, 牟成旭,李晶泽. 物理化学学报, 2014, 30 (10), 1787.] doi: 10.3866/PKU.WHXB201408052
(6)Mao, J. F.; Luo, C.; Gao, T.; Fan, X. L.; Wang, C. S. J. Mater. Chem. A 2015, 3, 10378. doi: 10.1039/C5TA01007A
(7)Xu, J.; Yang, D. Z.; Liao, X. Z.; He, Y. S.; Ma, Z. F. Acta Phys. -Chim. Sin. 2015, 31 (5), 913. [许 静, 杨德志, 廖小珍,何雨石, 马紫峰. 物理化学学报, 2015, 31 (5), 913.] doi: 10.3866/PKU.WHXB201503162
(8)Hu, Z.; Wang, L.; Zhang, K.; Wang, J.; Cheng, F.; Tao, Z.; Chen, J. Angew. Chem. Int. Edit. 2014, 53, 12794. doi: 10.1002/anie.201407898
(9)Li, H.; Wu, C.; Wu, F.; Bai, Y. Acta Chim. Sin. 2014, 72, 21.[李 慧, 吴 川, 吴 峰, 白 莹. 化学学报, 2014, 72, 21.]doi: 10.6023/A13080830
(10)Zhu, G. N.; Wang, Y. G.; Xia, Y. Y. Energy Environ. Sci. 2012,5, 6652. doi: 10.1039/c2ee03410g
(11)Senguttuvan, P.; Rousse, G.; Seznec, V.; Tarascon, J. M.;Palacín, M. R. Chem. Mater. 2011, 23, 4109.
(12)Zhang, Y.; Guo, L.; Yang, S. Chem. Commun. 2014, 50, 14029. doi: 10.1039/C4CC06451H
(13)Pan, H.; Lu, X.; Yu, X.; Hu, Y. S.; Li, H.; Yang, X. Q.; Chen, L. Q. Adv. Energy Mater. 2013, 3, 1186. doi: 10.1002/aenm.v3.9
(14)Yan, Z.; Liu, L.; Shu, H.; Yang, X.; Wang, H.; Tan, J.; Zhou, Q.;Huang, Z.; Wang, X. J. Power Sources 2015, 274, 8. doi: 10.1016/j.jpowsour.2014.10.045
(15)Wang, W.; Yu, C.; Lin, Z.; Hou, J.; Zhu, H.; Jiao, S. Nanoscale 2013, 5, 594. doi: 10.1039/C2NR32661B
(16)Zhang, C. L.; Jiang, W. J.; Zhang, J.; Qi, L. Acta Phys. -Chim. Sin. 2007, 23 (Supp), 31. [张春玲, 江卫军, 张 晶, 其 鲁.物理化学学报, 2007, 23 (Supp), 31.] doi: 10.3866/PKU.WHXB2007Supp08
(17)Cao, L. Y.; Diao, P.; Liu, Z. F. Acta Phys. -Chim. Sin. 2002, 18(12), 1062. [曹林有, 刁 鹏, 刘忠范. 物理化学学报, 2002, 18(12), 1062.] doi: 10.3866/PKU.WHXB20021202
(18)Zhang, K.; Han, X. P.; Hu, Z.; Zhang, X. L.; Tao, Z. L.; Chen, J. Chem. Soc. Rev. 2015, 44, 699. doi: 10.1039/C4CS00218K
(19)Wang, Y. Q.; Gu, L.; Guo, Y. G.; Li, H.; He, X. Q.; Tsukimoto, S.; Ikuhara, Y.; Wan, L. J. J. Am. Chem. Soc. 2012, 134, 7874. doi: 10.1021/ja301266w
(20)Guo, Y. J.; Chen, H.; Qi, L. Acta Phys. -Chim. Sin. 2007, 23(Supp), 89. [郭营军, 晨 辉, 其 鲁. 物理化学学报, 2007, 23(Supp), 89.] doi: 10.3866/PKU.WHXB2007Supp17
(21)Wang, S.; Wang, L.; Zhang, K.; Zhu, Z.; Tao, Z.; Chen, J. Nano Letters 2013, 13, 4404. doi: 10.1021/nl402239p
(22)Ye, F.; Wang, L.; Lian, F.; He, X. M.; Tian, G. Y.; Ouyang, M. G. Chem. Ind. Eng. Prog. 2013, 32, 1789. [叶 飞, 王 莉, 连芳, 何向明, 田光宇, 欧阳明高. 化工进展, 2013, 32, 1789.]
