Development of microfluidic devices for islet transplantation and islet physiologys
2016-11-12YuanXingKatherineXieManwanChanHevinPoonMaggieWangShusenWangMerigengQiYongWangDepartmentofSurgeryTransplantUniversityofIllinoisatChicagoChicagoIllinoisUSATheHonorsCollegeUniversityofIllinoisatChicagoChicagoIllinoisUSA
Yuan Xing,Katherine Xie,Manwan Chan,Hevin Poon,Maggie Wang,Shusen Wang,Merigeng Qi,Yong Wang(. Department of Surgery/Transplant,University of Illinois at Chicago,Chicago,Illinois,USA ;. The Honors College,University of Illinois at Chicago,Chicago,Illinois,USA ;. Department of organ transplantation,Key Laboratory for Critical Care Medicine of the Ministry of Health,Tianjin First Center Hospital,Tianjin 009,China ;.Department of Translational Research and Cellular Therapeutics,Diabetes and Metabolism Research Institute,Beckman Research Institute of City of Hope. Duarte,CA. USA.)
【Abstract】 This review discusses several microfluidic devices developed at the University of Illinois at Chicago(UIC)used for studying the physiology and pathophysiology of human islets and their applications in the human islet transplantation process. The review first introduces key issues found in the field of pancreatic islet transplantation as a clinical therapy for Type I diabetes. It then reviews microfluidic technologies that can be used to address those key issues, the unique features associated with each microfluidic device, and the application of each.Additionally, the review also briefly discusses the design and fabrication principles of UIC microfluidic devices.
【Key words】 Microfluidics;Pancreatic islet of langerhans;Islet perifusion ;Islet physiology;Human islet transplantation
Introduction
The pancreatic islets of Langerhans are made up of at least five different cell types. Approximately 65%-80% of the cells in the islet are beta-cells that secret insulin for maintaining glucose hemostasis.Insulin release displays biphasic and pulsatile profiles in response to glucose. Glucose first enters beta-cells via GLUT2. Glycolysis then generates pyruvate,which enters the TCA cycle and produces ATP,subsequently closing the ATP-sensitive K+(KATP)channels. This initiates plasma membrane depolarization and causes an increase in intracellular calcium concentration([Ca2+]i)via voltage-dependent calcium channels(VDCCs). Finally,the glucose-induced [Ca2+]i triggers the fusion of insulin granules to plasma membrane,resulting in the exocytosis of insulin1-4.The first phase of the biphasic profile corresponds to a prompt,marked insulin secretion(4-8 min). With continuous glucose stimulation,a secondary phase consisting of a gradual insulin increase is observed5-6.Alternate pathways of glucose-stimulated insulin secretion,independent of either KATP channels or[Ca2+]i,have been described7-8,but play a smaller role in insulin secretion.
Since the introduction of the Edmonton Protocol,human islet transplantation has emerged as a promising therapy for Type I diabetes mellitus(T1DM)and is currently the only cell-based therapy that can achieve tight glycemic control without insulin9-12.The advantage in islet transplantation is the ability of transplanted islets to maintain and/or regulate insulin secretion under a normal blood glucose range. Islet transplantation also has many advantages over whole pancreas transplantation,since it involves only a minor surgical procedure with much lower morbidity and mortality rates.
Islet transplantation has shown varying degrees of success in instances of both short-term and long-term insulin independence10-13,and much of this variability is associated with natural differences in∶the organ donor,pancreas procurement/preservation process,islet isolation process,the microenvironment of the islet transplant site(lower oxygen tension and delayed revascularization),beta-cell immunotoxicity,and long-term islet graft exhaustion. The U.S. Food and Drug Administration(FDA)define the islet product as a biological drug ;therefore,islets have to be prepared under FDA-approved guidelines for clinical therapy. Despite standardization of cGMP,lot-to-lot variability still cannot be avoided. To reduce the risk of transplanting low quality islets,appropriate product release tests are needed. While tests for identity,sterility,and purity are well established,to date there exists no reliable method to assess islet potency. This continues to be a key hurdle associated with variable clinical outcomes.
