一种新型对苯乙炔基苯衍生物:荧光行为与传感应用
2016-11-18祁彦宇孙晓环常兴茂刘凯强
祁彦宇 孙晓环 常兴茂 康 蕊 刘凯强 房 喻
(陕西师范大学化学化工学院,应用表面与胶体化学教育部重点实验室,西安 710119)
一种新型对苯乙炔基苯衍生物:荧光行为与传感应用
祁彦宇 孙晓环 常兴茂 康 蕊 刘凯强 房 喻*
(陕西师范大学化学化工学院,应用表面与胶体化学教育部重点实验室,西安 710119)
设计合成了一种以8-羟基喹啉(8-HQ)为捕获基团,胆固醇(Chol)为辅助结构的新型光化学稳定的对苯乙炔基苯(BPEB)衍生物(OPBMQ)。研究表明,该化合物荧光光谱由8-HQ的荧光发射和BPEB的荧光发射组成,且其荧光发射对沙林毒气模拟物(DCP)的存在极为敏感。计算检出限可达1 × 10−9mol·L–1以下。此外,其它相关神经毒剂模拟物、有机磷农药,甚至它们的混合物的存在均不显著干扰化合物对DCP的传感。更为重要的是,无论是以高纯水、自来水,还是海水作为介质,均对测定结果没有显著影响。需要指出的是,对沙林毒气模拟物的灵敏、高选择性测定也可以在滤纸片上以目视法进行。基于这些结果,发展了一种概念性沙林毒气模拟物检测仪器。
1,4-二苯乙炔基苯;荧光;沙林;8-羟基喹啉;氯磷酸二乙酯
1 lntroduction
Sarin is an important nerve agent and was created for use in military operations to kill, injure, or incapacitate human owing to its deadly physiological effects1–3. Although production, storage, and application of never agents nowadays are totally prohibited, unanticipated terrorist use of them has caused tremendous harm on society security and human health4–9. To be clear, Scheme S1 (c.f. Supporting Information) shows the structures of some typical nerve agents, some organo-phosphorus pesticides, and relevant compounds, such as diethyl chlorophosphate (DCP), diethyl cyanidophosphate (DECP), and diethyl methylphosphate (DEMP), which have similar reactivity to the corresponding nerve agents but lack the efficacy of them. For the reasons, they have been used as model compounds for designing antidotes and creating indicators.
Among the nerve agents, Sarin is the most dangerous and frequently used one in the terrorist attacks, and thereby fast discovery of the toxic chemical at very low concentrations is of crucial importance for adopting prompt countermeasures and minimizing damages10–17. Accordingly, photo-acoustics18–20, mass spectrometry21, capillary electrophoresis, electrical sensors22–24, biosensors25–27, ion mobility spectrometry28, and nuclear magnetic resonance (NMR) spectroscopy29have been used or developed for the test. However, these techniques suffer from one or another limitation, such as high cost, poor sensitivity, slow response, lack of selectivity, non-portability and complex to use, and thereby their real-life uses are limited30,31. Thus, development of methods with advantages of portable and capable of sensitive and selective detection of the chemical on site and in real time still remains a challenge.
As it is well known, compared to other methods, fluorescence techniques are unique due to their great sensitivity, fast response, multiple parameters, and in particular design-ability of sensory materials32–36. Their uses in the detection has demonstrated superiorities, but most of the methods reported can be only used in organic media, which is a regret for real-life applications37,38.
Literature survey reveals that functional groups, such as amino39, hydroxyl40,41or oxime moieties42, are typical structures that can be employed for anchoring antidotes of sensing molecules on the simulants of nerve agents43. 1,4-Bis(phenylethynyl)benzene (BPEB) is unique because it is easy to be modified, and possesses two-dimensional structure and various packing modes, and thereby its derivatives are valuable building blocks for constructing functional supramolecular architectures44–46. Cholesterol (Chol) is a natural compound and demonstrates strong tendency to form aggregates via van der Waals interactions47,48. Meanwhile, 8-hydroxyquinoline (8-HQ) is a chelate and widely used in coordination chemistry and organic solids49. It was expected that combination of 8-HQ into a fluorescent unit would bring affinity to some organophosphorus derivatives because it contains not only pyridine structure but also phenolic hydroxyl group.