(23)Liu, H.; Yang, D.; Waclawik, E. R.; Ke, X.; Zheng, Z.; Zhu, H.;Frost, R. L. J. Raman Spectrosc. 2010, 41, 1792.
(24)Yuan, S.; Huang, X. L.; Ma, D. L.; Wang, H. G.; Meng, F. Z.;Zhang, X. B. Adv. Mater. 2014, 26, 2273.
(25)Liu, J.; Song, K.; Aken, P. A. V.; Maier, J.; Yu, Y. Nano Lett. 2014, 14, 2597. doi: 10.1021/nl5004174
(26)Hu, Z.; Zhu, Z.; Cheng, F.; Zhang, K.; Wang, J.; Chen, C.; Chen, J. Energy Environ. Sci. 2015, 8, 1309.
(27)Zhang, K.; Hu, Z.; Tao, Z.; Chen, J. Sci. China Mater. 2014, 57, 42. doi: 10.1007/s40843-014-0006-0
(28)Gao, P.; Jia, H.; Yang, J.; Nuli, Y.; Wang, J.; Chen, J. Phys. Chem. Chem. Phys. 2011, 13, 20108. doi: 10.1039/c1cp23062j
(29)Chen, C. C.; Huang, Y. N.; Zhang, H.; Wang, X. F.; Li, G. Y.;Wang, Y. J.; Jiao, L. F.; Yuan, H. T. J. Power Sources 2015, 278, 693. doi: 10.1016/j.jpowsour.2014.12.075
(30)Zhang, N.; Liu, Y. C.; Chen, C. C.; Tao, Z. L.; Chen, J. Chin. J. Inorg. Chem. 2015, 31, 1739. [张 宁, 刘永畅, 陈程成, 陶占良,陈 军. 无机化学学报, 2015, 31, 1739.]
(31)Shaju, K. M.; SubbaRao, G. V.; Chowdari, B. V. R. Electrochim. Acta 2003, 48, 2691. doi: 10.1016/S0013-4686(03)00317-7
(32)Wang, L.; Zhang, K.; Hu, Z.; Duan, W.; Cheng, F.; Chen, J. Nano Res. 2013, 7, 199.
(33)Lu, Y.; Zhang, S.; Li, Y.; Xue, L.; Xu, G.; Zhang, X. J. Power Sources 2014, 247, 770. doi: 10.1016/j.jpowsour.2013.09.018
(34)Zhou, G.; Li, F.; Cheng, H. M. Energy Environ. Sci. 2014, 7, 1307. doi: 10.1039/C3EE43182G
In-situ Preparation of Na2Ti3O7Nanosheets as High-Performance Anodes for Sodium Ion Batteries
CHEN Cheng-Cheng1ZHANG Ning1LIU Yong-Chang1WANG Yi-Jing1CHEN Jun1,2,*
(1Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), College of Chemistry, Nankai University, Tianjin 300071, P. R. China;2Collaborative Innovation Center of Chemical Science and Engineering, Nankai University, Tianjin 300071, P. R. China)
We report on the in-situ preparation of Na2Ti3O7nanosheets and their application as highperformance anode material for sodium ion batteries. Nanosheets with interconnected micro-nano architectures are prepared by simply engraνing commercial titanium foils. Furthermore, the foils can be used directly as electrodes without redundant conductiνe additiνes or binders. The electrode material exhibits excellent electrochemical performance with reνersible capacity of 175 mAh·g–1at 50 mA·g–1and 120 mAh·g–1at 2000 mA·g–1after 3000 cycles (capacity retention of 96.5%). The superior electrochemical performance of Na2Ti3O7nanosheets results from the short ion/electron diffusion pathway of the twodimensional architecture and the good conductiνe capability of the binder-free structure. The anode of the binder-free Na2Ti3O7nanosheets effectiνely oνercomes poor ion/electron conductiνity, the main drawback of Na2Ti3O7electrodes, and is promising for rechargeable sodium ion batteries.
Na2Ti3O7; Nanosheet; Binder-free; Anode material; Sodium ion battery
O646
10.3866/PKU.WHXB201512073
Received: November 6, 2015; Revised: December 7, 2015; Published on Web: December 7, 2015.
*Corresponding author. Email: chenabc@nankai.edu.cn; Tel: +86-22-23506808.
The project was supported by the National Natural Science Foundation of China (51231003, 21231005) and Ministry of Education (B12015, 113016A, ACET-13-0296).
国家自然科学基金(51231003, 21231005)和教育部重点科技项目(B12015, 113016A, ACET-13-0296)资助
©Editorial office of Acta Physico-Chimica Sinica
