碱性阴离子交换聚合物膜研究进展
2016-10-26苏州大学材料与化学化工学部江苏苏州215123
顾 梁, 孙 哲, 徐 丹, 严 锋(苏州大学材料与化学化工学部,江苏苏州215123)
特约综述
碱性阴离子交换聚合物膜研究进展
顾 梁, 孙 哲, 徐 丹, 严 锋
(苏州大学材料与化学化工学部,江苏苏州215123)
碱性燃料电池(AFCs)是一种直接将化学能转化为电能的发电装置,因其高效、环保等优点,得到了科学界与工业界的广泛关注。阴离子交换聚合物膜作为碱性阴离子交换膜燃料电池的核心组成部分,要求其具备优异的电导率、良好的化学稳定性及力学强度。本文主要从聚合物主链及阳离子官能团的结构与性能之间的关系及调控方式方面,综述了碱性阴离子交换膜的研究进展。
碱性阴离子交换膜燃料电池;阳离子;耐碱稳定性;理论计算
聚电解质膜燃料电池是一种能够直接将燃料中的化学能转化为电能的发电装置,具有高能量转化效率、高比功率、高比能量、负荷响应快等特点,被认为是最有应用前景的清洁能源之一[1-3]。因此,研制无污染、高能效的燃料电池,对解决化石能源所造成的环境污染有着十分重要的意义。基于电解质的分类,聚电解质膜燃料电池主要分为质子交换膜燃料电池(PEMFCs)及碱性阴离子交换膜燃料电池(AEMFCs)等[4-6]。其中,PEMFCs具有功率密度高、启动速率快等优点,并已在车用动力能源、通讯设备用电源、移动电源等领域得到广泛应用[7]。然而,PFMFCs需要使用昂贵的铂等贵金属作为催化剂,限制了该燃料电池的大面积推广应用[8]。鉴于此,近年来,使用非贵金属(如Ag、Ni等)催化剂,且对CO2具有较强耐受性的AEMFCs开始受到人们的重视[9]。
AEMFCs主要由电极、催化剂、电解液及阴离子交换膜组成,其结构示意图如图1所示。在电池中,高浓度的强碱性溶液既可当作电解液,又可作冷却剂。碱性阴离子交换膜在燃料电池中起着分隔电极和传导氢氧根离子的作用,其性能的优劣直接影响着燃料电池的工作性能[11-12]。为满足碱性燃料电池的应用需求,阴离子交换膜必须具备较高的离子电导率、良好的热稳定性,优良的力学性能及碱性溶液中良好的耐碱稳定性能[13-14]。因此,研发性能优异的阴离子交换膜对碱性燃料电池的推广应用具有至关重要的意义[15-20]。

图1 碱性阴离子交换膜燃料电池结构示意图Fig.1 Structures of alkaline anion exchange membrane fuel cells
从化学结构上看,碱性阴离子交换膜主要由聚合物主链和阳离子基团组成。通常认为聚合物主链结构决定膜的力学性能、热稳定性能等;而阳离子基团则影响膜的电导率及耐碱稳定性。近年来,科研人员对阴离子交换膜进行了一系列的深入研究,分别从聚合物主链和阳离子官能团的设计入手,合成制备了高性能的新型阴离子交换膜[21-26]。本文主要论述碱性阴离子交换膜聚合物主链与阳离子官能团化学结构与性能之间的关系及其调控方法。
1 基于不同主链的阴离子交换聚合物膜
近年来,基于聚烯烃[27-30]、聚亚芳基[31-33]、聚苯醚[34-35]、聚亚苯基[36]和聚酰亚胺[37]以及聚(苯乙烯-丙烯腈)等共聚物的阴离子交换膜[38],由于具有优异的热稳定性、良好的力学性能以及较低的水溶胀性,已得到广泛研究,其结构示意图如图2所示。

图2 不同主链结构的阴离子交换膜结构Fig.2 Anion exchange membranes with various backbone structures
较之阳离子官能团,关于聚合物主链在碱性溶液中稳定性的研究较少。一般认为,聚合物主链对于碱性溶液的稳定性要优于阳离子官能团,但也有研究表明氢氧根离子的进攻会导致聚合物主链发生降解反应[9]。此外,聚合物主链结构也决定着阴离子交换膜的力学强度、水溶胀性能及离子电导率[39]。