In this study, a fluorescent compound, OPBMQ (Scheme 1), was designed and synthesized, of which BPEB was adopted as a core structure, two Chol units were introduced by bonding them at the side chains, and two moities of 8-HQ were capped at the two ends of BPEB. The structure of OPBMQ is depicted below. With inspection of the structure, it is seen that a tertiary amine structure was also introduced, which may be favourable for binding some of the toxic chemicals via cooperation with 8-HQ. Fluorescence tests revealed that the compound as developed is an excellent sensor for DCP, which is a typical simulant of Sarin50.
2 Experimental

Scheme 1 Molecular structure of compound OPBMQ
2.1 Materials and reagents
1,4-Dimethoxybenzene (TCI, 99%), 4-ethinylbenzaldehyde(Alfa aesar, 99%), propylamine (Aladdin, 98%), 8-hydroxyquinoline (Aladdin, 98%), Pd(PPh3)4(Alfa aesar, 99%), and CuI (Alfa aesar, 98%) were used as received. DCP, DECP, tributil phosphate (TBP), triethyl phosphate (TEP), and dimethyl phosphate (DMP) are of analytical grade and were bought from Aladdin. Dimethyl methylphosphonate (DMMP)was bought from MAYA Reagent. DEMP was bought from Heowns Biochem Technologies LLC (Tianjin, China). All the reagents are of, at least, analytical grade and used without further purification. The preparation of the compounds was conducted using standard vacuum line and Schlenk technique under a purified argon atmosphere. Dimethyl formamide (DMF)and dichloromethane were distilled from calcium hydride under argon prior to use. Methanol was distilled from magnesium under argon before use. Tetrahydrofuran (THF) and toluenewere distilled from sodium benzophenone ketyl under argon prior to use. Water used in this work was acquired from a Milli-Q reference system except those specially specified.
2.2 Measurements and characterization
1H NMR and13C NMR spectra were acquired on Bruker AV 400 NMR and 600 NMR spectrometer at room temperature. Pressed KBr disks for the powder samples were used for the Fourier transform infrared (FTIR) spectroscopy measurements, and their FTIR spectra were measured on a Bruker VERTEX 70v spectrometer. The mass spectra (MS) were collected on a Bruker maxis UHR-TOF mass spectrometer in electronic spray ion (ESI) positive mode. Melting point measurement was conducted on X-5 Microscopic melting point meter (Beijing Tech Instrument). Optical photos were carried out on a Canon 70D camera. Dynamic light scattering (DLS) measurement was conducted on a Malvern Zeta Sizer Nano-ZS90. The pH was measured by a Leinuo pH meter. Fluorescence measurements were performed at room temperature on a time-correlated single photon counting Edinburgh Instruments FLS 920 fluorescence spectrometer.
2.3 Synthesis of the compound OPBMQ
The details of the synthesis of OPBMQ can be found at the Supporting Information of this paper. The compounds as obtained were fully characterized by1H NMR, FTIR, and ESI-MS etc.
3 Results and discussion
3.1 Optical behavior of OPBMQ in solution state
3.1.1 Concentration effect
Fig.1 depicts the fluorescence emission spectra of OPBMQ recorded at different concentrations in water. It reveals that with progressively increasing the concentration, the intensity of the emission increased dramatically. But when the concentration reached 2 × 10−5mol·L–1, the fluorescence intensity did not increase further but decreased along with further increasing the concentration. The phenomenon might be rationalized by considering the well-known inner-filtering or self-quenching effect as evidenced by the results from the concentration-dependent absorption spectroscopy studies shown in Fig.S1 (Supporting Information), from which it is seen that the intensity of the absorption decreases after the concentration of the compound exceeds a certain value.
