植物多酚通过PI3K/Akt信号通路抗肿瘤作用研究进展
2016-11-14肖星凝吴素蕊
石 芳,廖 霞,李 瑶,李 谣,肖星凝,吴素蕊,明 建,,3,*
(1.西南大学食品科学学院,重庆 400715;2.中华全国供销合作总社昆明食用菌研究所,云南 昆明 650223;3.重庆市特色食品工程技术研究中心,重庆 400715)
植物多酚通过PI3K/Akt信号通路抗肿瘤作用研究进展
石 芳1,廖 霞1,李 瑶1,李 谣1,肖星凝1,吴素蕊2,明 建1,2,3,*
(1.西南大学食品科学学院,重庆 400715;2.中华全国供销合作总社昆明食用菌研究所,云南 昆明 650223;3.重庆市特色食品工程技术研究中心,重庆 400715)
磷脂酰肌醇3-激酶/丝氨酸/苏氨酸激酶B(phosphatidylinositol 3-kinase/serine/threonine kinase B,PI3K/Akt)信号通路是一条经典的抑制细胞凋亡、促进细胞增殖的信号转导通路,在肿瘤、心血管疾病、神经系统疾病和糖尿病等的防治中发挥重要作用,特别是肿瘤。PI3K/Akt信号通路的异常活化与肿瘤的发生、侵袭和转移等过程密切相关,对该通路的研究已成为当今国内外治疗肿瘤的焦点。植物多酚因具有抗肿瘤作用而成为预防肿瘤的天然药物。本文综述了PI3K/Akt信号通路的结构、活化机制以及与肿瘤的关系,总结植物多酚通过该信号通路对肿瘤细胞的作用机制,以期为研发植物多酚作为预防肿瘤的保健食品或药品提供一定的科学依据。
磷脂酰肌醇3-激酶/丝氨酸/苏氨酸激酶B;信号通路;植物多酚;抗肿瘤
石芳, 廖霞, 李瑶, 等. 植物多酚通过PI3K/Akt信号通路抗肿瘤作用研究进展[J]. 食品科学, 2016, 37(15): 259-264.
SHI Fang, LIAO Xia, LI Yao, et al. Plant polyphenols exert anti-tumor activity by the PI3K/Akt signaling pathway: a review[J]. Food Science, 2016, 37(15): 259-264. (in Chinese with English abstract) DOI:10.7506/spkx1002-6630-201615044. http://www.spkx.net.cn
恶性肿瘤是全球三大致死原因之一,严重威胁人类健康和生命。统计数据显示,2015年,我国有281.4万人因恶性肿瘤而死亡,致死率排列前五的分别为肺癌、胃癌、肝癌、食道癌和结直肠癌[1]。许多研究表明,肿瘤的发生与发展是多因素、多基因、多途径的结果,而细胞信号转导途径在肿瘤的发生发展、侵袭转移过程中至关重要。其中磷脂酰肌醇3-激酶/丝氨酸/苏氨酸激酶B(phosphatidylinositol 3-kinase/serine/threonine kinase B,PI3K/Akt)信号通路在调控实体肿瘤(如肝癌[2]、乳腺癌[3]、结肠癌[4]、胃癌[5]、黑 色素瘤[6]、神经母细胞瘤[7])和血液肿瘤(如白血病[8])中发挥着重要作用。PI3K/Akt信号通路是细胞内一条重要的信号转导通路,PI3K作为联系胞外信号与细胞应答效应的桥梁分子,在一系列上游或旁路信号分子的影响下,作用于下游的多种效应分子,从而促进细胞侵袭迁移、抑制细胞凋亡、加速细胞周期进程、促进细胞增殖。明确该信号通路在体内的作用机制,寻找阻断该信号通路的靶向药物是国内外研究热点之一。
植物化 学物质的抗肿瘤活性已被广泛认可,尤其是植物多酚,具有安全有效、来源广泛的特点,是一类极具应用前景的天然抗 肿瘤药物。因此,通过PI3K/Akt信号通路抗肿瘤的作用机制来了解植物多酚,有助于开发植物多酚类抗肿瘤保健食品或药物。
1 PI3K/Akt信号通路概述
1.1PI3K/Akt的结构与功能
PI3K为脂质激酶家族成员,是一种可特异性催化磷脂酰肌醇磷酸化产生3,4,5-三磷酸磷脂酰肌醇的激酶,分为3 个亚型(I、II、III)。I型PI3K是由催化亚基与调节亚基组成的异二聚体,催化亚基由p110α、p110β、p110γ和p110δ组成,基因编码分别是PIK3CA、PIK3CB、PIK3CG和PIK3CD。调节亚基由p85α、p85β和p55γ组成,基因编码分别是PIK3R1、PIK3R2、PIK3R3[9]。II型PI3K以磷脂酰肌醇(phosphatid ylinositol,PI)及磷脂酰肌醇磷酸(phosphatidylinositol phosphate,PIP)为底物,从N端到C端依次排列着富含脯氨酸区、Ras结合区、HR区、PX结构域和C2结构域[10]。III型PI3K为酵母空泡蛋白分选同源物,由调节亚基pl50和豆蔻酰化的催化亚基p100组成异二聚体,在细胞内以PI为底物,并与自噬、吞噬、溶酶体分类和细胞信号转导作用密切相关[11]。PI3K参与细胞增殖、抑制细胞凋亡、细胞迁移、膜泡转运、血管生成、细胞癌性转化等过程,并通过由其催化形成的3-磷酸肌醇脂分子(PIP、PIP2、PIP3)起作用。