最早应用于制备阴离子交换聚合物膜的是烯烃类聚合物。Varcoe课题组[40]首先报道了基于氟化聚链烯烃阴离子交换膜。该类聚合物膜在60~100℃具有较好的热稳定性。Coates[41]通过开环易位聚合制备了聚烯烃的交联聚合物膜材料。化学交联所形成的聚合物链网络结构有利于氢氧根离子的传导。聚亚芳醚聚合物包括聚亚芳基醚砜和聚亚芳基醚酮。这类聚合物具有较高的玻璃化转变温度(Tg)和优异的热稳定性,并且在有机溶剂中具有良好的溶解性能,因此在阴离子交换膜研究领域得到了广泛关注[42-43]。Yan等[44]使用N-溴代丁二酰亚胺对聚砜主链进行溴化,制备了主链结构为聚砜的阴离子交换膜。该结构可以通过控制聚砜主链的溴化程度调节膜的吸水能力和离子电导率(图3)。Watanabe等[45]制备了一系列含有季铵化芴基取代基的聚亚芳基醚阴离子交换膜,该类聚合物膜在碱性燃料电池的应用中展现出了优异的电导率和力学性能。

图3 基于聚砜主链阴离子交换膜Fig.3 Anion exchange membranes based on polysulfone backbone
作为优异的工程塑料,聚(2,6-二甲基-1,4-苯醚)同样因具有高玻璃化转变温度(Tg)和优异的化学稳定性而备受关注。Xu等[46]制备了基于聚苯醚的阴离子交换膜,进一步将该阴离子交换膜与含氯代乙酰基团的聚苯醚交联反应,从而提高其力学性能。Hickner[47]合成了聚亚苯基主链的阴离子交换膜,研究表明亚苯环结构的主链因其非共面的特性形成了刚性结构,使聚合物膜在500℃下仍保持优良的热稳定性和力学性能。Hibbs[48]通过Diels-Alder反应制备了聚亚苯基为主链的阴离子交换膜,其在水溶液中的离子电导率达到了50 mS/cm,并且在高温碱性溶液中具有良好的碱稳定性(图4)。聚酰亚胺具有优异的热稳定性、化学稳定性和力学性能,故被广泛应用于膜材料的制备。Chen等[49]制备了聚(醚酰亚胺)阴离子交换膜,该聚合物膜在室温条件下的8 mol/L碱溶液中仍具备相当高的化学稳定性。

图4 Diels-Alder聚合反应制备的聚亚苯基主链阴离子交换膜Fig.4 Poly(phenylene)type anion exchange membranes prepared by Diels-Alder polymerization
近年来,利用几种单体共聚所制备的阴离子交换膜得到了广泛的研究。严锋课题组[50]将苯乙烯、丙烯腈与咪唑盐单体共聚制备了交联型的阴离子交换膜。该阴离子交换聚合物膜具备较高的力学性能和电导率(在室温条件下达到了10 mS/cm以上),并且显示出良好的耐碱稳定性。该课题组还通过光交联1-乙烯基-3-甲基咪唑、苯乙烯和丙烯腈制备了新型交联阴离子交换膜。经400 h强碱溶液中稳定性测试后,电导率没有明显降低,展现出了优异的化学稳定性[38]。
阴离子交换膜在碱性燃料电池中起着传导氢氧根离子的作用,传统的提高离子交换容量(IEC,ionic exchange capacity)的方法,虽然能够增加膜的电导率,但同时也会使膜在水溶液中吸收过量的水,从而导致明显的水溶胀,所以更为有效的方式是提高氢氧根离子的有效迁移率[39]。由于氢氧根离子的传导主要依赖于聚合物主链中亲水基团附近存在的水分子,因此聚合物主链的结构框架会对氢氧根离子的迁移率产生明显影响。Nafion膜优异的离子传导率正是因为其形态结构上形成的相分离:聚合物主链结构上同时存在的疏水碳链骨架和包含离子基团的亲水链促进了亲水-疏水相分离结构的形成,并由亲水区域的相互重叠形成了内部的离子通道[51]。因此,在聚合物主链结构上构建相分离可以有效地提高阴离子交换膜的离子电导率。
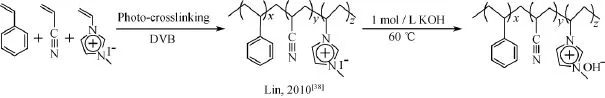
图5 1-乙烯基-3-甲基咪唑、苯乙烯和丙烯腈通过原位交联制备的阴离子交换膜Fig.5 Anion exchange membranes prepared via in situ cross-linking of 1-vinyl-3-methyl-imidazolium iodide([VMIm][I])with styrene and acrylonitrile