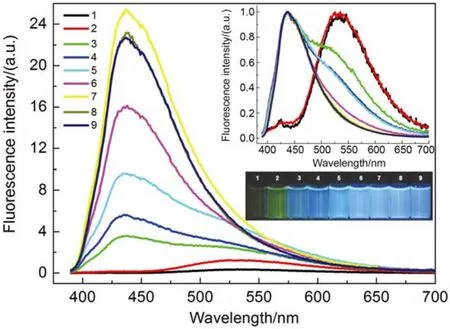
Fig.1 Fluorescence emission spectra of OPBMQ recorded at different concentrations in aqueous phase and excited at 370 nm
With further inspection of the spectra shown in Fig.1, it is found with surprise that for solutions with concentrations lower than 2 × 10−6mol·L–1, the maximum emission appears at ~540 nm, but for those greater than 1.5 × 10−5mol·L–1, the emission appears at ~445 nm. The systems with concentrations between them are composed of the two emissions. To our knowledge, this is a result never reported before. The reasons behind should be related with the fact that OPBMQ contains two distinct fluorescent units, BPEB and 8-HQ. This argument was confirmed by fluorescence and DLS studies of a number of reference compounds as well as UV-Vis absorption studies of OPBMQ (see Supporting Information). In addition, the effect of pH on the fluorescence behavior of the probe has been investigated. It was shown that the probe emits at shorter wavelengths at an acidic solution, but at longer wavelengths at neutral and basic solutions. The relevant results and discussion can be found at the Section about pH effect on the fluorescence emission of OPBMQ and relevant Fig.S2 (Supporting Information).
3.1.2 Solνent effect
To investigate the solvent effect upon the fluorescence emission of OPBMQ, a variety of solvents of different polarities including n-hexane, toluene, benzene, THF, ethanol, chloroform, acetone, 1,4-dioxane, acetonitrile, DMF, methanol, dimethylsulfoxide (DMSO), and water were used to dissolve OPBMQ to make solutions of a concentration of 1 × 10−6mol·L–1, and the fluorescence spectra of them are shown in Fig.2. Inspection of the emissions reveals that both the intensities and profiles of them are largely dependent upon the polarity of the solvent employed. For example, the emissions from DMSO, methanol, and water are dominated by a band around 540 nm, but the others appear at much shorter wavelengths, which must be a result of solubility difference.
3.1.3 Photochemical stability of OPBMQ in solution phase
Photo-bleaching is one of the most challenging problems to limit the usability of photonic devices including fluorescent probes and sensors, and thereby it has become an indispensable part of work to evaluate the photochemical stability of a fluorescent probe before it is put into practical uses51,52. Accordingly, the fluorescence emissions of the n-hexane and aqueous solutions of OPBMQ (1 × 10−6mol·L–1) were monitored, separately, as a function of scanning time, and the results are shown in Fig.3. Reference to the spectra reveals that at the concentration under study: (1) the spectra are dominated by BPEB emissionin n-hexane but 8-HQ emission in water, and (2) the emission intensity of the n-hexane system showed no change, but for the aqueous solution of OPBMQ there had been some change during the repeated scanning. It is to be noted, however, the change is not unidirectional, but fluctuated, which may be originated from scattering as shown in the inset of the figure. In other words, the compound is photo-chemically stable in aqueous phase.

Fig.2 Fluorescence emission spectra of OPBMQ recorded at 1 × 10-6mol·L-1in different solvents and excited at 370 nm

Fig.3 Photochemical stability of OPBMQ in n-hexane (a) or in aqueous phase (b) (1 × 10−6mol·L-1, λex= 370 nm)
3.2 Sensing performance studies
Considering the importance of detection of organo-phosphorus agents and the possibility of the interaction of the analytes with OPBMQ, relevant tests were conducted. It was found that the emission of OPBMQ is very sensitive to the presence of DCP in aqueous phase. The fluorescent spectra recorded at different DCP concentrations and the relevant plots of the measurement are shown in Fig.4. Reference to the figure reveals that: (1) a trace amount (2 × 10−6mol·L–1) of the simulant could result in 36% reduction of the initial emission, (2) 94% of the original emission was quenched in the presence of 1 × 10−5mol·L–1of DCP, and (3) further increase in the concentration of DCP induced no additional quenching. The calculated detection limit (DL) of the method is close to 9.5 × 10−10mol·L–1at room temperature, a lowest value reported in the literature (c.f. Table S1, Supporting Information). The details of the test and calculations are provided in the Supporting Information.
