石山巴豆枝叶的化学成分研究
2016-11-11夏梦雯宁德生黄思思吕仕洪潘争红
夏梦雯 , 宁德生, 黄思思, 程 玲, 吕仕洪,罗 蕾, 潘争红*
( 1. 广西植物功能物质研究与利用重点实验室,广西壮族自治区中国科学院 广西植物研究所, 广西 桂林 541006;2. 云南师范大学 化学化工学院,昆明 650500 )
石山巴豆枝叶的化学成分研究
夏梦雯1, 2, 宁德生1, 黄思思1, 程玲1, 2, 吕仕洪1,罗蕾2, 潘争红1*
( 1. 广西植物功能物质研究与利用重点实验室,广西壮族自治区中国科学院 广西植物研究所, 广西 桂林 541006;2. 云南师范大学 化学化工学院,昆明 650500 )
石山巴豆(Crotoneuryphyllus)为大戟科(Euphorbiaceae)巴豆属(Croton)植物,主要分布于西南各省的岩溶石山地区,民间用于杀虫和治疗跌打损伤。为了阐明其化学物质基础,该研究采用硅胶柱层析、Sephadex LH-20、HPLC等方法对石山巴豆枝叶醇提物进行分离纯化。结果表明:共分离得到16个化合物,分别鉴定为异毛叶巴豆萜 (1), jatrophoidin (2), 山藿香定 (3), 异山藿香素 (4), 山藿香素 (5), 赖百当-13-烯-8,15-二醇 (6), 7-酮基-β-谷甾醇 (7), (22E)-5α,8β-表二氧麦角甾-6,22-二烯-3β-醇 (8), 豆甾烷-4-烯-6β-醇-3-酮 (9), 齐墩果烷-12-烯-2α,3β-二醇 (10), 东莨菪内酯 (11), 催吐萝芙木醇 (12), lyratol F (13), 罗布麻酚A (14), 芹菜素 (15), 金色酰胺醇酯 (16)。所有化合物均为首次从该植物中分离得到,其中化合物8-16为首次从巴豆属中发现。
石山巴豆, 化学成分, 二萜, 甾醇, 结构鉴定
石山巴豆(Crotoneuryphyllus)为大戟科(Euphorbiaceae)巴豆属(Croton)植物,主要分布于我国西南各省,其中在广西石山地区十分常见。种子供药用,其性味辛、热,有大毒,作峻泻药,外用于恶疮、疥廯等;根、叶入药,治风湿骨痛等;民间用枝、叶作杀虫药或毒鱼等(China Flora Editorial Board, 1996)。吴新安等(2004)研究表明二萜及其内酯类、酚类是该属植物的主要化学成分,其中二萜化合物最常见,为该属植物抗癌、抗炎、杀虫等活性的有效成分。迄今为止关于石山巴豆化学成分和药理活性的研究尚未见报道。在前期的研究中,我们发现石山巴豆枝叶的粗提物有明显的抗癌作用。为了填补该植物的化学研究空白,同时为该植物的药用提供理论基础,本研究对石山巴豆枝叶的化学成分进行了研究,共分离鉴定了16个化合物,结构类型涉及萜类(单萜、二萜、三萜)、甾醇、黄酮、香豆素、酰胺等,其中化合物8-16为首次从巴豆属中发现。
1 材料与方法
1.1 材料与仪器
石山巴豆(Crotoneuryphyllus)采自广西平果县,由广西植物研究所吕仕洪副研究员鉴定并保存于广西植物功能物质研究与利用重点实验室。
硅胶(100~200目、200~300目)及TLC检测用的硅胶GF254(青岛海洋化工厂);BS110S赛多利斯电子天平(北京赛多利斯天平有限公司);Agilent 1200半制备型高效液相色谱仪;LC/MS-IT-TOF system (Shimadzu, Tokyo, Japan);瑞士Bruker AVANCE Ⅲ HD-500 MHz 超导核磁共振仪;所有试剂均为分析纯。
1.2 提取与分离
干燥的石山巴豆枝叶15 kg,用95%的乙醇浸提3次,将提取液浓缩至无醇味,将浸膏分散于水中,分别用石油醚,乙酸乙酯,正丁醇萃取,得到3个部分。乙酸乙酯部分(400 g)经硅胶柱层析(100~200目),用石油醚-乙酸乙酯(25∶1~0∶1)梯度洗脱,得到5个组分Fr. B1~Fr. B5。Fr. B2经过反复硅胶柱层析(200~300目,石油醚-乙酸乙酯9∶1~0∶1梯度洗脱),分别得到化合物6(5 mg)、7(50 mg)、8(35 mg)、9(35 mg)和10(15 mg)。Fr. B3经重结晶得到化合物1(115 mg),母液经过反复硅胶柱(200~300目,石油醚-丙酮9∶1~0∶1梯度洗脱)和凝胶Sephadex LH-20层析 (氯仿-甲醇, 1∶1),分别得到化合物2(8 mg)、3(13 mg)、4(15 mg)、5(20 mg)和16(95 mg)。Fr. B4经过硅胶柱(200~300目),石油醚-丙酮(9∶1~0∶1)梯度洗脱后,重结晶得到化合物15(55 mg),母液经HPLC半制备(乙腈/水,50%~65%,v/v)纯化,分别得到化合物11(12 mg)、12(20 mg)、13(30 mg)和14(8 mg)。