常见的构建相分离结构的方式有增加聚合物主链与阳离子基团之间的碳链长度,例如将季铵阳离子通过长支链连接到聚合物主链上,但是该方法通常需要使用高度致癌的试剂如氯甲基乙醚。Chen等[52]将季铵盐类的亲水性离子基团按一定顺序和规律分布在疏水性的聚合物主链上,离子基团与聚合物主链两者相互作用形成了微小的离子通道,形成了明显的微相分离。图6(a)与图6(d)分别是干燥状态下的季铵聚砜(QAPSF)和支链阳离子季铵聚砜(p QAPSF)的TEM照片。图6(a)中阳离子分布在聚砜主链上,亲水基团均匀地分散在干燥的膜表面,表明膜中无微相分离存在。通过将支链阳离子引入季铵聚砜主链(图6(d)),亲水基团出现了明显的聚集并且不均匀地分布在疏水主链上,形成了明显的微相分离。通过粗粒化分子动力学(coarse-grainedmoleculardynamics)进一步对QAPSF和p QAPSF模拟后表明,QAPSF无论在干燥还是湿润状态(图6(c))下都无明显的微相分离结构存在。相比较而言,p QAPSF的干燥(图6(e))和湿润状态(图6(f))则存在明显的微相分离结构。该微相分离结构的离子交换膜可以在聚合物主链附近形成亲水区域,并增加此区域的氢氧根浓度,使聚合物膜在碱性溶液中的离子电导率提高,同时也使离子交换膜兼具良好的耐水溶胀性能和优良的力学性能。

图6 QAPSF和p QAPSF膜的透射电子显微镜照片(a,d);QAPSF和p QAPSF在干燥状态(b,e)和在湿润状态(c,f)下的CGMD模拟照片Fig.6 TEM images for QAPSF and p QAPSF membrane in dry states(a,d);CGMD simulation images of QAPSF and p QAPSF in dry states(b,e)and in wet states(c,f)
Chao等[53]制备并合成了苯乙烯和异戊二烯嵌段的阴离子交换膜。该主链结构中的微相分离使其与传统的阴离子交换膜相比,呈现出了优异的离子电导率。另外,对于聚合物膜还可以采取化学交联的方法提高其力学性能和耐水溶胀性。例如,严锋课题组[54]以二乙烯基苯(DVB)作为交联剂,所制备的聚合物阴离子交换膜具有优良的力学性能,并且在碱性溶液中也具备优良的耐碱性。
2 基于不同阳离子官能团的阴离子交换聚合物膜
阳离子官能团作为阴离子交换膜的一个重要组成部分,对于其结构性能的研究必不可少,它的耐碱稳定性决定着离子交换膜的耐碱稳定性。在已有的研究报道中,基于N原子的季铵盐,由于具有合成简单,易于分子设计等特点而成为研究最广泛的一类阳离子[55]。然而,由于季铵盐阳离子化合物(QAs)在碱性溶液中易于被氢氧根进攻而发生霍夫曼降解反应以及SN2亲核取代反应 (图7),因此,近年来研究人员致力于探索合成基于新型阳离子的阴离子交换聚合物膜。

图7 季铵盐阳离子化合物在碱性溶液中的降解机理Fig.7 Degradation mechanism of QAs in basic solution
近年来,科研人员对包括基于季铵盐[56-58]、季磷盐[59]、胍盐[60]、咪唑盐[61]、金属阳离子[62]以及苯并咪唑[63]等阳离子的阴离子交换膜进行了一系列的研究(图8),通过选择设计不同的阳离子结构,提高阴离子交换膜的耐碱稳定性。

图8 常见的应用于阴离子交换膜的阳离子官能团Fig.8 Cation groups used in the anion exchange membrane
Ramani教授课题组[64]用三甲基膦代替三甲胺修饰氯甲基化聚砜,实验研究中观察到,用三甲基膦修饰的聚合物耐碱性反而降低。Yan课题组[65]合成了新型三甲氧基苯基磷型季磷阳离子碱性阴离子交换膜,结果表明苯环具有明显的位阻效应,可以阻碍氢氧根的亲核进攻,从而提高季磷盐阳离子的耐碱稳定性。长春应化所张所波教授课题组[66]合成了基于胍盐的碱性阴离子交换聚合物膜,胍盐的共振结构使得阳离子电子云分布均匀,减弱了氢氧根的亲核进攻,从而提高了阳离子的耐碱性。