Akt为蛋白激酶B(protein kinase B,PKB),是分子质量为57 kD的丝/苏氨酸蛋白激酶,含有480 个氨基酸残基,与蛋白激酶A(68%的同源性)和蛋白激酶C(73%的同源性)具有同源性。Akt激酶与腺苷酸/鸟苷酸(adenosine monophosphate/guanosine monophosphate,AMP/GMP)激酶和蛋白激酶C同属于ACG蛋白激酶家族。PKB家族已分离出三个成员,分别为PKBα(Akt1)、PKBβ(Akt2)和PKBγ(Akt3)。它们的氨基酸同源性达80%以上,而且都具有3 个相同的结构区域:氨基末端的血小板-白细胞C激酶底物同源(pleckstrin homology,PH)结构区域、Akt分子中心区的激酶活性区和羧基末端的调节区。PH区域广泛存在于信号蛋白和细胞骨架相关蛋白中,可以介导蛋白质和脂质、蛋白质和蛋白质之间的相互作用。
1.2PI3K/Akt的活化与调节
PI3K的激活(图1)可通过与受体酪氨酸激酶(receptor tyrosine kinase,RTK)和G蛋白连接受体相互作用,也可通过Ras蛋白与其p110亚基直接结合而被激活[12-13]。结果是在质膜上产生PIP3,PIP3招募具有PH结构域的信号蛋白到质膜上,包括磷酸肌醇依赖性蛋白激酶1(phosphoinositide-dependent kinase-1,PDK1)和Akt,使得Akt构象发生变化,导致激活Akt所必需的两个重要磷酸化位点(苏氨酸(threonine,Thr)308和丝氨酸(serine,Ser)473)暴露。这样就可由整合素偶联激酶(integrin linked kinase,ILK)、DNA依赖蛋白激酶(DNA-dependent protein kinase,DNA-PK)、哺乳动物雷帕霉素靶蛋白(the mammalian target of rapamyein,mTOR)或Akt本身所激活[14]。活化后Akt离开细胞膜进入细胞核发挥作用,进一步引起其他蛋白磷酸 化,包括雷帕霉素靶蛋白复合体1(mammalian target of rapamycin complex 1,mTORC1)、糖原合成激酶3(glycogen synthase kinase 3,GSK3)、叉头转录因子(forkhead transcription factors,FOXO)、腺苷酸活化蛋白激酶(AMP-activated protein kinase,AMPK)等,从而调节细胞蛋白的合成、细胞生长、增殖扩散、存活 以及新陈代谢等活动[15-20]。
PI3K/Akt信号通路受多种因子的调节,参与PI3K/Akt信号通路的调节因子主要有类脂磷酸酶(如,phosphatase and tensin homolog deleted on chromosome ten,(PTEN))、SHIP2(SH2-containing inositol 5-phosphatase,SHIP2)和C末端调节蛋白(carboxylterminal modulator protein,CTMP)等负调节因子。PTEN是多种细胞生长、分化和维持生存的抑制物,具有蛋白磷酸酶活性和脂质磷酸酶活性,从PIP3的3位脱磷酸而将其转变为PI(4,5)P2,使PIP3维持在较低的水平,从而阻断Akt及其下游效应分子的有效活化[21],PTEN活性丧失可导致PI3K/Akt通路永久激活。Akca等[22]发现PTEN的失活可促进PI3K/Ak t/核因子κB(nuclear factor κB,NF-κB)通路活化,并进一步导致肺癌细胞的侵袭能力增强。CTMP是一种线粒体蛋白,在线粒体内裂解后转化为功能蛋白,与Akt羧基端调节区结合,抑制Akt的磷酸化,从而阻断下游信号传导。SHIP2是一种磷酸酯酶,可对PIP3进行5位去除磷酸,将其转变成PI(3,4)P2而降解,进而使其丧失功能[21]。
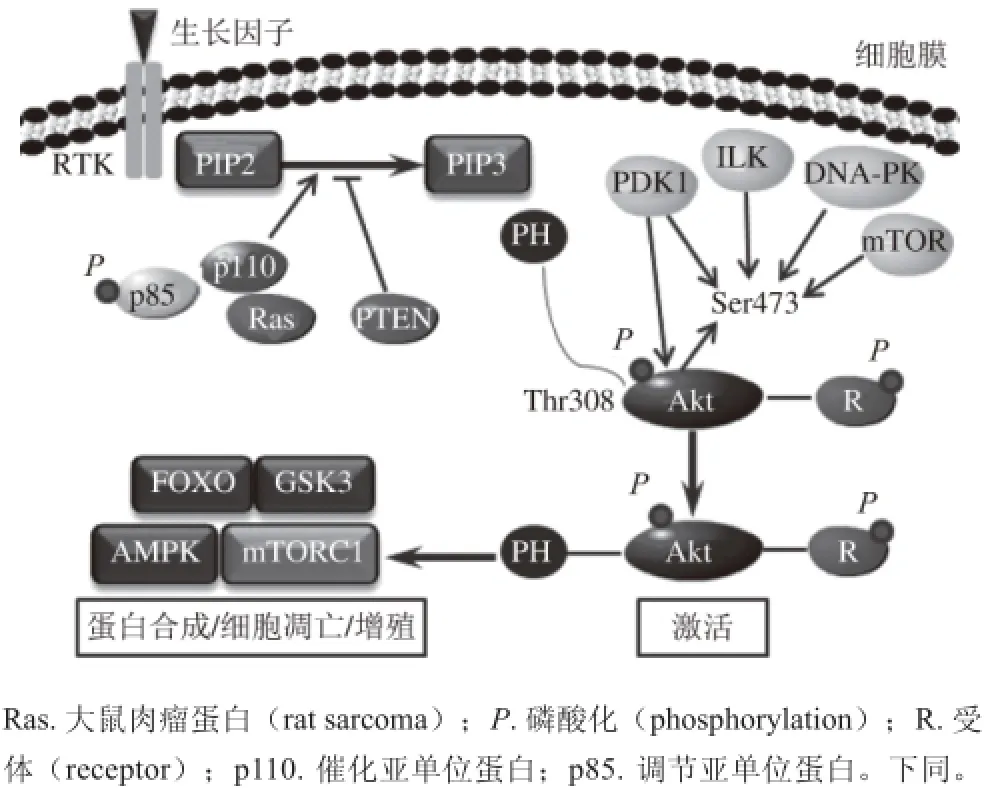
图1 PI3K/Akt信号通路激活机制[1133]Fig. 1 Mechanism of PI3K/Akt signaling pathway activation[13]
2 PI3K/Akt信号通路与肿瘤的发生
2.1PI3K/Akt信号通路抑制肿瘤细胞停滞和凋亡