Standard assays to assess islet function and viability include static glucose-stimulated insulin secretion(GSIS)for potency and inclusive and exclusive dyes for viability. Both assays have low correlation with transplant outcomes14-18. The GSIS only measures“bulk” insulin release and consequently,fails to quantify the dynamic nature of beta-cell insulin secretory kinetics;in addition,it fails to provide useful information regarding key insulin stimulator-secretion coupling factors. Clinicians depend more on islet cell mass(IEq)to determine the suitability of an islet cell preparation for transplantation. Since the isolated islets depend on passive diffusion to sense ambient glucose changes16,19,smaller islets often exhibit better GSIS results20-21. Paradoxically,larger islets often contribute to a higher IEq. On the other hand,an in vivo potency assay conducted by transplanting human islets into immunodeficient nude mice has high correlation with clinical transplant outcomes. However,it takes several weeks to complete and therefore,only provides a retrospective indication of islet function,rendering this assay impractical as a pre-transplant assessment22-25.
To address this problem,a variety of in vitro assays have been investigated including the measurement of the oxygen consumption rate(OCR)16-17,26-28,ROS18,and ADP/ATP ratios29-30. The advantages and disadvantages of those assays have been well documented and their predictions of in vivo islet function remain controversial.
Microfluidic and applicationin the field of diabetes
Microfluidic technology is a special class of Biomicroelectromechanical systems(Bio-MEMS). This technology has emerged as a valuable tool for a wide range of biological applications. The small-scaled nature of microfluidic devices allows for leveraging of microscale phenomena such as laminar flow and rapid diffusion31-32,while consuming minimal amounts of reagents and analysts. More importantly,microfluidics permits easier implementation of new experimental modalities currently not possible with tools on the macroscale. In addition,multiple tasks can be integrated onto a single device to improve experimental throughput33-34. Almost 10 000 microfluidics papers have been published over the last 20 years,and the number of new publications continues to increase annually. The general advantages of microfluidic tools in biological research have been reviewed elsewhere.To date,the application of microfluidics in islet and/or beta-cell studies are very limited,as only a handful of laboratories have pursued research of this nature.Their contributions have been reviewed elsewhere35.In the scope of this review,we will focus specifically on the current state of microfluidics developed in our laboratory and their applications.
The principle of microfluidic device design and fabrication
The detailed review on microfluidic design and fabrication for islet study have been described elsewhere36-37. In general,microfluidic devices are designed using computer-aided software,such as AutoCAD,and printed on a high-resolution(16 000 dpi)transparency film that is then used as a photomask to selectively crosslink photoresist(SU-8)pre-spun to a desired thickness on a silicon wafer. Once coated,the wafers undergo pre-exposure baking at 65°C for 5 min,90°C for 2 hrs,and 65°C for 1 min. Finally,the high-resolution photomask is placed in contact with the resist-covered wafer and irradiated with 365 nm filtered UV light to initiate crosslinking. The irradiated water is further cross-linked on a hotplate at 95°C for 1 hr before the wafer is placed in a developer solution in which any unpolymerized SU-8 is dissolved.The completed master is then cleaned with isopropyl alcohol and water,dried with compressed N2gas,and dehydration baked. After completing the master,PDMS(polydimethylsiloxane)is prepared for the molding,or the soft-lithography process. PDMS precursor(usually Sylgard 184)and cross-linking agent is added to a weighing boat in a 10∶1 ratio by mass and thoroughly mixed. A vacuum chamber is then used to extract bubbles,after which the PDMS mixture is poured over the SU8 master. After overnight curing at room temperature or 2 hours on an 85℃ hotplate,the PDMS is completely cured and retains the channel structures from the master. The bulk PDMS is then cut into separate devices,inlet and outlet ports are punched,and then the PDMS layers are bonded through plasma treatment to finalize the working device.
Microfluidic application for studying islet physiology and pathophysiology
Microfluidic islet perifusion biochip
The device is composed of three layers of PDMS36-37.The top layer(500 μm in height)has an inlet and an outlet channel(2 mm wide),the middle layer is a perifusion chamber(7 μm in diameter and 3 μm in depth),And the bottom layer(150 μm in height)contains microwells for islet immobilization,which are 100 μm apart and each 500 μm in diameter(Figure 1A).The unique features associated the device are∶① capability of multiple islets immobilization. The pocket design allows islets to passively sit without islet fixation or dissociation. ② creation of uniformed flow mixing and dynamics. As shown in Figure 1B,a CFD-GEOM computer simulation demonstrates uniform flow distribution in the perifusion chamber with most of the flow reaching bottom where islets are trapped in microwells without significant fluid shunting.③ capability of generating and maintaining various chemical gradients with a high level of complexity and consistency(Figure 2). ④ in addition to serving as islet perifusion,fluorescence-based analytical approaches were integrated that significantly increase its analytical power with good spatiotemporal resolution of the measured parameters(Figure 3).