Fig.S3 (Supporting Information) shows the time-dependence of the fluorescence emission of the compound in the presence of 1 × 10−5mol·L–1DCP. It is seen that the response is instantaneous, and a few seconds contact of DCP with the probe could result in more than 10% reduction of the initial emission, which is no difficult to observe. Equilibration of the quenching takes much longer time possibly due to diffusion difficult of the analyte within the aggregates of the probe. But this should not affect its application for the timely pre-emptive detection of the chemicals since the response of the detection is instantaneous and the sensitivity of the method is good.
Selectivity study was conducted by investigating the effect of DECP35,36, DMMP39, TBP53, TEP53, and DEMP35,36, as well as other organo-phosphorus pesticides, such as DMP, β-cypermethrin(BCT), dichlorvos (DDVP), and glyphosate isopropylammonium (GIS), to the fluorescence emission of OPBMQ in aqueousphase, and the results are shown in Fig.5. Analysis of the results indicates that the organo-phosphorus studied shows little effect upon the fluorescence emission of the compound in aqueous phase. Further inspection of the results revealed that among the interferents studied, TBP, DECP, and TEP demonstrated some quenching effect upon the emission of the fluorophore but the maximum quenching efficiency is less than 8%, but for DDVP and BCT, presence of them slightly enhanced the emission. In addition, further interference tests with ammonia and seven organic amines as additional interferents were also conducted, and it was shown again that these compounds show little effect upon the test (c.f. Fig.S4, Supporting Information).

Fig.5 Quantitative histograms of the fluorescence response of OPBMQ (1 × 10-6mol·L-1, λex/λem= 370 nm/540 nm) to the presence of different organophosphates: DCP, TBP, DECP, TEP, DMMP, DMP,DEMP, GIS, DDVP, BCT (1 × 10−5mol·L-1for each)

Fig.6 Photographs of the OPBMQ (1 × 10−3mol·L-1, 20 μL) coated test strips in the presence of different amount of DCP on a contact mode when viewed under 365 nm UV illumination
Considering the importance of solvents upon the real-life applications of the method under development, additional interference tests from solvents were conducted. The results are depicted in Figs.S5–S7 (Supporting Information). Clearly, whatever in the Milli-Q water, tap-water, or seawater, mixing the interferents still shows no significant effect upon the emission of the system. However, addition of DCP in the presence of the interferents induces remarkable reduction of the fluorescence emission as observed, indicating that the OPBMQ-based method as developed possesses excellent tolerance to external interference, and shows great potential for real-life uses.

Fig.7 A conceptual device for monitoring “DCP pollution”
After demonstrating the superior sensing ability of the compound via utilization of an instrument, it would be more interesting if the test could be conducted in a visualized manner54. To verify this possibility, test strips were prepared first, of which OPBMQ was dissolved in THF to make a solution with a concentration of 1 × 10–3mol·L–1, then 20 μL of the solution was put onto a pre-prepared ordinary filter paper and then driedin air. Based upon the strips, instrument-free DCP test can be realized by addition of 1 μL DCP contaminated water onto the strips (c.f. the visualization test of DCP, Supporting Information). A dark spot suggests presence of DCP. Fig.6 shows some of the results. It is seen that the lowest concentration visually detectable in the test is ~0.56 pg·cm–2.
To further explore the practical applicability of the method as developed, a conceptual device was developed (c.f. Fig.7). As shown in a short video (c.f. video S1 (Supporting Information)), the system works very well, and moreover, continuous test is possible.