较之于季铵盐类阳离子,咪唑盐阳离子因其环状共轭结构,降低了阳离子电子云密度,减弱了对氢氧根离子的吸引,从而增强了阳离子的耐碱稳定性。严锋课题组系统地研究了取代基对咪唑盐阳离子耐碱稳定性的影响[38,54,67]。研究结果表明,取代基的吸-供电子能力、离域效应和位阻效应等都会影响OH-对阳离子的进攻。进一步的研究表明,咪唑环上取代基的位置对其稳定性的影响,即取代基在咪唑环的C-2位上比在N-3位上能更有效地提高其耐碱稳定性[68]。Zhang等[69]制备了聚砜主链上接枝了甲基咪唑阳离子的阴离子交换聚合物膜,其电导率、IEC、热稳定性都优于传统的季铵盐阴离子交换膜。最近,严锋课题组[70]还考察了基于杂环吡咯烷阳离子的阴离子交换聚合物膜。研究表明,1-乙基-1-甲基吡咯阳离子具有很好的碱稳定性。核磁共振结果表明,其在80℃下、4 mol/L的NaOH溶液中浸泡96 h后,降解不足8%,最终制得的阴离子交换膜,其电导率在80℃下,于1 mol/L NaOH中浸泡了18 d后并无明显下降。
3 理论计算与阳离子的耐碱稳定性
仅仅通过实验表征手段较难预测阳离子官能团的化学稳定性。鉴于此,很多研究采用理论计算与实验表征相结合的方法研究阳离子官能团的耐碱稳定性。应用得比较多的计算方法是密度泛函理论(DFT,density functional theory)。鉴于阳离子在强碱溶液中发生降解反应的复杂性和多样性,运用密度泛函理论(DFT)对阳离子进行理论计算,可以有效预测阳离子的降解程度和耐碱性。严锋课题组[54,67]对咪唑盐阳离子在碱性环境下的稳定性进行计算。研究结果表明,可以通过阳离子LUMO的大小判断咪唑阳离子耐碱稳定性。阳离子的LUMO越高,咪唑盐阳离子越难在碱性条件下降解。计算结果与实验测试结果很好地吻合(表1)。

表1 80℃下,咪唑阳离子的LUMO与其在碱溶液中的降解率[67]Table 1 LUMO value and degradation of imidazolium salts in basic solutions at 80℃[67]
阳离子官能团的耐碱性与氢氧根离子反应的能量壁垒也有关,因此可以通过计算阳离子在氢氧根进攻下过渡态(transition state)与初始状态(initial state)的能量差(Ebarrier)来预测(图9)。Ebarrier越大,则氢氧根对于阳离子的进攻难度越大,即阳离子的耐碱稳定性越好。其他的理论计算还有Mulliken电荷布居分析等,这些理论计算都大大丰富了阳离子耐碱稳定性的检测手段。
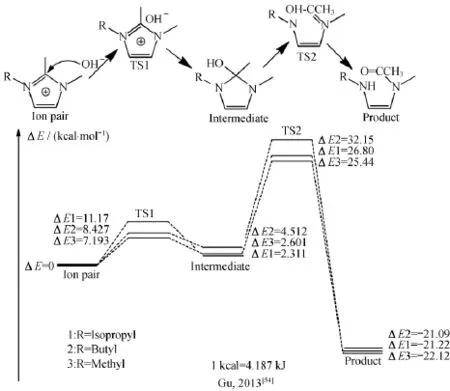
图9 咪唑阳离子反应的能量壁垒(1 kcal=4.187 kJ)Fig.9 Reaction energy barriers for imidazolium cations(1 kcal=4.187 kJ)
4 展 望
目前,阴离子交换膜因碱性燃料电池在能源领域颇受关注而得到了快速地发展。理想的阴离子交换膜需要满足较高的电导率、力学性能、耐水溶胀性、耐碱稳定性等性能。其中,耐碱稳定性成为聚合物阴离子交换膜能否实用化的一个主要参数之一。在关注聚合物主链结构影响的同时,也深入研究阳离子官能团的分子结构对其化学稳定性的影响。将实验检测手段与理论计算相结合是研究阴离子交换膜化学稳定性的有效方法。相信随着高电导率、高稳定性阴离子交换膜的研制成功,将推动碱性阴离子交换膜燃料电池的实际应用。
[1] STEELE B C,HEINZEL A.Materials for fuel-cell technologies[J].Nature,2001,414(6861):345-352.