Figure 1 Microfluidic islet perifusion biochip and flow dynamics.(A)Islet perifusion biochip.(B)FITC computer simulation of flow dynamics

Figure 2 The creation of various glucose temporal gradients in the microfluidic network vs. expected values.(A)Symmetric-bell shape at range of 2-14 mmol/L.(B)Square-shape of 5-14 mmol/L glucose.(C)Linear 5-25 mmol/L glucose.
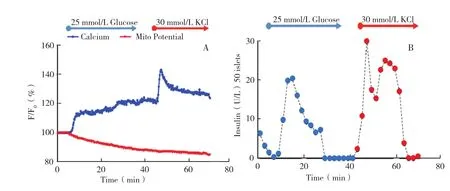
Figure 3 Human islet response to glucose and high potassium stimulation( A)A representative of traces of calcium influx and mitochondrial potential changes of human islets in response to 25 mmol/L glucose and 30 mmol/L potassium chloride.( B)A representative of trace of insulin secretion profile of 50 human islets in response to 25 mmol/L glucose and 30 mmol/L potassium chloride.
In UIC,we have used the aforementioned microfluidic biochip to thoroughly evaluate 150 human islet preparations. We have shown that the microfluidic parameters(calcium influx,mitochondrial potentials,and insulin secretion kinetics)can provide better predictive values for in vivo islet graft function and viability in the nude mouse transplant model(data now shown). Further clinical evaluation for predicting human islet in vivo graft function is under investigation.
Microfluidic Islet Array
Two major challenges presented by previous devices are the limitations in the number of islets that can be assessed(around 50 islets)and the inability to assess the heterogeneous property of individual islets.Examination of heterogeneous islet properties often provides more detailed physiological information than when solely utilizing averaging-based methodologies.For example,it enables a better understanding of human islet functionality,which in turn provides a better predictive value for the islet transplantation outcome. With this goal in mind,we developed a new microfluidic islet array based on a hydrodynamic trapping mechanism.
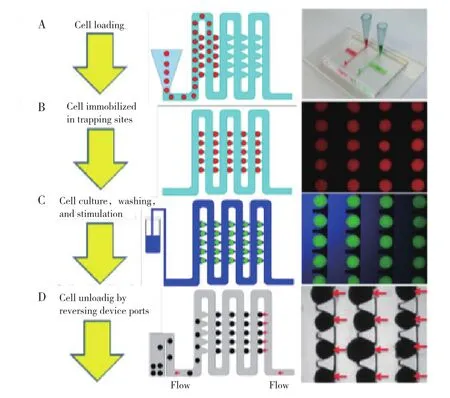
Figure 4 Islet loading, stimulation,and retrieval.(A)Schematics of islet loading and a photo image of the islet array.(B)Schematic of islet trapping and trapped fluorescence beads(200-240 μm).(C)Schematic of islet stimulation and trapped fluorescence beads(200-240 μm).(D)Schematic of islet retrieval and human islets
The microfluidic platform we developed is a onelayer PDMS device as shown in Figure 4. The array device utilizes the hydrodynamic trapping principle38to immobilize islets. The device contains an array consisting of two rows and ten columns. In each column,there exist a total of 300 traps. The site of the trap is a U-shaped pocket(250 μm in diameter and 275 μm in depth),superimposed onto a loop channel that is used for the delivery of fluids and islets. There is also a cross-flow channel(45 μm in width)at the apex of the U- pocket. Based on differences in flow rate between the U-shaped pocket and the loop channel,the flow encounters less resistance in the unoccupied U-shaped pocket. When an islet in solution flows proximal to the gap between the U-shaped pocket and the main channel,it becomes trapped in the U-shaped pocket due to the difference in flow resistance between the U-shaped pocket and the loop channel. The newly trapped islet causes increased resistance in the U-shaped pocket,directing the flow back into the loop channel. The COMSOL fluid-flow simulation results depict this phenomenon in Figure 5-specifically,they show that the Q1 velocity is significantly larger than the Q2 velocity once the leading islet is trapped in the U-cup pocket.