2 结构鉴定
化合物1ESI-MS (m/z) 327 [M+H]+,分子式为C19H18O5。1H NMR (500 MHz, CDCl3)δ: 4.89 (1H, m, H-6), 2.24 (1H, m, H-10), 5.02 (1H, t,J= 1.5 Hz, H-11), 5.17 (1H, s, H-14), 1.14 (3H, d,J= 4.0 Hz, Me-17);13C NMR (125 MHz, CDCl3)δ: 23.8 (C-1), 21.1 (C-2), 20.1 (C-3), 127.7 (C-4), 162.3 (C-5), 76.4 (C-6), 35.5 (C-7), 36.6 (C-8), 57.7 (C-9), 37.0 (C-10), 103.8 (C-11), 147.1 (C-12), 115.5 (C-13), 107.2 (C-14), 144.5 (C-15), 141.5 (C-16), 13.7 (C-17), 172.6 (C-18), 177.4 (C-20)。以上数据与Chatterjee et al(1978)的报道一致,故鉴定为异毛叶巴豆萜 (isocrotocaudin)。
化合物2ESI-MS (m/z) 409 [M+Na]+,分子式为C21H22O7。1H NMR (500 MHz, CDCl3)δ: 5.25 (1H, m, H-6), 2.72 (1H, m, H-1), 2.66 (1H, m, H-11), 2.44 (1H, m, H-8), 5.43 (1H, m, H-12), 6.37 (1H, m, H-14), 7.26 (1H, s, H-15), 7.45 (1H, s, H-16), 3.80 (3H, s, COOCH3), 1.01 (3H, d,J= 5.5 Hz, Me-17);13C NMR (125 MHz, CDCl3)δ: 29.3 (C-1), 20.8 (C-2), 20.0 (C-3), 129.5 (C-4), 158.0 (C-5), 78.3 (C-6), 35.1 (C-7), 33.9 (C-8), 52.5 (C-9), 56.4 (C-10), 37.1 (C-11), 72.0 (C-12), 124.6 (C-13), 108.0 (C-14), 144.4 (C-15), 139.7 (C-16), 17.8 (C-17), 172.5 (C-18), 170.8 (C-19), 174.7 (C-20), 53.3 (COOMe)。以上数据与Mbwambo et al(2009)的报道一致,故鉴定为jatrophoidin。
化合物3ESI-MS (m/z) 351 [M+Na]+,分子式为C19H20O5。1H NMR (500 MHz, CDCl3)δ: 2.04 (1H, m, H-1α), 2.00 (1H, m, H-2α), 2.35 (2H, m, H-3β, 7β), 5.01 (1H, m, H-6α), 3.27 (1H, m, H-10α), 2.60 (1H, m, H-11B), 5.36 (1H, t,J=7.0, 13.5 Hz, H-12), 6.35 (1H, t,J= 2.0 Hz, H-14), 7.43 (1H, s, H-15), 7.44 (1H, s,H-16), 1.35 (3H, d,J= 6.0 Hz, Me-17);13C NMR (125 MHz, CDCl3)δ: 23.5 (C-1), 21.5 (C-2), 20.2 (C-3), 127.9 (C-4), 162.3 (C-5), 76.2 (C-6), 35.8 (C-7), 38.8 (C-8), 52.2 (C-9), 35.9 (C-10), 39.1 (C-11), 72.0 (C-12), 125.4 (C-13), 108.0 (C-14), 144.5 (C-15), 139.6 (C-16), 14.4 (C-17), 172.7 (C-18), 177.7 (C-20)。以上数据与Rodriguez et al(2004)的报道一致,故鉴定为山藿香定 (teucvidin)。