[2] DIAT O,GEBEL G.Fuel cells:Proton channels[J].Nature Materials,2008,7(1):13-14.
[3] COUTURE G,ALAAEDDINE A,BOSCHET F,et al.Polymeric materials as anion-exchange membranes for alkaline fuel cells[J].Progress in Polymer Science,2011,36(11):1521-1557.
[4] DIAT O,GEBEL G.Fuel cells:Proton channels[J].Nature Materials,2008,7(1):13-14.
[3] 乔宗文,高保娇,陈涛.侧链末端为磺酸根基团的磺化改性聚砜的制备及其阳离子交换膜的基本性能[J].功能高分子学报,2014,27(4):399-407.
[5] WU B,LIN X,GE L,et al.A novel route for preparing highly proton conductive membrane materials with metal-organic frameworks[J].Chemical Communications,2013,49(2):143-145.
[6] BAKANGURA E,GE L,MUHAMMAD M,et al.Sandwich structure SPPO/BPPO proton exchange membranes for fuel cells:Morphology-electrochemical properties relationship[J].Journal of Membrane Science,2015,475:30-38.
[7] LI N,YAN T,LI Z,et al.Comb-shaped polymers to enhance hydroxide transport in anion exchange membranes[J]. Energy and Environmental Science,2012,5(7):7888-7892.
[8] VARCOE J R,SLADE R C,YEE E L H.An alkaline polymer electrochemical interface:A breakthrough in application ofalkaline anion-exchange membranes in fuel cells[J].Chemical Communications,2006,(13):1428-1429.
[9] MERLE G,WESSLING M,NIJMEIJER K.Anion exchange membranes for alkaline fuel cells:A review[J].Journal of Membrane Science,2011,377(1):1-35.
[10] JANNASCH P,WEIBER E A.Configuring anion-exchange membranes for high conductivity and alkaline stability by using cationic polymers with tailored side chains[J].Macromolecular Chemistry and Physics,2016,DOI:10.1002/ macp.201500481
[11] CHEN D,HICKNER M A.Degradation of imidazolium-and quaternary ammonium-functionalized poly(fluorenyl ether ketone sulfone)anion exchange membranes[J].ACS Applied Materials and Interfaces,2012,4(11):5775-5781.
[12] LI N,ZHANG Q,WANG C,et al.Phenyltrimethylammonium functionalized polysulfone anion exchange membranes[J]. Macromolecules,2012,45(5):2411-2419.
[13] VARCOE J R,ATANASSOV P,DEKEL D R,et al.Anion-exchange membranes in electrochemical energy systems[J]. Energy and Environmental Science,2014,7(10):3135-3191.
[14] LI N,GUIVER M D,BINDER W H.Towards high conductivity in anion-exchange membranes for alkaline fuel cells[J]. Chem Sus Chem,2013,6(8):1376-1383.
[15] YU E H,SCOTT K.Development of direct methanol alkaline fuel cells using anion exchange membranes[J].Journal of Power Sources,2004,137(2):248-256.
[16] AN L,ZHAO T,LI Y.Carbon-neutral sustainable energy technology:Direct ethanol fuel cells[J].Renewable and Sustainable Energy Reviews,2015,50:1462-1468.
[17] DONG X,ZHUANG J,HUANG N,et al.Development of anion-exchange membrane for anion-exchange membrane fuel cells[J].Materials Research Innovations,2015,19:38-41.
[18] LI Y,XU T.Fundamental studies of a new series of anion exchange membranes:Membranes prepared from bromomethylated poly(2,6-dimethyl-1,4-phenylene oxide)and 4-vinylpyridine[J].Journal of Applied Polymer Science,2009,114(5):3016-3025.