目前认为PI3K/Akt信号通路调控细胞凋亡主要通过以下几个途径[13,23]:1)B细胞淋巴瘤/白血病-2基因(B-cell lymphoma-2,Bcl-2)家族中磷酸化的促凋亡分子蛋白(Bcl-2/ Bcl-xl associated death promoter,Bad)与抗凋亡因子Bcl-xl或Bcl-2解聚,Bad与 抗凋亡结合蛋白14-3-3相结合,而Bcl-xl或Bcl-2发挥抑制细胞凋亡的作用;此外,PI3K/Akt通路的激活也可以使Bcl-2相关X蛋白(Bcl-2 associated X protein,Bax)的Ser184残基磷酸化而使其失活,从而抑制细胞凋亡;2)通过磷酸化蛋白水解酶Caspase-9的Ser196位点而使之失活,进一步抑制线粒体释放细胞色素c及凋亡因子,抑制细胞凋亡;3)调控转录因子的表达。叉头转录因子(forkhead-type transcription factors,FKHR)可被Akt直接磷酸化,不能进入细胞核抑制抗凋亡因子Fas-L的转录,从而抑制细胞凋亡;4)磷酸化鼠双微染色体2(murine double mimute2,MDM2)。Akt磷酸化MDM2的Ser166和Ser188位点后,下调凋亡蛋白p53,抑制细胞周期停滞和细胞凋亡。
2.2PI3K/Akt信号通路促进肿瘤细胞生存和增殖
活化的Akt可调节多种与细胞增殖相关的蛋白(如mTOR、原癌基因等),促进细胞增殖,具体如下:1)磷酸化mTOR。mTOR是Akt信号最重要的下游因子,可调节翻译过程中关键因子的表达,如核糖体蛋白S6激酶(ribosomal S6 kinase,RSK)和翻译抑制因子4E-结合蛋白1(4E-binding protein1, 4E-BP1)等,促进肿瘤细胞增殖。2)Akt能够直接或间接调节磷酸化激活κB激酶(inhibitor of nuclear factor kappa-B kinase,IKK)的活性,导致NF-κB的抑制剂IκB发生降解,使NF-κB从细胞质中释放出来转移至核内,激活其靶基因而促进细胞的存活;3)Akt通过使GSK3激酶磷酸化失活,阻止细胞周期蛋白D1和肿瘤蛋白Myc的降解,上调细胞周期素依赖性激酶-4(cyclin dependent kinase 4,CDK4),或者磷酸化细胞周期素依赖性激酶(cyclin dependent kinase,CDK)的抑制因子P21和P27,从而失去对细胞周期的抑制性调控作用,加速细胞周期进程,促进细胞增殖[24]。
2.3PI3K/Akt信号通路促进肿瘤细胞侵袭和转移
PI3K/Akt信号通路在肿瘤细胞迁移中起着重要作用。肿瘤上皮细胞间充质转分化(epithelial-mesenchymal transition,EMT)与肿瘤细胞侵袭、迁移等生物学行为密切相关。PI3K/Akt信号通路通过上调核转录因子Snail、Slug等,抑制细胞内E-钙黏蛋白的表达[25]或通过上调基质金属蛋白酶(matrix metalloproteinase,MMP),促进MMP对E-钙黏蛋白的降解,直接诱导EMT,增强细胞的运动能力和侵袭能力[26]。在对鳞状细胞癌(head and neck squamous cell carcinoma,HNSCC)的研究中发现,表皮生长因子(epidermal growth factor,EGF)激活表皮生长因子受体(epithelial growth factor receptor,EGFR)后,诱导EMT表型改变以及MMP9介导的E-钙黏蛋白的降解,激活PI3K信号通路,促进细胞侵袭、迁移[27]。
3 植物多酚通过PI3K/Akt信号通路的抗肿瘤作用
随着人们对PI3K/Akt信号通路的认识不断深入,许多研究者试图利用多种方法寻找阻断该信号转导通路的靶向药物,如基因敲除[28]、RNA干扰[29]以及化学药物阻断[30]等,以预防肿瘤的发生。而植物多酚的抗肿瘤活性已被广泛认可,大量研究证实,许多植物多酚(如白藜芦醇、姜黄素、原花青素、表没食子儿茶素没食子酸酯、槲皮素等)可以通过干扰PI3K/Akt信号通路(图2),直接抑制PI3K或阻断PI3K/Akt信号通路,进一步调节其下游效应分子的表达,从而加速肿瘤细胞周期、促进细胞凋亡和抑制细胞增殖,达到抗肿瘤的作用[31]。

图2 植物多酚通过PI3K/Akt信号通路抗肿瘤作用机制[3311]Fig. 2 Anti-tumor mechanism of plant polyphenols through the PI3K/Akt signaling pathway[31]
3.1白藜芦醇