Figure 5 Computer simulation of flow stream and velocity profiles with and without particles trapping
We further analyzed the impact of varying geometries on trapping efficacy. Our results show that when Q1/Q2 =5.5,the resistance ratio is high and multiple islets are trapped per site(Figure 6A,Table 1). When Q1/Q2=0.7,the solution going into the gap exhibits a resistance too low for optimal loading(Figure 6C,Table 1). By modifying the resistance of the straight channel(Q1/Q2=2.8),we achieved individual islet occupancy in each U-shaped pocket trap(Figure 6B,Table 1). We found that at the ratio of 2.8,(99.0±2.5)% of the sites in our microfluidic device are filled;with(95±2)% of the filled sites containing only one individual islet.
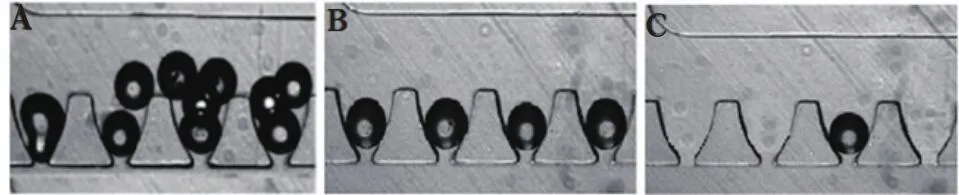
Figure 6 Determination of optimal loading parameter.(A)Loading efficacy at Q1/Q2=5.5.(B)Loading efficacy at Q1/Q2 =2.8.(C)Loading efficacy at Q1/Q2=0.7

Table 1 Optimal loading parameter
Used in combination with real-time fluorescence imaging,the array can be used for islet flow cytometry to track the dynamic physiological and pathophysiological behavior of individual islets. The heterogeneous responses of intracellular calcium and mitochondrial potential changes from isolated human islets are shown in Figures 7A and 7B. Human islets displayed heterogeneous calcium profiles in response to glucose and KCI 〔25 mmol/L glucose ∶(144.20±1.74)%,Max∶149.9%,Min∶139.9% ∶30 mmol/L KCI∶( 176.20±1.54)%,Ma∶179.1%,Min∶173.5%〕 and heterogeneous mitochondrial potential changes in response to glucose〔 25 mmol/L glucose∶(71.30±2.02)%,Max∶76.8%,Min∶62.3%〕.
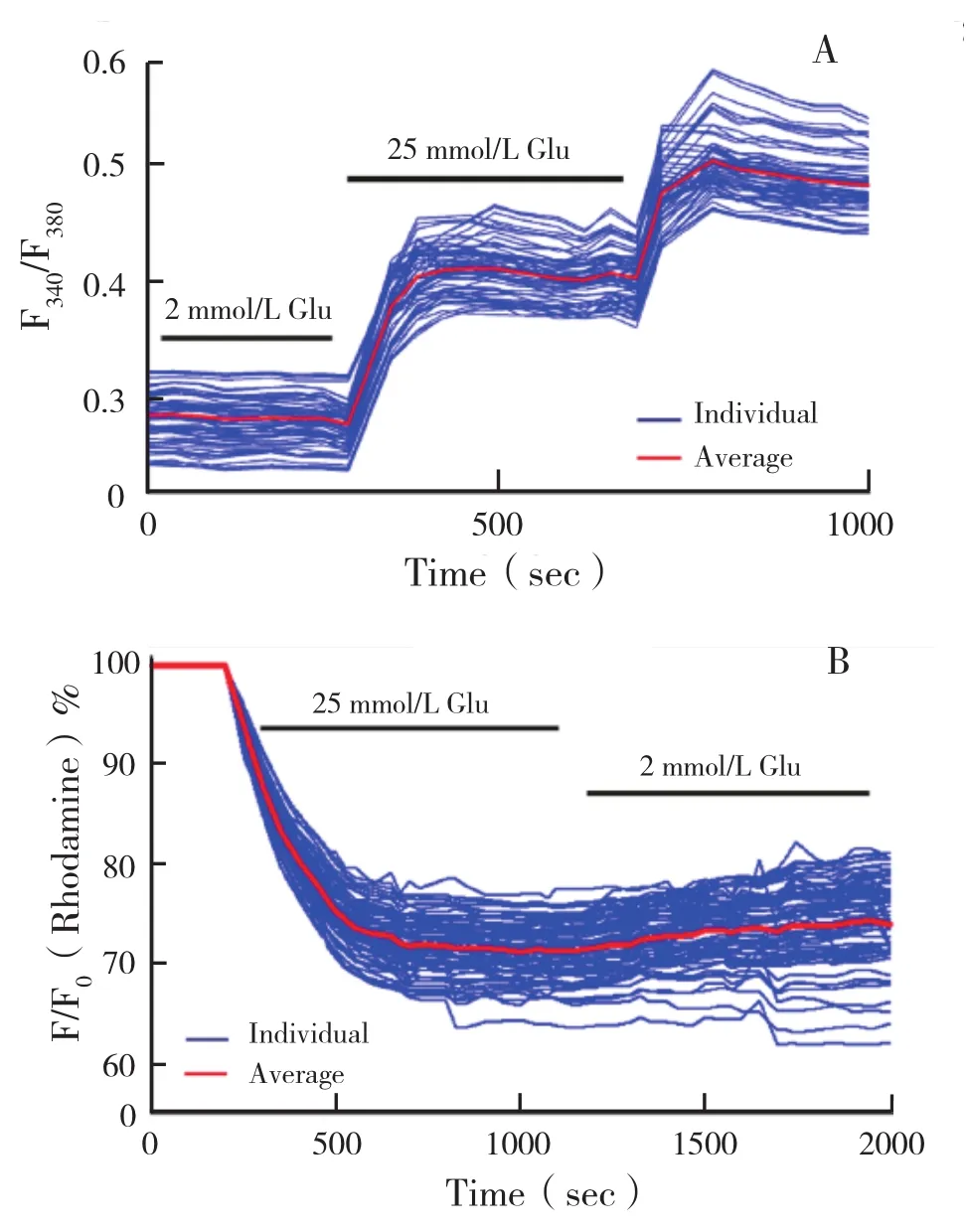
Figure 7 Heterogeneous responses of human islet in response to insulin secretion secretagogues.( A)Intracellular calcium signaling of islets to 25 mmol/L glucose and 30 mmol/L KCI(n=100 islets).( B)Mitochondrial potential changes of human islets in response to 25 mmol/L glucose( n=100 islets)
Microfluidic Encapsulated Islet Array
Islet transplantation provides tight glycemic control for those with Type I diabetes;however,the transplant recipient is required to take immunosuppressant,which inevitably has unwanted side effects on both the patient and the islet graft. To avoid the need for immunosuppression,the immunoisolation of islets in biocompatible microcapsules has been investigated. If successful,this strategy would play a significant role in islet transplantation outcomes. Despite initial promising results obtained in both small animal and nonhuman primate transplant models,only short-term and partial graft function has been achieved in clinical trials39-40.Several factors have been proposed to explain possible factors causing graft loss∶insufficient biocompatibility of the encapsulation material,limited immunoprotective properties,hypoxic environment,and suboptimal insulin release40-41. While research has focused heavily on biocompatibility of materials and immunoprotection of islets,a comprehensive understanding of the physiological changes found in microencapsulated islets is often constrained due to the availability of research tools.To combat this challenge,we developed a three-layer microfluidic array to assess microencapsulated islets based on the same hydrodynamic trapping principle(Figure 8)41. In addition to the aforementioned design,we integrated an oxygenation channel to study the pathophysiological changes under hypoxia,one factor that was proposed to contribute to the graft failure of microencapsulated islets. Oxygenation efficacy in the microfluidic channel was evaluated through both gaseous(diffused)and aqueous(dissolved)states(Figure 9). Through diffusion into the channel,as shown in Figure 9A and 9C,the device was capable of creating and maintaining the targeted oxygen concentrations with high consistency. When using the cyclic oxygenation protocol( 21%5%21%),the time needed to switch from a 21% oxygen concentration to a 5% oxygen concentration was less than 40 seconds,as was the reversion back to 21% from 5%. Equally as important,both concentrations were well maintained over time 〔(21.21±0.05)% and(6.22±0.03)%〕.Similarly,the time needed to change from one oxygen concentration to another in a step-down protocol was also less than 40 seconds and again,was well-maintained over time〔(21.33±0.04)%,(11.53±0.05)%,(6.33±0.02)%,and(1.77±0.02)%,respectively〕.When utilizing oxygen in its dissolved state as shown in Figure 9C and 9D,the time needed to switch from 21% 〔(22.07±0.13)%〕 oxygen concentration to 5% 〔(6.83±0.08)%〕 oxygen concentration was approximately 120 seconds,approximately three times as long as that we observed when oxygen was diffused through the channel. The time needed to change from one particular oxygen concentration to another in the step-down protocol was less than 120 seconds and was well maintained over time〔(20.87±0.06)%,( 11.63±0.03)%,( 6.45±0.03)%,and( 1.68±0.03)%,respectively〕.
As explained before,hypoxia is widely considered to be another one of the primary factors associated with the loss of function in encapsulated islets and has also been linked to the failure of immunoisolation in microencapsulation. Isolated islets are exposed to hypoxic environments at many levels∶① isolated islets exhibit a disrupted vascular network and depend on diffusion for their oxygen supply,② the microencapsulation process further aggravates islet hypoxia through both preventing normal islet revascularization and increasing oxygen diffusion distances when microcapsule sizes are larger than 500 μm,and ③ the intraperitoneal space,a common transplant site for microencapsulated islets,has low oxygen tension,in which the O2concentration is approximately 3.5%-5% O2-levels significantly lower than those of the in situ pancreas. In addition to function loss,hypoxia may also attract macrophages and subsequently,cause fibrotic cell overgrowth on the surface of the microcapsule.

Figure 8 Schematics and photoimage of the three-layer microfluidic array.(A)Schematics of the microfluidic array and structure dimension.(B)Photoimage of the microfluidic array.(C)Photoimage of trapped encapsulated human islets

Figure 9 Characterization of device oxygenation in the microfluidic islet trapping array.(A and B)Wall-shape profiles of diffused oxygen and dissolved oxygen.(C and D)Step-down profiles of diffused oxygen and dissolved oxygen.

Figure 10 Hypoxia impaired [Ca2+]i signaling of microencapsulated human islets.(A)Representative trace of [Ca2+]i of microencapsulated human islets in response to 25 mmol/L glucose under varying hypoxic concentrations.(B)Statistics of[Ca2+]i changes under hypoxic concentration(n=65 from three experiments.aP<0.05)

Figure 11 Hypoxia impaired m changes of microencapsulated human islets.(A)Representative traces of Ψm changes of microencapsulated human islets in response to 25 mmol/L glucose under varying hypoxic concentrations.(B)Statistics of Ψm changes under varying hypoxic concentration(n=65 from three experiments. aP< 0.05)
As shown in Figures 10A and 10B,the changes in calcium influx of the microencapsulated human islets in response to the 25 mmol/L glucose stimulation were dependent on oxygen concentration and consequently,were inhibited by hypoxia. Under normoxia,the average intracellular calcium concentration increased by(10.00±4.16)% in response to the 25 mmol/L glucose stimulation,while hypoxic concentrations decreased intracellular calcium responses to the same stimulation∶(8.19±2.50)% in 10% O2,(3.57±1.18)% in 5% O2,and(1.70±0.64)% in 1% O2.(P < 0.01 when 21%vs. 5% and 1%,as well as P<0.01 when 10% vs.5% and 1%). Similarly,changes in mitochondrial potential,often used as an indicator of cellular energetic status,were also inhibited in an oxygen concentration-dependent manne(r 21%10%5%1%)∶(17.23±3.13)%,(8.83 ±3.53)%,(6.40±2.56)%,and(4.09±1.37)%(P<0.01 when comparing 21%vs. 10%,5% and 1%;P<0.01 when comparing 10%vs. 5% and 1%)as shown in Figure 11A and 11B.This device achieved a high trapping efficacy for microencapsulated islets 〔(99±2)%〕with minimal physical stress on islets. The integration of gas modulation allowed for rapid membrane-diffused oxygenation of islets at the microscale-level. This provided a practical tool useful for studying hypoxia in microencapsulated islets. This is the first report on real-time multiparametric imaging of metabolic changes of microencapsulated islets under hypoxia,a feat previously unachievable using either large hypoxic chambers or existing microfluidic devices. In the future,this device may be used to improve the longterm function and viability of microencapsulated islets prior to transplantation. Specifically,we can consider having encapsulated islets undergo intermittent hypoxia preconditioning(IH)or chemical preconditioning In a previous study,we applied IH preconditioning(1 min/1 min 5-21% cycling for 1 hour)and successfully diminished hypoxic injury in naked islets and improved naked islet insulin secretion42.Additionally,this array-based study laid out the groundwork for developing future assessments in the area of islet microencapsulation and may potentially act as a screening tool for therapeutic agents.In conclusion,this mini review summarized three different microfluidic devices developed in our laboratory used for studying human islet physiology.Microfluidic technology has a potential application in the field of diabetes research and treatment.Future research should focus on the development of microfluidic devices that can be more easily utilized in research laboratories. Specifically,efforts should be directed towards developing pumpless devices and further integrating analytical tools such as electrical components,online hormone assays,and smartphone technologies.