[19] ZHANG Q,ZHANG Q,WANG J,et al.Synthesis and alkaline stability of novel cardo poly(aryl ether sulfone)s with pendent quaternary ammonium aliphatic side chains for anion exchange membranes[J].Polymer,2010,51(23):5407-5416.
[20] 钱文静,袁超,郭江娜,等.聚离子液体功能材料研究进展[J].化学学报,2015,73(4):310-315.
[21] LI N,LENG Y,HICKNER M A,et al.Highly stable,anion conductive,comb-shaped copolymers for alkaline fuel cells [J].Journal of the American Chemical Society,2013,135(27):10124-10133.
[22] WANG J,ZHAO Z,GONG F,et al.Synthesis of soluble poly(arylene ether sulfone)ionomers with pendant quaternary ammonium groups for anion exchange membranes[J].Macromolecules,2009,42(22):8711-8717.
[23] LI N,ZHANG F,WANG J,et al.Dispersions of carbon nanotubes in sulfonated poly[bis (benzimidazobenzisoquinolinones)]and their proton-conducting composite membranes[J].Polymer,2009,50(15):3600-3608.
[24] WANG J,LI N,CUI Z,et al.Blends based on sulfonated poly[bis(benzimidazobenzisoquinolinones)]and poly(vinylidene fluoride)for polymer electrolyte membrane fuel cell[J].Journal of Membrane Science,2009,341(1):155-162.
[25] YAN X,HE G,WU X,et al.Ion and water transport in functionalized PEEK membranes[J].Journal of Membrane Science,2013,429:13-22.
[26] YAN X,HE G,GU S,et al.Imidazolium-functionalized polysulfone hydroxide exchange membranes for potential applications in alkaline membrane direct alcohol fuel cells[J].International Journal of Hydrogen Energy,2012,37(6):5216-5224.
[27] SHERAZI T A,SOHN J Y,LEE Y M,et al.Polyethylene-based radiation grafted anion-exchange membranes for alkaline fuel cells[J].Journal of Membrane Science,2013,441:148-157.
[28] WAGNER N,SCHULZE M,GÜLZOW E.Long term investigations of silver cathodes for alkaline fuel cells[J].Journal of Power Sources,2004,127(1):264-272.
[29] 马敬骥,郝健.一种具有氟碳骨架的阴离子交换膜的制备[J].功能高分子学报,1992,5(1):70-75.
[30] 栾英豪,张恒,张永明,等.全氟磺酸离子交换膜的制备与性能研究[J].功能高分子学报,2005,18(1):62-66.
[31] GOPI K H,PEERA S G,BHAT S,et al.Preparation and characterization of quaternary ammonium functionalized poly(2,6-dimethyl-1,4-phenylene oxide)as anion exchange membrane for alkaline polymer electrolyte fuel cells[J].International Journal of Hydrogen Energy,2014,39(6):2659-2668.
[32] JANARTHANAN R,HORAN J L,CAIRE B R,et al.Understanding anion transport in an aminated trimethyl polyphenylene with high anionic conductivity[J].Journal of Polymer Science Part B:Polymer Physics,2013,51(24):1743-1750.
[33] LI N,WANG C,LEE S Y,et al.Enhancement of proton transport by nanochannels in comb-shaped copoly(arylene ether sulfone)s[J].Angewandte Chemie,2011,123(39):9324-9327.
[34] YANG Y,KNAUSS D M.Poly(2,6-dimethyl-1,4-phenylene oxide)-b-poly(vinylbenzyltrimethylammonium)diblock copolymers for highly conductive anion exchange membranes[J].Macromolecules,2015,48(13):4471-4480.
[35] RAN J,WU L,RU Y,et al.Anion exchange membranes(AEMs)based on poly(2,6-dimethyl-1,4-phenylene oxide)(PPO)and its derivatives[J].Polymer Chemistry,2015,6(32):5809-5826.
[36] HAN J,LIU Q,LI X,et al.An effective approach for alleviating cation-induced backbone degradation in aromatic etherbased alkaline polymer electrolytes[J].ACS Applied Materials and Interfaces,2015,7(4):2809-2816.
[37] WANG G,WENG Y,ZHAO J,et al.Preparation of a functional poly(ether imide)membrane for potential alkaline fuel cell applications:Chloromethylation[J].Journal of Applied Polymer Science,2009,112(2):721-727.