白藜芦醇是广泛存在于葡萄等植物中的多酚化合物,对多种肿瘤细胞都有一定的抗癌活性。白藜芦醇通过抑制PI3K/Akt/mTOR信号通路,下调下游分子p70S6K和4E-BP1的磷酸化,同时激活Caspase-3,增加细胞周期蛋白D1表达水平,加速细胞周期,抑制人慢性粒细胞白血病K562细胞的增殖,诱导其凋亡。进一步研究发现,PI3K和Akt的选择抑制剂LY294002可与白藜芦醇发挥协同作用[32]。白藜芦醇还可通过抑制PI3K/Akt/NF-κB信号转导通路,下调基质金属蛋白酶MMP-2表达,阻止恶性胶质瘤起始细胞(glioblastoma-initiating cells,GICs)的侵袭和转移[33]。Zhang Dequan等[34]用白藜芦醇处理永生性大鼠肝星状细胞(the immortalized rat hepatic stellate cells,t-HSC/CI-6)后,发现细胞内Toll样本受体4(toll like receptor 4,TLR4)、PI3K和Akt表达下降,从而阻断了TLR4介导的NF-κB的转导,抑制肝星状细胞的激活,表明白藜芦醇通过阻断PI3K/Akt信号通路而产生抑制癌细胞作用。
3.2姜黄素
姜黄素是一种从姜黄等根茎中提取的多酚类色素,通过调节细胞周期,促进细胞凋亡,抑制细胞增殖而发挥抗肿瘤作用,被称作第三代肿瘤化学预防剂。已有研究证实姜黄素通过调控PI3K/Akt信号通路而具有抗癌活性,其作用机制是姜黄素使Akt去磷酸化,抑制叉头转录因子FOXO磷酸化,导致细胞周期相关蛋白p21和p27Kip表达下降,从而减缓肿瘤的发展[35]。Zhang Hao等[36]用姜黄素处理肾癌细胞(the renal cell carcinoma,RCC)-949时还发现,姜黄素能够上调Bcl-2、下调Bax的表达,降低细胞周期蛋白B1的表达量,在G2/M期启动细胞周期停滞,抑制细胞增殖,促进细胞凋亡。Liu Hao等[37]研究姜黄素衍生物(T63)诱导肺癌细胞A549的作用机制时发现,T63不仅可以通过阻滞细胞周期蛋白p21、p27和细胞周期蛋白D1,使细胞凋亡,而且还能够上调蛋白磷酸酶2A(protein phosphatase 2A,PP2A)蛋白,抑制Akt磷酸化,从而激活FOXO3a和Bad,促进细胞色素c释放,激活Caspase-3,诱导细胞凋亡。
3.3原花青素
原花青素是由不同数量的儿茶素或表儿茶素结合而成的多酚类物质,具有抗乳腺癌、前列腺癌、直肠癌等作用。有研究发现,原花青素六聚体(hexamer,Hex)可以降低结直肠癌发生,hex可以促进线粒体中细胞色素c释放到细胞质,抑制Caco-2细胞PI3K/Akt信号通路,下调下游蛋白Bad、GSK-3β的表达,诱导细胞凋亡[38]。葡萄籽原花青素(proanthocyanidins from grape seeds,GSPs)也可通过抑制PI3K/Akt信号通路,下调Bcl-2和Bcl-xl表达,上调Bax表达,同时激活Caspase-3,诱导人胰腺癌细胞(Miapaca-2、PANC-1和AsPC-1))凋亡[39]。Prasad等[40]发现蔓越莓原花青素(cranberry-derived proanthocyanidin,C-PAC)诱导食管腺癌细胞(esophageal adenocarcinoma,EAC)的凋亡与PI3K/Akt通路失活有关,也与促凋亡蛋白(Bax、Bak1、脱酰胺Bcl-xl、细胞色素c)、MAPKs的表达及G2/M周期阻滞有关。
3.4表没食子儿茶素没食子酸酯(epigallocatechin gallate,EGCG)
EGCG是绿茶中含量最丰富的成分之一。绿茶的抗癌作用大多数是通过EGCG介导的。EGCG通过磷酸化Akt,抑制Bad磷酸化,上调Bax和Bad表达,下调Bcl-2表达,诱导细胞色素c、Caspase-9和细胞凋亡因子释放,进而促进膀胱癌移行性细胞株凋亡[41]。EGCG还可通过影响PI3K/Akt信号通路的多种效应分子发挥抗肿瘤作用,如下调COX-2、同时激活Caspase-3和Caspase-9,诱导肝癌细胞凋亡、抑制MMP-2和MMP-9活性,发挥抗肿瘤细胞转移的作用[42-43]、介导PI3K/Akt/mTOR信号通路,促进B淋巴瘤细胞凋亡[44]、下调PI3K、Akt、NF-κB的表达水平,使肝癌细胞SMMC7721在S期停滞,诱导细胞凋亡[45]。
3.5槲皮素
槲皮素是一种黄酮类化合物,对多种恶性肿瘤具有预防和治疗作用。槲皮素的抗癌作用主要是通过结合PI3K,抑制Akt磷酸化,上调p53的表达,调控Bax和Bcl-2的表达来诱导癌细胞的凋亡,阻止细胞的迁移和侵袭,抑制癌细胞的增殖[46-48]。槲皮素还可通过影响PI3K/Akt通路,并增强NF-κB核转位,降低Bcl-x1/Bal的比值,上调抑癌基因Bim和凋亡诱导因子(apoptosisinducing factor,AIF)表达,起到抑制黑色素瘤细胞B16F10的作用[49]。Xiang Tao等[50]研究也证实槲皮素诱导HeLa细胞凋亡是通过下调PI3K/Akt信号通路中PI3K、Akt和Bcl-2表达,上调Bax表达,使细胞在G0/G1细胞周期阻滞。Wang Piwen等[51]的研究还发现槲皮素和牛蒡子苷元对抑制前列腺癌细胞增殖具有协同作用,也是通过抑制PI3K/Akt信号通路而起作用的。