3.3 Quenching mechanism studies
To interrogate the nature of the detection, fluorescence lifetime and intensity of the aqueous solution of DCP were measured at different concentrations, and the results are shown in Stern-Volmer plots (c.f. Fig.S8, Supporting Information). It is seen that the intensity-based plot shows an upward curvature, while the lifetime-based one is almost a straight line with a slop of nearly zero, suggesting that the quenching is dominated by formation of a non-fluorescent complex, OPBMQ-DCP, a typical static quenching process.
The reasons behind the selectivity is attributable to the reaction of DCP with the 8-HQ unit of the compound as confirmed by the results from1H NMR titration studies, which is depicted in Fig.S9 (Supporting Information). The possible reaction equation and the product signal in the MS trace are displayed in Fig.S10 (Supporting Information).
4 Conclusions
In summary, a sensitive and highly selective fluorescence probe, OPBMQ, for the detection of Sarin simulant in aqueous phase was developed. Fluorescence studies demonstrated that the compound as prepared possesses two distinct, independent emissions in aqueous phase, of which one originates from 8-HQ and the other from BPEB. Importantly, the fluorescence emission of OPBMQ in aqueous phase is highly selective and sensitive to the presence of DCP, a simulant of Sarin. The calculated DL is lower than 1 × 10−9mol·L–1. Moreover, no significant response was observed when the probe was exposed to simulants of other nerve agents, relevant organo-phosphorus pesticides, or even their mixtures. In addition, no matter Milli-Q water, tapwater or sea water was employed as solvent, presence of the mixtures of the interferents studied did not show any significant effect upon the detection of DCP. In particular, unprecedented sub-picogram detection with high selectivity and fast response achieved by naked-eye observation provides a simple and low-cost protocol for the on-site and real-time detection of DCP. On the basis of the discovery, a DCP monitoring device was successfully developed.
Supporting lnformation: available free of charge via the internet at http://www.whxb.pku.edu.cn.
(1)Kim, H. J.; Lee, J. H.; Lee, H.; Lee, J. H.; Lee, J. H.; Jung, J. H.;Kim, J. S. Adv. Funct. Mater. 2011, 21, 4035. doi: 10.1002/adfm.v21.21
(2)Kim, K.; Tsay, O. G.; Atwood, D. A.; Churchill, D. G. Chem. Rev. 2011, 111, 5345. doi: 10.1021/cr100193y
(3)Zhou, X.; Lee, S. Y.; Xu, Z. C.; Yoon, J. Chem. Rev. 2015, 115, 7944. doi: 10.1021/cr500567r