[38] LIN B,QIU L,LU J,et al.Cross-linked alkaline ionic liquid-based polymer electrolytes for alkaline fuel cell applications [J].Chemistry of Materials,2010,22(24):6718-6725.
[39] PAN J,CHEN C,ZHUANG L,et al.Designing advanced alkaline polymer electrolytes for fuel cell applications[J]. Accounts of Chemical Research,2011,45(3):473-481.
[40] VARCOE J R,SLADE R C,LAM HOW YEE E,et al.Poly(ethylene-co-tetrafluoroethylene)-derived radiation-grafted anion-exchange membrane with properties specifically tailored for application in metal-cation-free alkaline polymer electrolyte fuel cells[J].Chemistry of Materials,2007,19(10):2686-2693.
[41] CLARK T J,ROBERTSON N J,KOSTALIK H A,et al.A ring-opening metathesis polymerization route to alkaline anion exchange membranes:Development of hydroxide-conducting thin films from an ammonium-functionalized monomer[J]. Journal of the American Chemical Society,2009,131(36):12888-12889.
[42] ROY A,HICKNER M A,EINSLA B R,et al.Synthesis and characterization of partially disulfonated hydroquinone-based poly(arylene ether sulfone)s random copolymers for application as proton exchange membranes[J].Journal of Polymer Science Part A:Polymer Chemistry,2009,47(2):384-391.
[43] QI Y,GAO Y,TIAN S,et al.Synthesis and properties of novel benzimidazole-containing sulfonated polyethersulfones for fuel cell applications[J].Journal of Polymer Science Part A:Polymer Chemistry,2009,47(7):1920-1929.
[44] YAN J,HICKNER M A.Anion exchange membranes by bromination of benzylmethyl-containing poly(sulfone)s[J]. Macromolecules,2010,43(5):2349-2356.
[45] TANAKA M,KOIKE M,MIYATAKE K,et al.Synthesis and properties of anion conductive ionomers containing fluorenyl groups for alkaline fuel cell applications[J].Polymer Chemistry,2011,2(1):99-106.
[46] WU L,XU T.Improving anion exchange membranes for DMAFCs by inter-crosslinking CPPO/BPPO blends[J].Journal of Membrane Science,2008,322(2):286-292.
[47] FUJIMOTO C H,HICKNER M A,CORNELIUS C J,et al.Ionomeric poly(phenylene)prepared by diels-alder polymerization:Synthesis and physical properties of a novel polyelectrolyte[J].Macromolecules,2005,38(12):5010-5016.
[48] HIBBS M R,FUJIMOTO C H,CORNELIUS C J.Synthesis and characterization of poly(phenylene)-based anion exchange membranes for alkaline fuel cells[J].Macromolecules,2009,42(21):8316-8321.
[49] WANG G,WENG Y,CHU D,et al.Preparation of alkaline anion exchange membranes based on functional poly(etherimide)polymers for potential fuel cell applications[J].Journal of Membrane Science,2009,326(1):4-8.
[50] QIU B,LIN B,QIU L,et al.Alkaline imidazolium-and quaternary ammonium-functionalized anion exchange membranes for alkaline fuel cell applications[J].Journal of Materials Chemistry,2012,22(3):1040-1045.
[51] LI N,GUIVER M D.Ion transport by nanochannels in ion-containing aromatic copolymers[J].Macromolecules,2014,47 (7):2175-2198.
[52] CHEN C,PAN J,HAN J,et al.Varying the microphase separation patterns of alkaline polymer electrolytes[J].Journal of Materials Chemistry A,2016,4(11):4071-4081.
[53] LEE H C,LIU K L,TSAI L D,et al.Anion exchange membranes based on novel quaternized block copolymers for alkaline direct methanol fuel cells[J].Rsc Advances,2014,4(21):10944-10954.
[54] GU F,DONG H,LI Y,et al.Highly stable N3-substituted imidazolium-based alkaline anion exchange membranes:Experimental studies and theoretical calculations[J].Macromolecules,2013,47(1):208-216.
[55] MARINO M,KREUER K.Alkaline stability of quaternary ammonium cations for alkaline fuel cell membranes and ionic liquids[J].Chem Sus Chem,2015,8(3):513-523.
[56] ZHANG M,KIM H K,CHALKOVA E,et al.New polyethylene based anion exchangemembranes(PE-AEMs)with high ionic conductivity[J].Macromolecules,2011,44(15):5937-5946.