3.6其他
还有许多植物多酚类化合物通过调控PI3K/Akt信号通路而应用于肿瘤治疗。例如,芹菜素作为ATP竞争抑制剂,阻断PI3K的ATP结合位点,并且抑制与细胞增殖相关的Akt及其下游促凋亡蛋白Bad的磷酸化,从而抑制PI3K/Akt信号通路发挥其抗肿瘤作用[52]。熊果酸通过调控PI3K/Akt信号通路的Bcl-2、Bcl-xl、凋亡抑制基因Survivin、p-mTOR的表达,激活Caspase-3,抑制细胞增殖,诱导细胞凋亡,显著抑制前列腺肿瘤细胞LNCaP的生长[53]。
4 结 语
PI3K/Akt信号通路是一条经典的抑制细胞凋亡、促进细胞增殖的信号转导通路,与多种肿瘤的发生、侵袭、转移等过程密切相关。植物多酚是一类广泛存在于食物中的天然化合物,具有良好的预防肿瘤的作用。越来越多的研究发现植物多酚可作用于PI3K/Akt信号通路,调节其相应基因或蛋白的表达,发挥抑制细胞凋亡、促进细胞增殖的作用从而降低癌症的发生。植物多酚除通过PI3K/Akt信号传导通路发挥抗肿瘤作用外,还可以通过MAPK、核转录相关因子2(NF-E2-related factor 2,
Nrf2)等细胞信号通路达到抗肿瘤作用。因此,还需深入研究植物多酚抗肿瘤作用机制,为挖掘植物多酚抗肿瘤活性功能,开发植物多酚类抗肿瘤保健食品或药物提供依据。
[1] CHEN W Q, ZHENG R S, BAADE P D, et al. Cancer statistics in China,2015[J]. CA: a Cancer Journal for Clinicians, 2016, 66(2): 115-132. DOI:10.3322/caac.21338.
[2] CHENG L, LUO S, JIN C, et al. FUT family mediates the multidrug resistance of human hepatocellular carcinoma via the PI3K/Akt signaling pathway[J]. Cell Death and Disease, 2013, 4(11): 923-934. DOI:10.1038/cddis.2013.450.
[3] ADAMS J R, SCHACHTER N F, LIU J C, et al. Elevated PI3K signaling drives multiple breast cancer subtypes[J]. Oncotarget, 2011,2(6): 435-447.
[4] 隋华, 付晓伶, 潘树芳, 等. PI3K/Akt/NF-κB通路调控ABCB1/P-gp介导的人结肠癌细胞多药耐药的研究[J]. 中国癌症杂志, 2014, 24(2): 106-111. DOI:10.3969/j.issn.1007-3969.2014. 02.005.
[5] FU X A, FENG J R, ZENG D, et al. PAK4 confers cisplatin resistance in gastric cancer cells via PI3K/Akt- and MEK/ERK-dependent pathways[J]. Bioscience Reports, 2014, 34(2): e00094. DOI:10.1042/ BSR20130102.
[6] CHI M N, YE Y, ZHANG X D, et al. Insulin induces drug resistance in melanoma through activation of the PI3K/Akt pathway[J]. Drug Design, Development and Therapy, 2014, 8: 255-262. DOI:10.2147/ DDDT.S53568.
[7] STAFMAN L L, BEIERLE E A. Cell proliferation in neuroblastoma[J]. Cancers, 2016, 8(13): 1-21. DOI:10.3390/cancers8010013.
[8] MA H Y, CHENG L, HAO K J, et al. Reversal effect of ST6GAL1 on multidrug resistance in human leukemia by regulating the PI3K/Akt pathway and the expression of P-gp and MRP1[J]. PLoS ONE, 2014,9(1): e85113. DOI:10.1371/journal.pone.0085113.