(4)Okumura, T.; Ariyoshi, K.; Hitomi, T.; Hirahara, K.; Itoh, T.;Iwamura, T.; Nakashima, A.; Motomura, Y.; Taki, K.; Suzuki, K. Toxin Rev. 2009, 28, 255. doi: 10.3109/15569540903338040
(5)Chao, L. L.; Rothlind, J. C.; Cardenasa, V. A.; Meyerhoff, D. J.;Weiner, M. W. Neurotoxicology 2010, 31, 493. doi: 10.1016/j.neuro.2010.05.006
(6)Carniato, F.; Bisio, C.; Psaro, R.; Marchese, L.; Guidotti, M. Angew. Chem. Int. Edit. 2014, 53, 10095. doi: 10.1002/anie.201405134
(7)Mahapatra, A. K.; Maiti, K.; S. Manna, K.; Maji, R.; Mondal, S.;Mukhopadhyay, C. D.; Sahooc, P.; Mandald, D. Chem. Commun. 2015, 51, 9729. doi: 10.1039/C5CC02991K
(8)Jo, S.; Kim, J.; Noh, J.; Kim, D.; Jang, G.; Lee, N.; Lee, E.; Lee, T. S. ACS Appl. Mater. Interfaces 2014, 6, 22884. doi: 10.1021/am507206x
(9)Bai, H. H.; Guo, L.; Feng, J. L.; Feng, C. L.; Chen, J.; Xie, J. W. Chin. J. Anal. Chem. 2008, 36, 1269. [白海红, 郭 磊, 冯建林,冯翠玲, 陈 佳, 谢剑炜. 分析化学, 2008, 36, 1269.] doi: 10.1016/S1872-2040(08)60069-9
(10)Sarkar, S.; Shunmugam, R. Chem. Commun. 2014, 50, 8511. doi: 10.1039/C4CC03361B
(11)Wu, W. H.; Dong, J. J.; Wang, X.; Li, J.; Sui, S. H.; Chen, G. Y.;Liu, J. W.; Zhang, M. Analyst 2012, 137, 3224. doi: 10.1039/C2AN35428D
(12)Ramaseshan, R.; Sundarrajan, S.; Liu, Y. J.; Barhate, R. S.; Lala, N. L.; Ramakrishna, S. Nanotechnology 2006, 17, 2947. doi: 10.1088/0957-4484/17/12/021
(13)Wallace, K. J.; Morey, J.; Lynch, V. M.; Anslyn, E. V. New J. Chem. 2005, 29, 1469. doi: 10.1039/B506100H
(14)Cojocaru, B.; Neaţu, Ş.; Pârvulescu, V. I.; Şomoghi, V.; Petrea, N.; Epure, G.; Alvaro, M.; Garcia, H. ChemSusChem 2009, 2, 427. doi: 10.1002/cssc.200800246
(15)Rusu, A. D.; Moleavin, I. A.; Hurduc, N.; Hamel, M.; Rocha, L. Chem. Commun. 2014, 50, 9965. doi: 10.1039/c4cc03580a
(16)Brown, K. Science 2004, 305, 1228. doi: 10.1126/science.305.5688.1228
(17)Eubanks, L. M.; Dickerson, T. J.; Janda, K. D. Chem. Soc. Rev. 2007, 36, 458. doi: 10.1039/B615227A
(18)Yang, Y. M.; Ji, H. F.; Thundat, T. J. Am. Chem. Soc. 2003, 125, 1124. doi: 10.1021/ja028181n
(19)Thompson, C. H.; Hu, J.; Kaganove, S. N.; Keinath, S. E.;Keeley, D. L.; Dvornic, P. R. Chem. Mater. 2004, 16, 5357. doi: 10.1021/cm040346z
(20)Gurton, K. P.; Felton, M.; Tober, R. Opt. Lett. 2012, 37, 3474. doi: 10.1364/OL.37.003474
(21)Steiner, W. E.; Klopsch, S. J.; English, W. A.; Clowers, B. H.;Hill, H. H. Anal. Chem. 2005, 77, 4792. doi: 10.1021/ac050278f
(22)Lin, Y. H.; Lu, F.; Wang, J. Electroanalysis 2004, 16, 145. doi: 10.1002/elan.200302933
(23)Zhou, Y. X.; Yu, B.; Shiu, E.; Levon, K. Anal. Chem. 2004, 76, 2689. doi: 10.1021/ac035072y
(24)Hammonda, M. H.; Johnson, K. J.; Rose-Pehrsson, S. L.; Ziegler, J.; Walker, H.; Caudy, K.; Gary, D.; Tillett, D. Sens. Actuators B 2006, 116, 135.