[57] 郑三燕,吕德水,江秀明,等.季铵盐型阳离子聚合物的合成及其应用[J].功能高分子学报,2004,17(4):703-709.
[58] YANG Z,ZHOU J,WANG S,et al.A strategy to construct alkali-stable anion exchange membranes bearing ammonium groups via flexible spacers[J].Journal of Materials Chemistry A,2015,3(29):15015-15019.
[59] COTANDA P,SUDRE G,MODESTINO M A,et al.High anion conductivity and low water uptake of phosphonium containing diblock copolymer membranes[J].Macromolecules,2014,47(21):7540-7547.
[60] LI W,WANG S,ZHANG X,et al.Degradation of guanidinium-functionalized anion exchange membrane during alkaline environment[J].International Journal of Hydrogen Energy,2014,39(25):13710-13717.
[61] DONG H,GU F,LI M,et al.Improving the alkaline stability of imidazolium cations by substitution[J].Chem Phys Chem,2014,15(14):3006-3014.
[62] ZHA Y,DISABB-MILLER M L,JOHNSON Z D,et al.Metal-cation-based anion exchange membranes[J].Journal of the American Chemical Society,2012,134(10):4493-4496.
[63] LIN X,LIANG X,POYNTON S D,et al.Novel alkaline anion exchange membranes containing pendant benzimidazolium groups for alkaline fuel cells[J].Journal of Membrane Science,2013,443:193-200.
[64] ARGES C G,PARRONDO J,JOHNSON G,et al.Assessingthe influence of different cation chemistries on ionic conductivity and alkaline stability of anion exchange membranes[J].Journal of Materials Chemistry,2012,22(9):3733-3744.
[65] GU S,CAI R,LUO T,et al.A soluble and highly conductive ionomer for high-performance hydroxide exchange membrane fuel cells[J].Angewandte Chemie International Edition,2009,48(35):6499-6502.
[66] WANG J,LI S,ZHANG S.Novel hydroxide-conducting polyelectrolyte composed of an poly(arylene ether sulfone)containing pendant quaternary guanidinium groups for alkaline fuel cell applications[J].Macromolecules,2010,43(8):3890-3896.
[67] LIN B,DONG H,LI Y,et al.Alkaline stable C2-substituted imidazolium-basedanion-exchange membranes[J].Chemistry of Materials,2013,25(9):1858-1867.
[68] SI Z,SUN Z,GU F,et al.Alkaline stable imidazolium-based ionomers containing poly(arylene ether sulfone)side chains for alkaline anion exchange membranes[J].Journal of Materials Chemistry A,2014,2(12):4413-4421.
[69] ZHANG F,ZHANG H,QU C.Imidazolium functionalized polysulfone anion exchange membrane for fuel cell application [J].Journal of Materials Chemistry,2011,21(34):12744-12752.
[70] GU F,DONG H,LI Y,et al.Base stable pyrrolidinium cations for alkaline anion exchange membrane applications[J]. Macromolecules,2014,47(19):6740-6747.
Progress of Alkaline Anion Exchange Membranes
GU Liang, SUN Zhe, XU Dan, YAN Feng
(College of Chemistry,Chemical Engineering and Materials Science,Soochow University,Suzhou 215123,Jiangsu,China)
Alkaline fuel cells(AFCs),a kind of devices which can convert chemical energy into electric energy directly,have attracted considerable attention in the fields of science and industry with the advantages of high energy-conversion efficiencies and environmental protection.As the key component of AFCs,the anion exchange membranes require excellent ionic conductivity,high chemical stability,mechanical strength and low water swelling.This review reports the research progress of the anion exchange membranes,especially on the structure-alkaline stability relationships of polymer backbones and cations.
alkaline anion exchange membrane fuel cells;cations;alkaline stability;theoretical calculations
O63
A
1008-9357(2016)02-0153-010DOI: 10.14133/j.cnki.1008-9357.2016.02.003
2016-04-19
国家杰出青年基金(21425417);国家自然科学基金(21274101);江苏高校优势学科建设工程资助项目
顾 梁(1991-),男,江苏苏州人,硕士生,主要研究方向为碱性燃料电池阴离子交换膜。E-mail:18351086532@163.com
严 锋,E-mail:fyan@suda.edu.cn