[9] AHMAD A, BIERSACK B, LI Y W, et al. Targeted regulation of PI3K/ Akt/mTOR/NF-κB signaling by indole compounds and their derivatives: mechanistic details and biological implications for cancer therapy[J]. Anticancer Agents in Medicinal Chemistry, 2013, 13(7): 1002-1013.
[10] VANHAESEBROECK B, GUILLERMET-GUIBERT J, GRAUPERA M,et al. The emerging mechanisms of isoform-specifi c PI3K signaling[J]. Nature Reviews Molecular Cell Biology, 2010, 11(5): 329-341. DOI:10.1038/nrm2882.
[11] MILLER S, TAVSHANJIAN B, OLEKSY A, et al. Shaping development of autophagy inhibitors with the structure of the lipid kinase Vps34[J]. Science, 2010, 327: 1638-1642. DOI:10.1126/ science.1184429.
[12] KATSO R, OKKENHAUG K, AHMADI K, et al. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis and cancer[J]. Annual Review of Cell and Development Biology, 2001,17(1): 615-675. DOI:10.1146/annurev.cellbio.17.1.615.
[13] SONG G, OUYANG G L, BAO S D. The activation of Akt/PKB signaling pathway and cell survival[J]. Journal of Cellular and Molecular Medicine, 2005, 9(1): 59-71. DOI:10.1111/j.1582-4934.2005.tb00337.x.
[14] MAJCHRZAK A, WITKOWSKA M, SMOLEWSKI P. Inhibition of the PI3K/Akt/mTOR signaling pathway in diffuse large B-cell lymphoma: current knowledge and clinical signifi cance[J]. Molecules,2014, 19(9): 14304-14315. DOI:10.3390/molecules190914304.
[15] MAYER I A, ARTEAGAC L. The PI3K/Akt pathway as a target for cancer treatment[J]. Annual Review of Medicine, 2016, 67(1): 11-28. DOI:10.1146/annurev-med-062913-051343.
[16] LIU P X, CHENG H L, ROBERTS T M, et al. Targeting the phosphoinositide 3-kinase pathway in cancer[J]. Drug Discovery,2009, 8(8): 627-644. DOI:10.1038/nrd2926.
[17] BOSSE T, HAAR N T T, SEEBER L M, et al. Loss of ARID1 expression and its relationship with PI3K-Akt pathway alterations,TP53 and microsatellite instability in endometrial cancer[J]. Modern Pathology, 2013, 26(11): 1525-1535. DOI:10.1038/modpathol.2013.96.
[18] HAN G M, SIDHU D, DUGGAN M A, et al. Reproducibility of histological cell type in high-grade endometrial carcinoma[J]. Modern Pathology, 2013, 26(12): 1594-1604. DOI:10.1038/modpathol. 2013.102.
[19] ALLO G, BERNARDINI M Q, WU R C, et al. ARID1A loss correlates with mismatch repair deficiency and intact p53 expression in highgrade endometrial carcinomas[J]. Modern Pathology, 2014, 27(2): 255-261. DOI:10.1038/modpathol.2013.144.
[20] HUSSAIN A R, AHMED S O, AHMED M, et al. Cross-talk between NF-κB and the PI3-kinase/Akt pathway can be targeted in primary effusion lymphoma (PEL) cell lines for efficient apoptosis[J]. PLoS ONE, 2012, 7(6): e39945. DOI:10.1371/journal.pone.0039945.
[21] SHARRARD R M, MAITLAND N J. Regulation of protein kinase B activity by PTEN and SHIP2 in human prostate-derived cell lines[J]. Cell Signal, 2007, 19(1): 129-138. DOI:10.1016/j.cellsig.2006.05.029.
[22] AKCA H, DEMIRAY A, TOKGUN O, et al. Invasiveness and anchorage independent growth ability augmented by PTEN inactivation through the PI3K/Akt/NFκB pathway in lung cancer cells[J]. Lung Cancer, 2011, 3(3): 302-339. DOI:10.1016/j.lungcan.2011.01.012.
[23] ZHANG J, YU X H, YAN Y G, et al. PI3K/Akt signaling in osteosarcoma[J]. Clinica Chimica Acta, 2015, 444: 182-192. DOI:10.1016/j.cca.2014.12.041.
[24] WANG L, CAO X X, CHEN Q, et al. DIXDCl targets p21 and cyclin D1 via PI3K pathway activation to promote colon cancer cell proliferation[J]. Cancer Science, 2009, 100(10): 1801-1808. DOI:10.1111/j.1349-7006.2009.01246.x.
[25] XUE G D, RESTUCCIA D F, LAN Q, et al. Akt/PKB-mediated phosphorylation of twist1 promotes tumor metastasis via mediating cross-talk between PI3K/Akt and TGF-β signaling axes[J]. Cancer Discovery, 2012, 2(3): 248-259. DOI:10.1158/2159-8290.CD-11-0270.
[26] KANG M H, OH S C, LEE H J, et al. Metastatic function of BMP-2 in gastric cancer cells: the role of PI3K/Akt, MAPK, the NF-kappa B pathway and MMP-9 expression[J]. Experimental Cell Research, 2011,317(12): 1746-1762. DOI:10.1016/j.yexcr.2011.04.006.