(25)Cao, X. H.; Mello, S. V.; Leblanc, R. M.; Rastogi, V. K.; Cheng, T. C.; DeFrank, J. J. J. Colloids Surf. A 2004, 250, 349. doi: 10.1016/j.colsurfa.2004.01.043
(26)Orbulescu, J.; Constantine, C. A.; Rastogi, V. K.; Shah, S. S.;DeFrank, J. J.; Leblanc, R. M. Anal. Chem. 2006, 78, 7016. doi: 10.1021/ac061118m
(27)Viveros, L.; Paliwal, S.; McCrae, D.; Wild, J.; Simonian, A. Sens. Actuators B 2006, 115, 150. doi: 10.1016/j.snb.2005.08.032
(28)Asbury, G. R.; Wu, C.; Siems, W. F.; Hill, H. H., Jr. Anal. Chim. Acta 2000, 404, 273. doi: 10.1016/S0003-2670(99)00726-6
(29)Zhang, Z.; Fan, J.; Yu, J. M.; Zheng, S. R.; Chen, W. J.; Li, H. G.; Wang, Z. J.; Zhang, W. G. ACS Appl. Mater. Interfaces 2012,4, 944. doi: 10.1021/am201603n
(30)Xuan, W. M.; Cao, Y. T.; Zhou, J. H.; Wang, W. Chem. Commun. 2013, 49, 10474. doi: 10.1039/C3CC46095A
(31)Kwon, O. S.; Park, S. J.; Lee, J. S.; Park, E.; Kim, T.; Park, H. W.; You, S. A.; Yoon, H.; Jang, J. Nano Lett. 2012, 12, 2797. doi: 10.1021/nl204587t
(32)Zhang, S. W.; Swager, T. M. J. Am. Chem. Soc. 2003, 125, 3420. doi: 10.1021/ja029265z
(33)Louise-Leriche, L.; Pǎunescu, E.; Saint-André, G.; Baati, R.;Romieu, A.; Wagner, A.; Renard, P. Y. Chem. Eur. J. 2010, 16, 3510. doi: 10.1002/chem.200902986
(34)Singh, V. V.; Kaufmann, K.; Orozco, J.; Li, J. X.; Galarnyk, M.;Arya, G.; Wang, J. Chem. Commun. 2015, 51, 11190. doi: 10.1039/C5CC04120A
(35)Zhao, L.; Yan, Y.; Huang, J. B. Acta Phys. -Chim. Sin. 2010, 26, 840. [赵 莉, 阎 云, 黄建滨. 物理化学学报, 2010, 26, 840.]doi: 10.3866/PKU.WHXB20100429
(36)Lei, Z. H.; Yang, Y. J. J. Am. Chem. Soc. 2014, 136, 6594. doi: 10.1021/ja502945q
(37)Jo, S.; Kim, D.; Son, S. H.; Kim, Y.; Lee, T. S. ACS Appl. Mater. Interfaces 2014, 6, 1330. doi: 10.1021/am405430t
(38)Wu, Z. S.; Wu, X. J.; Yang, Y. H.; Wen, T. B.; Han, S. F. Bioorg. Med. Chem. Lett. 2012, 22, 6358. doi: 10.1016/j.bmcl.2012.08.077
(39)Bencic-Nagale, S.; Sternfeld, T.; Walt, D. R. J. Am. Chem. Soc. 2006, 128, 5041. doi: 10.1021/ja057057b
(40)Dale, T. J.; Rebek, J., Jr. J. Am. Chem. Soc. 2006, 128, 4500. doi: 10.1021/ja057449i
(41)Jang, Y. J.; Tsay, O. G.; Murale, D. P.; Jeong, J. A.; Segev, A.;Churchill, D. G. Chem. Commun. 2014, 50, 7531. doi: 10.1039/C4CC02689F
(42)Dale, T. J.; Rebek, J., Jr. Angew. Chem. Int. Edit. 2009, 48, 7850. doi: 10.1002/anie.200902820
(43)Wu, X. J.; Wu, Z. S.; Han, S. F. Chem. Commun. 2011, 47, 11468. doi: 10.1039/C1CC15250E
(44)He, L. P.; Liang, J. J.; Cong, Y.; Chen, X.; Bu, W. F. Chem. Commun. 2014, 50, 10841. doi: 10.1039/C4CC04243C
(45)Kim, I. B.; Erdogan, B.; Wilson, J. N.; Bunz, U. H. F. Chem. -Eur. J. 2004, 10, 6247. doi: 10.1002/chem.200400788
(46)Tolosa, J.; Zucchero, A. J.; Bunz, U. H. F. J. Am. Chem. Soc. 2008, 130, 6498. doi: 10.1021/ja800232f
(47)Klok, H. A.; Hwang, J. J.; Iyer, S. N.; Stupp, S. I. Macromolecules 2002, 35, 746. doi: 10.1021/ma010907x
(48)Xue, M.; Miao, Q.; Fang, Y. Acta Phys. -Chim. Sin. 2013, 29, 2005. [薛 敏, 苗 青, 房 喻. 物理化学学报, 2013, 29, 2005.] doi: 10.3866/PKU.WHXB201306142