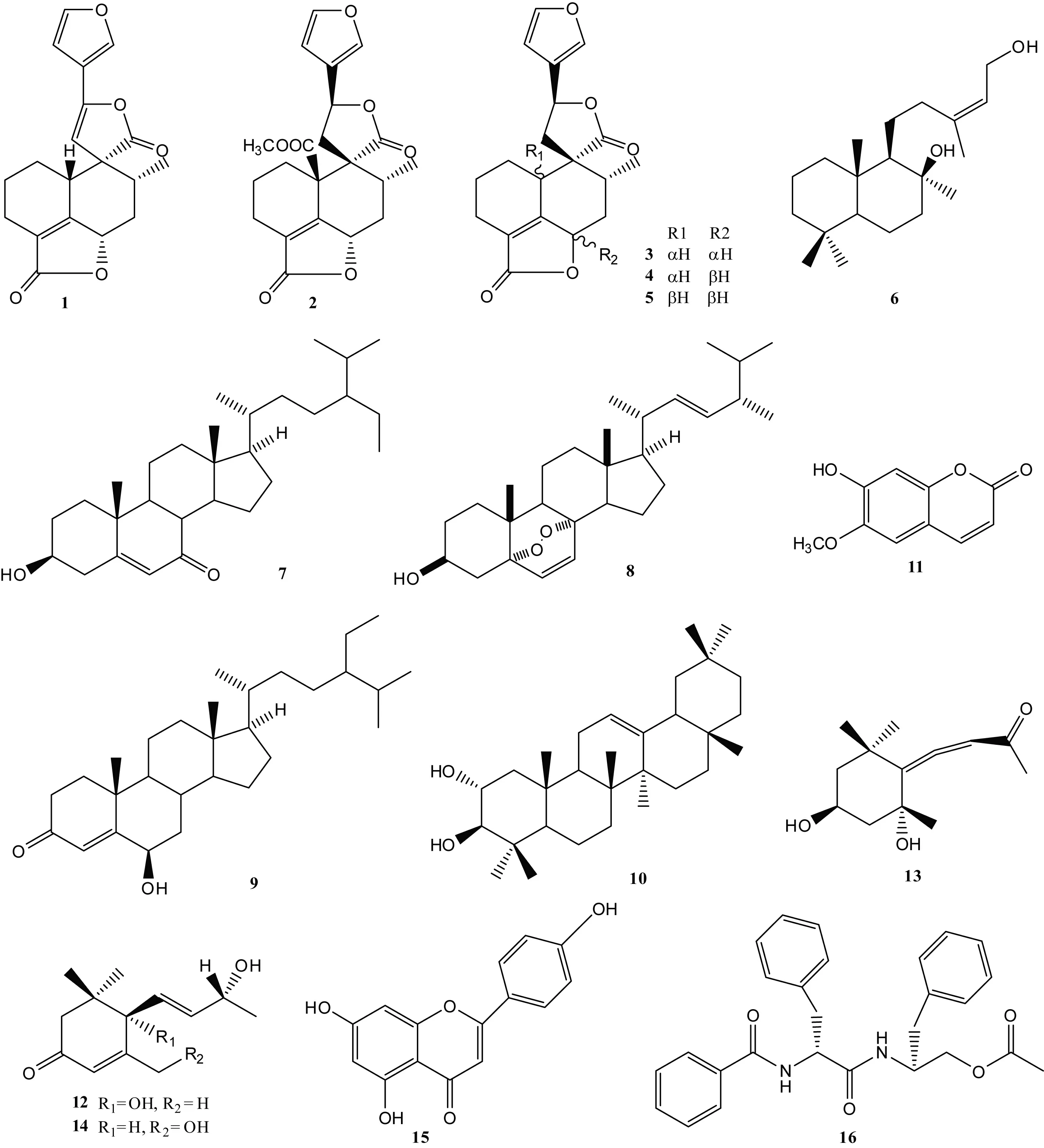
图 1 化合物1-16的结构图Fig. 1 Structure of compounds 1-16
化合物4ESI-MS (m/z) 351 [M+Na]+,分子式为C19H20O5。1H NMR (500 MHz, CDCl3)δ: 4.82 (1H, d,J= 11.0 Hz, H-6), 2.93 (1H, m, H-10), 2.71 (1H, m, H-11), 5.49 (1H, d,J= 7.0 Hz, H-12), 6.35 (1H, s, H-14), 7.43 (1H, s, H-15), 7.46 (1H, s, H-16), 1.08 (3H, d,J= 5.5 Hz, Me-17);13C NMR (125 MHz, CDCl3)δ: 23.1 (C-1), 22.5 (C-2), 19.2 (C-3), 125.2 (C-4), 166.1 (C-5), 77.0 (C-6), 34.2 (C-7), 39.0 (C-8), 47.3 (C-9), 37.8 (C-10), 40.7 (C-11), 71.8 (C-12), 126.2 (C-13), 107.9 (C-14), 144.7 (C-15), 138.9 (C-16), 19.2 (C-17), 173.1 (C-18), 176.9 (C-20)。以上数据与Mbwambo et al(2009)的报道一致,故鉴定为异山藿香素 (isoteucvin)。
化合物5ESI-MS (m/z) 351 [M+Na]+,分子式为C19H20O5。1H NMR (500 MHz, CDCl3)δ: 4.77 (1H, m, H-6β), 2.69 (1H, m, H-10β), 2.56 (2H, m, H-11A, 11B), 5.46 (1H, t,J= 7.0, 15.0 Hz, H-12), 6.39 (1H, s, H-14), 7.44 (1H, s, H-15), 7.46 (1H, s, H-16), 1.07 (3H, d,J= 6.0 Hz, Me-17);13C NMR (125 MHz, CDCl3)δ: 24.9 (C-1), 21.8 (C-2), 19.7 (C-3), 126.6 (C-4), 161.7 (C-5), 78.4 (C-6), 35.4 (C-7), 36.0 (C-8), 53.6 (C-9), 42.2 (C-10), 41.0 (C-11), 71.9 (C-12), 125.0 (C-13), 108.1 (C-14), 144.4 (C-15), 139.7 (C-16), 17.1 (C-17), 173.2 (C-18), 175.8 (C-20)。以上数据与Rodriguez et al(2004)的报道一致,故鉴定为山藿香素 (teucvin)。
化合物6ESI-MS (m/z) 309 [M+H]+,分子式为C20H36O2。1H NMR (500 MHz, CDCl3)δ: 5.45 (1H, d,J= 4.5 Hz, H-14), 1.66 (3H, s, H3-16), 1.36 (3H, s, H3-17), 0.93 (3H, s, H3-18), 0.86 (3H, s, H3-19), 1.06 (3H, s, H3-20);13C NMR (125 MHz, CDCl3)δ: 39.4 (C-1), 16.6 (C-2), 42.2 (C-3), 33.4 (C-4), 56.1 (C-5), 18.5 (C-6), 42.4 (C-7), 73.4 (C-8), 59.0 (C-9), 39.1 (C-10), 18.4 (C-11), 43.5 (C-12), 140.6 (C-13), 123.3 (C-14), 59.6 (C-15), 24.1 (C-16), 30.4 (C-17), 33.6 (C-18), 21.8 (C-19), 15.3 (C-20)。以上数据与Albert et al(2007)的报道一致,故鉴定为赖百当-13-烯-8,15-二醇 (labd-13E-ene-8β, 15-diol)。
化合物7ESI-MS (m/z) 451 [M+Na]+,分子式为C29H48O2。1H NMR (500 MHz, CDCl3)δ: 5.38 (1H, m, H-6), 0.64 (3H, s, H-18), 1.03 (3H, s, H-19), 0.89 (3H, m, H-21), 0.88 (3H, m, H-27), 0.85 (3H, m, H-29);13C NMR (125 MHz, CDCl3)δ: 36.5 (C-1), 31.4 (C-2), 70.7 (C-3), 42.0 (C-4), 165.2 (C-5), 126.3 (C-6), 202.4 (C-7), 45.6 (C-8), 50.2 (C-9), 38.4 (C-10), 21.4 (C-11), 38.9 (C-12), 43.3 (C-13), 50.1 (C-14), 26.5 (C-15), 28.7 (C-16), 54.9 (C-17), 12.1 (C-18), 17.5 (C-19), 36.2 (C-20), 18.9 (C-21), 34.1 (C-22), 26.3 (C-23), 46.0 (C-24), 29.3 (C-25), 19.1 (C-26), 20.0 (C-27), 23.2 (C-28), 12.1 (C-29)。以上数据与马晓莉等(2009)的报道一致,故鉴定为7-酮基-β-谷甾醇 (7-oxo-β-sitosterol)。
化合物8ESI-MS (m/z) 429 [M+H]+,分子式为C28H44O3。1H NMR (500 MHz, CDCl3)δ: 4.02 (1H, m, H-3α), 4.89 (1H, m, H-22), 5.24 (1H, m, H-23), 0.81 (3H, s, Me-18), 0.88 (3H, s, Me-19), 1.00 (3H, d,J= 5.5 Hz, Me-21), 0.84 (3H, m, Me-26), 0.78 (3H, m, Me-27), 0,92 (3H, d,J= 3.0 Hz, Me-28);13C NMR (125 MHz, CDCl3)δ: 34.9 (C-1), 30.3 (C-2), 66.6 (C-3), 37.1 (C-4), 82.3 (C-5), 135.6 (C-6), 130.9 (C-7), 79.6 (C-8), 51.3 (C-9), 37.1 (C-10), 20.8 (C-11), 39.5 (C-12), 44.7 (C-13), 51.9 (C-14), 23.6 (C-15), 28.8 (C-16), 56.4 (C-17), 13.0 (C-18), 18.3 (C-19), 39.9 (C-20), 21.0 (C-21), 135.4 (C-22), 132.5 (C-23), 42.9 (C-24), 33.2 (C-25), 19.8 (C-26), 20.1 (C-27), 17.7 (C-28)。以上数据与姜北等(2002)的报道一致,故鉴定为(22E)-5α, 8β-表二氧麦角甾-6, 22-二烯-3β-醇 ((22E)-5α, 8β-epidioxyergosta-6, 22-dien-3β-ol)。
化合物9ESI-MS (m/z) 451 [M+Na]+,分子式为C29H48O2。1H NMR (500 MHz, CDCl3)δ: 5.81 (1H, s, H-4), 4.34 (1H, s, H-6), 0.74 (3H, s, Me-18), 1.37 (3H, s, Me-19), 0.93 (3H, d,J= 5.5 Hz, Me-21), 0.83 (1H, s, H-26), 0.82 (3H, d,J= 6.0 Hz, Me-27), 0.85 (3H, d,J= 6.0 Hz, Me-29);13C NMR (125 MHz, CDCl3)δ: 37.3 (C-1), 34.4 (C-2), 200.5 (C-3), 126.5 (C-4), 168.6 (C-5), 73.4 (C-6), 38.8 (C-7), 29.9 (C-8), 53.8 (C-9), 38.2 (C-10), 21.1 (C-11), 39.8 (C-12), 42.7 (C-13), 56.1 (C-14), 24.3 (C-15), 28.3 (C-16), 56.2 (C-17), 12.1 (C-18), 20.0 (C-19), 36.3 (C-20), 18.9 (C-21), 34.1 (C-22), 26.3 (C-23), 46.0 (C-24), 29.3 (C-25), 19.7 (C-26), 19.2 (C-27), 23.3 (C-28), 12.2 (C-29)。以上数据与吴少华等(2008)的报道一致,故鉴定为豆甾烷-4-烯-6β-醇-3-酮 (stigmast-4-en-6β-ol-3-one)。
化合物10ESI-MS (m/z) 465 [M+Na]+,分子式为C30H50O2。1H NMR (500 MHz, CDCl3)δ: 4.32 (1H, d,J= 12.5 Hz, H-2), 5.42 (1H, m, H-12), 1.58 (3H, s, Me-23), 0.87 (3H, s, Me-24), 1.34 (3H, s, Me-25), 1.20 (3H, s, Me-26), 1.66 (3H, s, Me-27), 0.94 (3H, s, Me-28), 1.10 (3H, s, Me-29), 1.15 (3H, s, Me-30);13C NMR (125 MHz, CDCl3)δ: 46.7 (C-1), 69.1 (C-2), 84.1 (C-3), 39.4 (C-4), 55.4 (C-5), 18.6 (C-6), 32.7 (C-7), 40.1 (C-8), 47.8 (C-9), 38.4 (C-10), 23.8 (C-11), 121.7 (C-12), 145.4 (C-13), 41.9 (C-14), 26.3 (C-15), 27.1 (C-16), 32.6 (C-17), 47.4 (C-18), 47.0 (C-19), 31.6 (C-20), 35.0 (C-21), 37.3 (C-22), 28.8 (C-23), 19.9 (C-24), 17.0 (C-25), 17.0 (C-26), 26.2 (C-27), 28.5 (C-28), 33.5 (C-29), 23.8 (C-30)。以上数据与Braca et al(2001)的报道一致,故鉴定为齐墩果烷-12-烯- 2α, 3β-二醇 (olean-12-en-2α, 3β-diol)。
化合物11ESI-MS (m/z) 193 [M+H]+,分子式为C10H8O4。1H NMR (500 MHz, (CD3)2CO)δ: 6.22 (1H, d,J= 7.5 Hz, H-3), 7.91 (1H, d,J= 8.0 Hz, H-4), 6.78 (1H, s, H-5), 7.21 (1H, s, H-8), 3.81 (3H, s, -OCH3);13C NMR (125 MHz, (CD3)2CO)δ: 160.8 (C-2), 111.8 (C-3), 144.6 (C-4), 109.6 (C-5), 145.3 (C-6), 151.2 (C-7), 102.8 (C-8), 149.6 (C-9), 110.6 (C-10), 56.1 (-CH3)。以上数据与喻蓉等(2003)的报道一致,故鉴定为东莨菪内酯 (scopoletin)。
化合物12ESI-MS (m/z) 225 [M+H]+,分子式为C13H20O3。1H NMR (500 MHz, CDCl3)δ: 2.44 (1H, d,J= 14.0 Hz, H-2), 2.23 (1H, d,J= 14.0 Hz, H-2), 5.89 (1H, s, H-4), 5.78 (1H, d,J= 13.0 Hz, H-7), 5.82 (1H, d,J= 4.5 Hz, H-8), 4.40 (1H, m, H-9), 1.28 (3H, d,J= 6.0 Hz, Me-10), 1.00 (3H, s, Me-11), 1.06 (3H, s, Me-12), 1.88 (3H, s, Me-13);13C NMR (125 MHz, CDCl3)δ: 41.3 (C-1), 49.8 (C-2), 198.5 (C-3), 126.9 (C-4), 163.4 (C-5), 79.1 (C-6), 135.8 (C-7), 129.1 (C-8), 68.1 (C-9), 23.8 (C-10), 23.0 (C-11), 24.2 (C-12), 19.1 (C-13)。以上数据与赵雪梅等(2008)的报道一致,故鉴定为催吐萝芙木醇(vomifoliol)。
化合物13ESI-MS (m/z) 247 [M+Na]+,分子式为C13H20O3。1H NMR (500 MHz, CDCl3)δ: 4.34 (1H, m, H-3), 5.83 (1H, s, H-8), 2.17 (3H, s, Me-10), 1.14 (3H, s, Me-11), 1.36 (3H, s, Me-12), 1.41 (3H, s, Me-13);13C NMR (125 MHz, CDCl3)δ: 36.2 (C-1), 48.8 (C-2), 63.9 (C-3), 49.0 (C-4), 72.4 (C-5), 118.8 (C-6), 198.7 (C-7), 100.9 (C-8), 209.8 (C-9), 26.5 (C-10), 29.2 (C-11), 31.0 (C-12), 31.8 (C-13)。以上数据与Miysse et al(1987)的报道一致,故鉴定为lyratol F。
化合物14ESI-MS (m/z) 247 [M+Na]+,分子式为C13H20O3。1H NMR (500 MHz, MeOD)δ: 2.13 (1H, d,J= 16.5 Hz, H-2a), 2.52 (1H, d,J= 16.5 Hz, H-2b), 6.17(1H, s, H-4), 2.69 (1H, d,J= 7.5 Hz, H-6), 5.68 (1H, m, H-7), 5.65 (1H, m, H-8), 4.28 (1H, m, H-9), 4.20 (1H, m, H2-11), 1.25 (3H, s, H3-10), 1.05 (3H, s, H3-12), 1.01 (3H, s, H3-13);13C NMR (125 MHz, MeOD)δ: 35.8 (C-1), 48.2 (C-2), 200.6 (C-3), 121.0 (C-4), 166.9 (C-5), 50.8 (C-6), 138.8 (C-7), 126.0 (C-8), 67.4 (C-9), 22.3 (C-10), 62.7 (C-11), 26.5 (C-12), 25.9 (C-13)。以上数据与李艳平(2013)的报道一致,故鉴定为罗布麻酚A (apocynol A)。
化合物15ESI-MS (m/z) 271 [M+H]+,分子式为C15H10O5。1H NMR (500 MHz, CDCl3)δ: 6.77 (1H, s, H-3), 6.19 (1H, d,J= 2.0 Hz, H-6), 6.48 (1H, d,J= 2.0 Hz, H-8), 7.92 (2H, d,J= 7.5 Hz, H-2′,6′), 6.93 (2H, d,J= 7.5 Hz, H-3′,5′), 12.95 (1H, s, 5-OH), 10.83 (1H, s, 7-OH), 10.36 (1H, s, 4′-OH);13C NMR (125 MHz, CDCl3)δ: 163.8 (C-2), 103.7 (C-3), 181.8 (C-4), 161.5 (C-5), 98.9 (C-6), 164.2 (C-7), 94.0 (C-8), 161.2 (C-9), 102.9 (C-10), 121.2 (C-1′), 128.5 (C-2′), 116.0 (C-3′), 157.3 (C-4′), 116.0 (C-5′), 128.5 (C-6′)。以上数据与王淑英(2013)的报道一致,故鉴定为芹菜素 (apigenin)。
化合物16ESI-MS (m/z) 467 [M+Na]+,分子式为C27H28O4N2。1H NMR (500 MHz, CDCl3)δ: 4.83 (1H, m, H-2), 3.22 (2H, m, H-3), 7.28 (5H, m, H-5,9), 7.72 (2H, m, H-3′, 7′), 7.44 (2H, m, H-4′, 6′), 7.53 (2H, m, H-5′), 4.34 (1H, m, H-1″), 2.76 (2H, m, H-2″), 7.07 (2H, m, H-4″, 8″), 7.17 (2H, m, H-5″, 7″), 3.22 (2H, m, H-9″);13C NMR (125 MHz, CDCl3)δ: 170.5 (C-1), 55.1 (C-2), 38.6 (C-3), 136.8 (C-4), 128.8 (C-5, 9), 129.5 (C-6, 8), 127.2 (C-7), 167.3 (C-1′), 133.8 (C-2′), 128.7 (C-3′, 7′), 127.2 (C-4′, 6′), 132.0 (C-5′), 49.6 (C-1″), 37.6 (C-2″), 136.9 (C-3″), 128.8 (C-4″, 8″), 129.3 (C-5″, 7″), 126.9 (C-6″), 64.7 (C-9″), 170.9 (-CO-), 20.9 (-CH3)。以上数据与顾晓洁等(2007)的报道一致,故鉴定为金色酰胺醇酯 (aurantiamide acetate)。
ALBERT WW,VAN WYK, MICHAEL T, 2007. Davies-Coleman. Semisynthesis of labdane diterpene metabolites from the nudibranchPleurobranchaeameckelii[J]. Tetrahedron, 63: 12179-12184.
BRACA A, SORTINO C, MENDEZ J,et al, 2001. Triterpenes fromLicanialicanieflora[J]. Phytochem Comm, 72: 585-587.
CHATTERJEE A, BANERJEE A, BOHLMANN F, 1978. Isocrotocaudin, a new norclerodane-type diterpene fromCrotoncaudatus[J]. Phytochemistry, 17: 1777-1779CHINA FLORA EDITORIAL BOARD, CHINESE ACADEMY OF SCIENCE, 1996. The Flora of China [M]. Beijing: Science Press: 133.
GU XJ, LI YB, LI P,et al, 2007. Studies on chemical constituents ofPrunellavulgaris[J]. Chin J Chin Mat Med, 32(10): 923-926. [顾晓洁,李友宾,李萍, 等, 2007. 夏枯草花惠化学成分研究 [J]. 中国中药杂志, 32(10): 923-926.]
JIANG B, ZHAO QS, PENG LY,et al, 2002. Constituents fromThamnoliavermicularis[J]. Acta Bot Yunnan, 24 (4): 525-530. [姜北,赵勤实,彭丽艳, 等,2002. 雪茶化学成分研究 [J]. 云南植物研究, 24 (4): 525-530.]
LI YP,2013. Studies on the chemical constituents and bioactivities of three medical plants [D]. Yunnan: Kunming University of Science and Technology. [李艳平,2013. 三种药用植物的化学成分和生物活性研究 [D]. 云南:昆明理工大学.]
MA XL, LIN WB, ZHANG GL,2009. Chemical condtituents ofOsmanthusyunnanensis[J]. Nat Prod Res Dev, 21(4): 593-599. [马晓莉,林文彬,张国林,2009. 野桂花化学成分研究 [J]. 天然产物研究与开发, 21(4): 593-599.]
MBWAMBO ZH, FOUBERT K, CHACHA M, et al, 2009. New furanoditerpenoids fromCrotonjatrophoides[J]. Plant Med, 75(3): 262-267. MIYSSE T,UENO A,TAKIZAWA N,et al, 1987. Studies on the Glycosides ofEpimediumgrandiflorumMorr. var.thunbergianum(Miq.) Mamai. I [J]. Chem Pharm Bull,35(3):1109-1117. RODRIGUEZ B, JIMENO ML, 2004.1H and13C NMR spectral assignments and conformational analysis of 14, 19-nor-neoclerodane diterpenoids [J]. Magn Reson Chem, 42(7): 605-616.
WANG SY,2013. Study on chemical compositions of the leaves ofDendrocalamus[D]. Beijing: Chinese Academy Forestry. [王淑英,2013. 牡竹属竹叶化学成分研究 [D]. 北京:中国林业科学研究院.]
WU SH, SHENG YM, CHEN YW,et al, 2008. Studies on chemical condtituents from stem bark ofTrewianudiflora[J]. Chin J Chin Mat Med, 33(13): 1566-1568. [吴少华,沈月毛,陈有为,等,2008. 滑桃树茎皮的化学成分研究 [J]. 中国中药杂志, 33(13): 1566-1568.]WU XA,ZHAO YM, 2004. Advance on chemical composition and pharmacological action ofCroton[J]. Nat Prod Res Dev, 16(5):467-472. [吴新安,赵毅民,2004. 巴豆属植物化学成分及药理作用研究进展 [J]. 天然产物研究与开发,16(5):467-472.]
YU R, XU Q, LI BG, et al, 2003. Chemical consitituents ofPranadiscifera[J]. Nat Prod Res Dev, 15(5): 405-407. [喻蓉,许庆,李伯刚,等,2003. 搭棚藤的化学成分研究 [J]. 天然产物研究与开发, 15(5): 405-407.]
ZHAO XM, YE XQ, ZHU DY, 2008. Anovel compound isolated from the peels ofCitruschangshan-huyouY. B. Chang [J]. Acta Pharm Sin, 43(12): 1208-1210. [赵雪梅,叶兴乾,朱大元,2008. 常山胡柚皮中的一个新化合物 [J]. 药学学报, 43(12): 1208-1210.]
Chemical constituents from the branches and leaves ofCrotoneuryphyllus
XIA Meng-Wen1,2, NING De-Sheng1, HUANG Si-Si1, CHENG Ling1,2,LÜ Shi-Hong1, LUO Lei2, PAN Zheng-Hong1*
( 1. Guangxi Key Laboratory of Functional Phytochemicals Research and Utilization, Guangxi Institute of Botany, Chinese Academy of Sciences,Guilin 541006, Guangxi, China; 2.FacultyofChemistryandChemicalEngineering,YunnanNormalUniversity, Kunming 650500, China )
Crotoneuryphyllus(Euphorbiaceae) is mainly distributed in the karst region of Southwest China and has been used as a folk medicine to cure traumatic injury and kill insects. In order to clarify the chemical constituents ofC.euryphyllus, the ethanol extract was separated by the chromatography (silica gel, Sephadex LH-20 and HPLC) and sixteen compounds were isolated. Their structures were elucidated as: isocrotocaudin (1), jatrophoidin (2), teucvidin (3), isoteucvin (4), teucvin (5), labd-13E-ene-8β,15-diol (6), 7-oxo-β-sitosterol (7), (22E)-5α, 8β-epidioxyergosta-6, 22-dien-3β-ol (8), stigmast-4-en-6β-ol-3-one (9), olean-12-en-2α,3β-diol (10), scopoletin (11), vomifoliol (12), lyratol F (13), apocynol A (14), apigenin (15), aurantiamide acetate (16). All compounds were firstly isolated from this plant, and compounds 8-16 were isolated from the genusCrotonfor the first time.
Crotoneuryphyllus, chemical constituents, diterpenoid, sterol, structure identification
10.11931/guihaia.gxzw201509026
2015-10-19
2015-12-29
国家自然科学基金(21102023);广西自然科学基金(2011GXNSFB018022);中国科学院“西部之光”人才培养计划项目(2012) [Supported by the National Natural Science Foundation of China(21102023); the Natural Science Foundation of Guangxi (2011GXNSFB018022); the West Light Foundation of the Chinese Academy of Sciences(2012)]。
夏梦雯(1989-),女,四川雅安人,硕士研究生,研究方向为天然产物研究,(E-mail) 1036978486@qq.com。
潘争红,博士,研究员,主要从事天然产物化学与功能研究,(E-mail) pan7260@126.com。
Q946.91
A
1000-3142(2016)10-1186-06
夏梦雯, 宁德生, 黄思思,等. 石山巴豆枝叶的化学成分研究 [J]. 广西植物,2016,36(10):1186-1191
XIA MW, NING DS, HUANG SS,et al. Chemical constituents from the branches and leaves ofCrotoneuryphyllus[J]. Guihaia,2016,36(10):1186-1191