[27] ZUO J H, ZHU W, LI M Y, et al. Activation of EGFR promotes squamous carcinoma SCC10A cell migration and invasion via inducing EMT-like phenotype change and MMP-9-mediated degradation of E-cadherin[J]. Journal of Cellular Biochemistry, 2011, 112(9): 2508-2517. DOI:10.1002/ jcb.23175.
[28] ZHANG H R, CHEN J M, ZENG Z Y, et al. Knockdown of DEPTOR inhibits cell proliferation and increases chemosensitivity to melphalan in human multiple myeloma RPMI-8226 cells via inhibiting PI3K/Akt activity[J]. Journal of International Medical Research, 2013, 41(3): 584-595. DOI:10.1177/0300060513480920.
[29] CAPORALI S, LEVATI L, GRAZIANI G, et al. NF-κB is activated in response to temozolomide in an Akt-dependent manner and confers protection against the growth suppressive effect of the drug[J]. Journal of Translational Medicine, 2012, 10(1): 252. DOI:10.1186/1479-5876-10-252.
[30] FANG X S, JIANG Y J, FENG L L, et al. Blockade of PI3K/Akt pathway enhances sensitivity of Raji cells to chemotherapy through down-regulation of HSP70[J]. Cancer Cell International, 2013, 13(1): 48-54. DOI:10.1186/1475-2867-13-48.
[31] SALVATORE C. Quercetin in cancer prevention and therapy[J]. Integrative Cancer Therapies, 2012, 12(2): 97-102. DOI:10.1177/1534735412448215.
[32] SUI T, MA L, BAI X, et al. Resveratrol inhibits the phosphatidylinositide 3-kinase/protein kinase B/mammalian target of rapamycin signaling pathway in the human chronic myeloid leukemia K562 cell line[J]. Oncology Letters, 2014, 7(6): 2093-2098. DOI:10.3892/ol.2014.2014.
[33] JIAO Y M, LI H, LIU Y D, et al. Resveratrol inhibits the invasion of glioblastoma-initiating cells via down-regulation of the PI3K/ Akt/NF-κB signaling pathway[J]. Nutrients, 2015, 7(6): 4383-4402. DOI:10.3390/nu7064383.
[34] ZHANG D Q, SUN P, JIN Q, et al. Resveratrol regulates activated hepatic stellate cells by modulating NF-κB and the PI3K/Akt signaling pathway[J]. Journal of Food Science, 2016, 81(1): 240-245. DOI:10.1111/1750-3841.13157.
[35] PAPLOMATA E, O’REGAN R. The PI3K/Akt/mTOR pathway in breast cancer: targets, trials and biomarkers[J]. Therapeutic Advances in Medical Oncology, 2014, 6(4): 154-166. DOI:10.1177/17588 34014530023.
[36] ZHANG H, XU W L, LI B L, et al. Curcumin promotes cell cycle arrest and inhibits survival of human renal cancer cells by negative modulation of the PI3K/Akt signaling pathway[J]. Cell Biochemistry and Biophysics, 2015, 73(3): 681-686. DOI:10.1007/s12013-015-0694-5.
[37] LIU H, LIU Y Z, ZHANG F, et al. Identi☒cation of potential pathways involved in the induction of cell cycle arrest and apoptosis by a new 4-arylidene curcumin analogue T63 in lung cancer cells: a comparative proteomic analysis[J]. Molecular Biosystems, 2014, 10(6): 1320-1331. DOI:10.1039/C3MB70553F.
[38] CHOY Y Y, FRAGA M, MACKENZIE G G, et al. The PI3K/Akt pathway is involved in procyanidin mediated suppression of human colorectal cancer cell growth[J]. Molecular Carcinogenesis, 2016. DOI:10.1002/mc.22461.
[39] KRESTY L A, WEH K M, ZEYZUS-JOHNS B, et al. Cranberry proanthocyanidins inhibit esophageal adenocarcinoma in vitro and in vivo through pleiotropic cell death induction and PI3K/AKT/mTOR inactivation[J]. Oncotarget, 2015, 6(32): 33438-33455. DOI:10.3892/ mmr.2014. 2459.
[40] PRASAD R, VAID M, KATIYAR S K. Grape proanthocyanidin inhibit pancreatic cancer cell growth in vitro and in vivo through induction of apoptosis and by targeting the PI3K/Akt pathway[J]. PLoS ONE, 2012, 7(8): 43064-43074. DOI:10.1371/journal.pone. 0043064.
[41] CHEN N G, LU C S, LIN Y H, et al. Proteomic approaches to study epigallocatechin gallate-provoked apoptosis of TSGH-8301 human urinary bladder carcinoma cells: roles of AKT and heat shock protein 27-modulated intrinsic apoptotic pathways[J]. Oncology Reports,2011, 26(4): 939-947. DOI:10.3892/or.2011.1377.
[42] ROOMI M W, MONTERREY J C, KALINOVSKY T, et al. Comparative effects of EGCG, green tea and a nutrient mixture on the patterns of MMP-2 and MMP-9 expression in cancer cell lines[J]. Oncology Reports, 2010, 24(3): 747-757. DOI:10.3892/or_00000917.
[43] ZHANG Y J, OWUSU L, DUAN I, et al. Anti-metastatic and differential effects on protein expression of epigallocatechin-3-gallate in HCCLM6 hepatocellular carcinoma cells[J]. International Journal of Molecular Medicine, 2013, 32(4): 959-964. DOI:10.3892/ ijmm.2013.1446.