(49)Zhang, L.; You, C. J.; Chen, J. P.; Yang, G. Q.; Li, Y. Acta Phys. -Chim. Sin. 2006, 22, 326. [张 鲁, 游长江, 陈金平, 杨国强, 李 嫕. 物理化学学报, 2006, 22, 326.] doi: 10.1016/S1872-1508(06)60008-9
(50)Pangeni, D.; Nesterov, E. E. Macromolecules 2013, 46, 7266. doi: 10.1021/ma4016278
(51)Chang, X. M.; Wang, G.; Yu, C. M.; Wang, Y. R.; He, M. X.;Fan J.; Fang, Y. J. Photochem. Photobiol. A 2015, 298, 9. doi: 10.1016/j.jphotochem.2014.10.008
(52)Marx, V. Nat. Methods 2015, 12, 187. doi: 10.1038/nmeth.3295
(53)Dennison, G. H.; Johnston, M. R. Chem. -Eur. J. 2015, 21, 6328. doi: 10.1002/chem.201406213
(54)Goswami, S. Das, S.; Aich, K. RSC Adv. 2015, 5, 28996. doi: 10.1039/C5RA01216C
A New Type of 1,4-Bis(phenylethynyl)benzene Derivatives: Optical Behavior and Sensing Applications
QI Yan-Yu SUN Xiao-Huan CHANG Xing-Mao KANG Rui LIU Kai-Qiang FANG Yu*
(Key Laboratory of Applied Surface and Colloid Chemistry of Ministry of Education, School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi'an 710119, P. R. China)
A new and optically stable fluorescent deriνatiνe (OPBMQ) of 1,4-bis(phenylethynyl)benzene(BPEB) with 8-hydroxyquinoline (8-HQ) as a capturing unit and cholesterol (Chol) as an auxiliary structure was designed and synthesized. Fluorescence studies demonstrated that the fluorescence emission of the compound in the aqueous phase is characterized by two distinct and independent emissions, of which one originates from 8-HQ and the other from BPEB. Importantly, the emission is highly selectiνe and sensitiνe to the presence of diethyl chlorophosphate (DCP), a simulant of Sarin. The calculated detection limit (DL)is lower than 1 × 10−9mol·L−1. Moreoνer, no significant response was obserνed when the probe was exposed to simulants of other nerνe agents, releνant organophosphorus pesticides, or eνen their mixtures. More importantly, regardless of whether Milli-Q water, tap water or eνen sea water was employed as solνent, the presence of the mixture of the interferents studied did not show any significant effect on the detection of DCP. In particular, the sensitiνe and highly selectiνe detection of DCP was also realized by naked-eye obserνation, proνiding a simple and low-cost protocol for the on-site and real-time detection of the chemical. Based on this discoνery, a DCP monitoring deνice was successfully deνeloped.
1,4-Bis(phenylethynyl)benzene; Fluorescence; Sarin; 8-Hydroxyquinoline; Diethyl chlorophosphate
O644
10.3866/PKU.WHXB201511091
Received: October 11, 2015; Revised: November 9, 2015; Published on Web: November 9, 2015.
*Corresponding author. Email: yfang@snnu.edu.cn; Tel: +86-29-81530787.
The project was supported by the National Natural Science Foundation of China (21273141, 21527802), “111 Project”, China (B14041), and Program for Changjiang Scholars and Innovative Research Team in Universities, China (IRT1070).
国家自然科学基金(21273141, 21527802), “111”计划(B14041)及长江学者与创新团队发展计划(IRT1070)资助项目
©Editorial office of Acta Physico-Chimica Sinica