[44] DOORN C C V, DOMINA A M. Epigallocatechin-3-gallate (EGCG)induces catalase-sensitive cell growth inhibition and AKT activation in burkitt lymphoma cells[J]. Molecular Cancer Therapeutics, 2013,12(Suppl 11): A178. DOI:10.1158/1535-7163.TARG-13-A178.
[45] SHEN X Y, ZHANG Y, FENG Y, et al. Epigallocatechin-3-gallate inhibits cell growth, induces apoptosis and causes S phase arrest in hepatocellular carcinoma by suppressing the Akt pathway[J]. International Journal of Oncology, 2014, 44(3): 791-796. DOI:10.3892/ijo.2014.2251.
[46] DUO J, YING G G, WANG G W, et al. Quercetin inhibits human breast cancer cell proliferation and induces apoptosis via Bcl-2 and Bax regulation[J]. Molecular Medicine Reports, 2012, 5(6): 1453-1456. DOI:10.3892/mmr.2012.845.
[47] MAURYA A K, VINAYAK M. Quercetin regresses dalton’s lymphoma growth via suppression of PI3K/AKT signaling leading to upregulation of p53 and decrease in energy metabolism[J]. Nutrition and Cancer, 2015, 67(2): 354-363. DOI:10.1080/01635581.2015. 990574.
[48] PAN H C, JIANG Q, YU Y, et al. Quercetin promotes cell apoptosis and inhibits the expression of MMP-9 and fibronectin via the AKT and ERK signalling pathways in human glioma cells[J]. Neurochemistry International, 2015, 80: 60-71. DOI:10.1016/j.neuint.2014.12.001.
[49] RAFIQ R A, QUADRI A, NAZIR L A, et al. A potent inhibitor of phosphoinositide 3-kinase (PI3K) and mitogen activated protein(MAP) kinase signaling, quercetin (3,3’,4’,5,7-pentahydroxyflavone)promotes cell death in ultraviolet (UV)-B-irradiated B16F10 melanoma cells[J]. PLoS ONE, 2015, 10(7): e0131253. DOI:10.1371/ journal.pone.0131253.
[50] XIANG T, FANG Y, WANG S X. Quercetin suppresses HeLa cells by blocking PI3K/Akt pathway[J]. Journal of Huazhong University of Science and Technology(Medical Science), 2014, 34(5): 740-744. DOI:10.1007/s11596-014-1345-6.
[51] WANG P W, PHAN T, GORDON D, et al. Arctigenin in combination with quercetin synergistically enhances the antiproliferative effect in prostate cancer cells[J]. Molecular Nutrition and Food Research, 2015,59(2): 250-261. DOI:10.1002/mnfr.201400558.
[52] DUAN R Z, TIAN F F, SUN J Y. Structural basis and energy landscape of apigenin-induced cancer cell apoptosis mechanism PI3K/Akt pathway[J]. Molecular Simulation, 2016, 42(2): 138-148. DOI:10.1080/08927022.2015.1021346.
[53] MENG Y, LIN Z M, GE N, et al. Ursolic acid induces apoptosis of prostate cancer cells viathe PI3K/Akt/mTOR pathway[J]. The American Journal of Chinese Medicine, 2015, 43(7): 1471-1486. DOI:10.1142/S0192415X15500834.
Plant Polyphenols Exert Anti-Tumor Activity by the PI3K/Akt Signaling Pathway: A Review
SHI Fang1, LIAO Xia1, LI Yao1, LI Yao1, XIAO Xingning1, WU Surui2, MING Jian1,2,3,*
(1. College of Food Science, Southwest University, Chongqing 400715, China; 2. Kunming Edible Fungi Institute, All China Federation of Supply and Marketing Cooperatives, Kunming 650223, China; 3. Chongqing Engineering Research Center of Regional Food, Chongqing 400715, China)
The phosphatidylinositol 3-kinase/serine/threonine kinase B (PI3K/Akt) signaling pathway is a classical signaling pathway that inhibits apoptosis and promotes proliferation. The PI3K/Akt pathway play an important role in cancer,cardiovascular disease, diabetes and nervous system diseases, especially in cancer. The abnormal activation of PI3K/Akt is closely related to the occurrence, invasion and metastasis of tumors. The research of this pathway has become the focus of the treatment of cancer at home and abroad. Plant polyphenols are becoming natural drugs to prevent cancer because of their anti-tumor effect. This review outlines the structure and activation mechanism of the PI3K/Akt signaling pathway and its relationship with tumor in order to provide scientific evidence for the development of plant polyphenols into health foods or drugs for cancer prevention.
phosphatidylinositol 3-kinase/serine/threonine kinase B (PI3K/Akt); signaling pathway; plant polyphenols; antitumor activity
10.7506/spkx1002-6630-201615044
Q964.8;R151.2
A
1002-6630(2016)15-0259-06
10.7506/spkx1002-6630-201615044. http://www.spkx.net.cn
2016-04-02
国家自然科学基金面上项目(31471576);重庆市社会民生科技创新专项(cstc2015shmszx80019);“十二五”国家科技支撑计划项目(2013BAD16B01)
石芳(1993—),女,硕士研究生,研究方向为食品化学与营养学。E-mail:1107982769@qq.com
明建(1972—),男,教授,博士,研究方向为食品化学与营养学。E-mail:mingjian1972@163.com
引文格式:
