Ag-containing Keggin-type germanomolybdate [NH4]2[Ag(H2O)(H2PDCA)]2[α-GeMo12O40]·12H2O
2016-10-25LIYanyanJINGuangfengGONGPeijunLUOJieCHAIYun
LI Yanyan, JIN Guangfeng, GONG Peijun, LUO Jie, CHAI Yun
(College of Chemistry and Chemical Engineering, Henan University, Kaifeng 475004, Henan, China)
Ag-containing Keggin-type germanomolybdate [NH4]2[Ag(H2O)(H2PDCA)]2[α-GeMo12O40]·12H2O
LI Yanyan, JIN Guangfeng, GONG Peijun, LUO Jie, CHAI Yun*
(CollegeofChemistryandChemicalEngineering,HenanUniversity,Kaifeng475004,Henan,China)
An organic-inorganic hybrid Ag-containing Keggin-type germanomolybdate [NH4]2[Ag(H2O)(H2PDCA)]2[α-GeMo12O40]·12H2O (1) (H2PDCA = 2,6-pyridinedicarboxylic acid) was prepared by a conventional solution synthesis and the structure characterized by elemental analyses, IR spectrum and single-crystal X-ray diffraction. X-ray crystallography reveals that the molecular structure consists of one classical Keggin [α-GeMo12O40]4-polyanion, two [Ag(H2O)(H2PDCA)]+coordination cations, two NH4+ions and twelve lattice water molecules. In addition each oxygen atom bonding to Ge atom in [α-GeMo12O40]4-polyanion is disordered over two positions, resulting in the cubic coordination geometry of the Ge atom. Interestingly, each [α-GeMo12O40]4-polyanion is combined with adjacent four [α-GeMo12O40]4-polyanions through four [Ag(H2O)(H2PDCA)]+bridges, giving rise to a 2-D sheet structure. Furthermore, the electrochemical properties of this compound have been investigated at room temperature.
germanomolybdate; Keggin structure; electrochemistry
Article ID: 1008-1011(2016)05-0537-07
In the past decades, the preparation and development of inorganic-organic hybrid materials based on polyoxometalate (POM) building blocks and transition-metal coordination compounds have received great attention owing to their unrivaled structures, charming properties and potential applications in medicine, catalysis, photochemistry, electrochemistry and materials science[1-4]. As is well known, POMs, which are a large variety of metal oxide clusters with unique structural characteristics and diversity of electronic structures[5-6], can act as unusually effective ligands to coordinate various metal-organic units[7-8]. On the other hand, Ag+as a d10transition-metal cation has attracted considerable interest due to its high affinity for N and O donors, versatile coordination behaviors and geometries such as “linear”, “T-type”, “seesaw”, “square pyramidal” and “trigonal bipyramidal” and its special photophysical and photochemical properties[9-12]. Moreover, either Ag+or POM has remarkable catalytic properties and their combination derivatives have also exhibited special properties such as photocatalysis, oxidation catalysis and so on[13-15]. Thus, the design and synthesis of organic-inorganic hybrid Ag-containing POM-based materials have drawn more and more attention on account of the appealing synergistic effects between organic components and POM (especially Keggin-type POMs) units. So far, some organic-inorganic hybrid Ag-containing Keggin-type POM-based materials (AKPMs) with neoteric structures and value-added properties have been successfully synthesized. Some typical examples include [Ag7(L1)4(H2O)(PMo12O40)] and [Ag5(L2)2(PMo12O40)]·H2O (HL1= 5-(4-imidazol-1-yl-phenyl)-2H-tetrazole, HL2= 5-(4-triazol-1-ylmethyl-phenyl)-2H-tetrazole)[16], [Ag2(mbpy)3][Ag(mbpy)2][PW12O40] and [Ag2(mbpy)3]2[SiW12O40] (mbpy = 4,4′-dimethyl-2,2′-dipyridyl)[17], [Ag2(H2biim)2]2(SiW12O40)·2H2O (H2biim = 2,2′-biimidazole)[18], [Ag2(4,4′-bpy)2(4,4′-Hbpy)(H2O)][PW12O40] (4,4′-bpy = 4,4′-bipyridine)[19], etc. However, we found that most of the previous reports were synthesized by means of the hydrothermal method and they are usually functionalized by single N-containing organic ligand, little work has been devoted to AKPMs containing multi-function organic ligands. In this background, we used (NH4)6Mo7O2·4H2O, GeO2, AgNO3and 2,6-pyridinedicarboxylic acid to construct the new AKPM materials. Herein, we report an organic-inorganic hybrid Ag-containing Keggin-type germanomolybdate [NH4]2[Ag(H2O) (H2PDCA)]2[α-GeMo12O40]·12H2O (1) (CCDC 1484695). Furthermore, the solid-state electrochemical properties of 1 have also been discussed.
1 Experimental section
1.1Physical measurements
All chemicals were obtained from commercial resources and used without further purification. The IR spectrum was recorded from solid sample powder palletized with KBr on a Nicolet 170 SXFT-IR spectrometer in the range of 400-4 000 cm-1. Cyclic voltammogram (CV) measurements were performed using a CS310 electrochemical workstation at room temperature. The as-synthesized bulk-modified carbon paste electrode (1-CPE) was worked as a working electrode. Platinum electrode was served as a counter electrode and Ag/AgCl was used as a referencing electrode.
1.2Synthesis of 1
GeO2(0.125 g, 1.195 mmol) and (NH4)6Mo7O2·4H2O (1.000 g, 0.809 mmol) were dissolved in 15 mL of distilled water. After the solution was stirred for about 15 min, the pH of the solution was adjusted to 0.65 using concentrated nitric acid. Subsequently, the resulting solution was kept at 60 ℃ in a water bath for 30 min. And then, AgNO3(0.205 g, 1.206 mmol) and 2,6-pyridinedicarboxylic acid (0.095 g, 0.569 mmol ) were added to the above solution, and the resulting mixture was stirred for ca. 20 min. The final pH value was 0.6 and then the mixture was kept in the 60 ℃ water bath for 2 h. After cooling to room temperature, the mixture was filtered and the filtrate was left to evaporate at room temperature. Orange block crystals were obtained after one week. Yield: ca. 30% (based on H2PDCA). Anal. calcd. (Found %) for 1 : C 6.22 (6.39), H 1.72 (1.85), N 2.07 (1.94).
1.3Preparation of 1-CPE
10 mg sample of compound 1 and 30 mg graphite powder were mixed and ground together using agate mortar and pestle to achieve a dry mixture. ca. 0.6 mL nujol was added into the mixture and stirred with a glass rod. Then the homogenized mixture was impressed into a glass tube with a 1.2 mm inner diameter and the surface was wiped smoothly with weighing paper. Electrical contact was established with a copper rod through the back of the electrode.
1.4X-ray crystallography
Diffraction data for 1 were collected at 296(2) K on Bruker APEX-II CCD detector using graphite monochromatized Mo Kαradiation (λ= 0.071 073 nm). Its structure was solved by direct methods and refined by full-matrix least-squares onF2utilizing the SHELXTL-97 software[20]. The H atoms associated with C and N atoms were geometrically added using a riding model and were refined isotropically using the default SHELXTL parameters. The H atoms attached to the water molecules were not located. All the non-H atoms were refined anisotropically except for O2W, O3W, O4W, O5W, O6W, O7W, O8W, O9W, O8, O12, O17, O18, O19, O20, O22 and N2. The crystal data and structure refinement of 1 are summarized in Table 1.
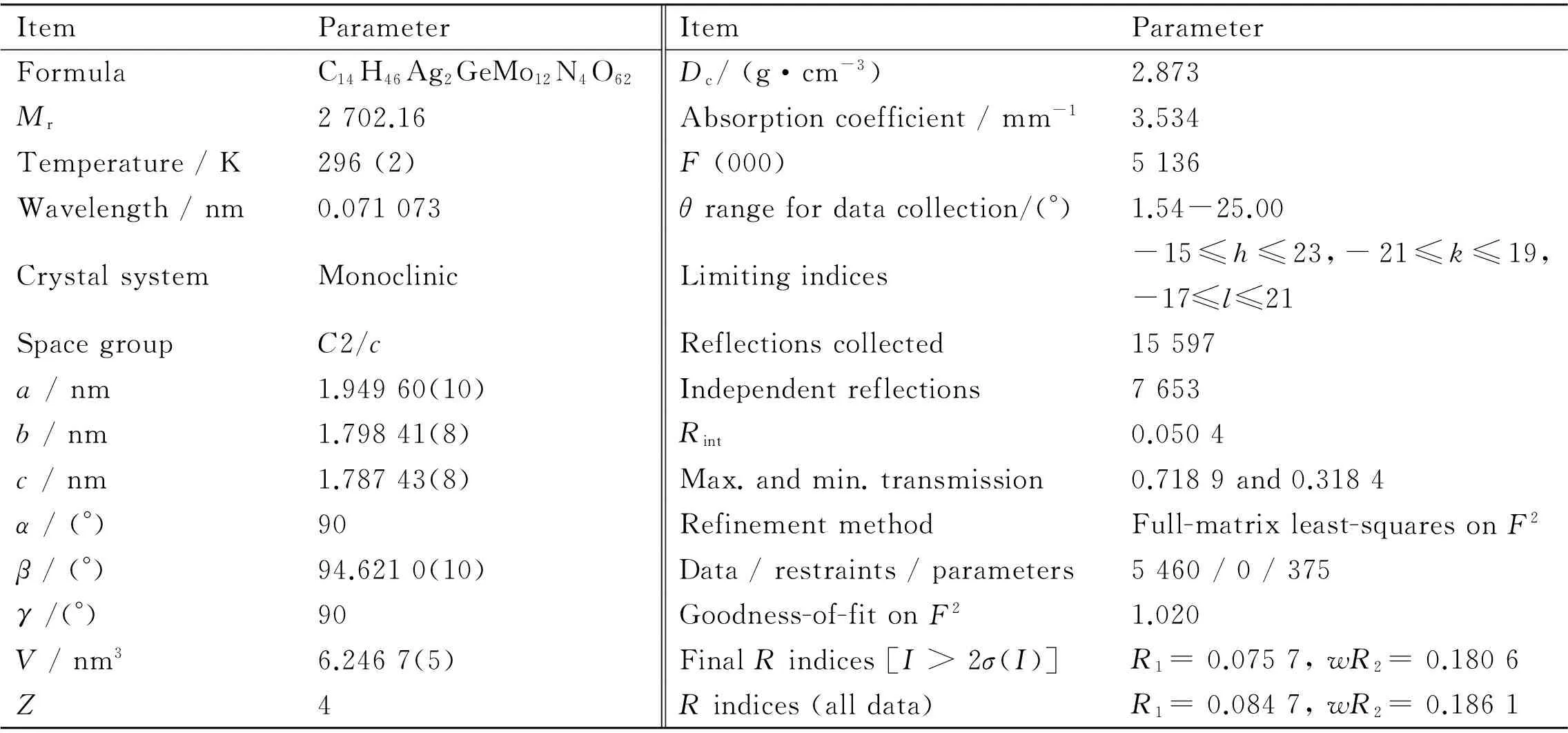
Table 1 Crystal data and structural refinements of 1
2 Results and discussion
2.1Description of crystal structure
X-ray single-crystal diffraction indicates that 1 crystallizes in the monoclinic space groupC2/cand the molecular structural unit of 1 (Fig.1a) consists of one typical Keggin [α-GeMo12O40]4-polyanion (Fig.1b), two [Ag(H2O)(H2PDCA)]+coordination cations, two NH4+ions and twelve crystallization water molecules. The [α-GeMo12O40]4-polyanion exhibits a classical Keggin-type structure, in which the central GeIVheteroatom is surrounded by four oxygen atoms in the position of the corner of the cube and each oxygen atom in a symmetrical position with half occupancy (Fig.1c). Notably, this similar phenomenon also coincident with the cubic geometry of the SiIVatom in [Cu(biim)2]4[α-SiW12O40]2·4H2O reported by LUO et al[21]. In addition, the Ge-O distances range from 0.171 0(15) nm to 0.177 2(16) nm. The distances between the disordered oxygen atom and the Mo atom are in the range of 0.230 8(15)-0.235 4(15) nm, which are much longer than those between the Mo atom and the terminal oxygen atoms [0.164 3(9)-0.166 3(9) nm]. Simultaneously, the disordered oxygen atoms make the geometry of Mo atom transform to a unique configuration (Fig.1d). So, we may come to a conclusion that the disordered oxygen atoms are different from the terminal oxygen atoms. As a result, the oxygen atoms of the [α-GeMo12O40]4-polyanion can be divided into three groups: the disordered four central oxygen atoms, Oa; twenty-four bridging oxygen atoms, O(b,c); twelve terminal oxygen atoms, Ot. The Mo-Oa, Mo-O(b,c)and Mo-Otdistances fall in the range of 0.230 8(15)-0.235 4(15) nm, 0.180 0(13)-0.203 5(17) nm and 0.164 3(9)-0.166 3(9) nm, respectively, which are in the normal ranges and consistent with those described in literatures[22-23]. In the [Ag(H2O)(H2PDCA)]+coordination cation, the Ag1+ion displays the six-coordinate severely distorted octahedral geometry, in which a coordination water molecule and two carboxylic oxygen atoms from a H2PDCA ligand and a nitrogen atom from a H2PDCA ligand build the basal plane [Cu-O: 0.219 7(13)-0.257 8(13) nm, Cu-N: 0.225 6(11) nm] and two bridging oxygen atoms from adjacent two [α-GeMo12O40]4-polyanions occupy two axial positions [Cu-O: 0.296 6(17)-0.304 7(18) nm]. The most intriguing structural characteristic is that each [α-GeMo12O40]4-polyanion is combined with adjacent four [α-GeMo12O40]4-polyanions through four [Ag(H2O)(H2PDCA)]+bridges, giving rise to a 2-D sheet structure (Fig.2).
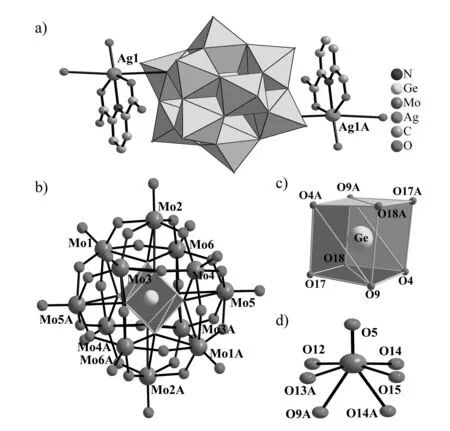
Fig.1 (a) Molecular structural unit of 1. Lattice water molecules and NH4+ ions are omitted for clarity. Symmetry code A: 0.5-x, 0.5-y, -z. (b) Ball-and-stick representation of the [α-GeMo12O40]4- polyanion. (c) Cubic coordination geometry of the Ge atom. (d) Unique geome-try of Mo atom

Fig.2 2-D sheet structure of 1
2.2IR spectrum
The IR spectrum of 1 has been collected from a solid sample palletized with KBr in the range of 4 000-400 cm-1(Fig.3). The broad peaks at about 3 432 and 1 602 cm-1are attributed to the stretching and bending modes of lattice and coordination water molecules. In the low-wave number domain, the characteristic peaks at 950, 878, 807, 777 cm-1for 1 are ascribed to theνas(Ge-Oa),νas(Mo-Ot),νas(Mo-Ob-Mo), andνas(Mo-Oc-Mo) vibration modes, respectively, being in accordance with those IR data of [α-GeMo12O40]4-except for slight changes in peak position[24], which indicates that the polyanion exhibits theα-Keggin structure. In addition, the symmetric and antisymmetric stretching vibrations of 2,6-pyridinedicarboxylic acid are obviously observed at about 1 399 and 1 629 cm-1[25], respectively, and the latter band is overlapping with the OH bend of free and coordination water molecules. Otherwise, bands at 1 450-1 600 cm-1correspond to the stretching vibrations of the heterocyclic picolinate ringν(C=C and C=N), respectively[26]. In brief, the results of the IR spectrum are in great agreement with those from the X-ray single-crystal structural analysis.

Fig.3 IR spectrum of 1
2.3Electrochemical property
Since TOTH et al. first reported the electrochemistry of POMs in 1989[27], considerable efforts have been paid to the study of the electrochemistry and electrocatalytic properties of POMs, primarily because POMs with empty orbitals in their structures, can go through a stepwise multi-electron reversible redox reaction without changing their original structures[28]. As is well-known, the bulk-modified carbon paste electrode(CPE) is inexpensive and easy to be prepared and handled[29]. Thus, in this article, compound 1 was used to prepare the CPE for the exploration of its electrochemical property. The cyclic voltammogram (CV) experiment of 1-CPE was performed in a 0.5 mol·L-1Na2SO4+ H2SO4buffer solution. As shown in Fig. 4a, three pairs of redox peaks (I-I′, II-II′ and III-III′) can be observed in the potential range of -0.4 to 1.0 V and their half-wave potentialsE1/2= (Epa+Epc)/2 are +0.015 8 ,+0.227 and +0.458 V, respectively, which can be attributed to the redox process of MoVIcenters in 1. Furthermore, the CV curves of 1-CPE were carried out in Na2SO4+ H2SO4buffer solution (pH = 3.23) at different scan rates. Obviously, the cathodic peak potentials shift toward the negative direction while the corresponding anodic peak potentials to the positive direction as scan rate increases (Fig. 4b). With the increase of the scan rates from 20 to 110 mV·s-1, the peak currents are proportional to the square root of the scan rates and the linear equation isIpc= -8.56×10-6ν1/2-1.50×10-5with the correlation coefficient of 0.994 (Fig. 4c), indicating that the electrode reaction is controlled by diffusion process. With the increase of the scan rates from 140-290 mV·s-1, the peak current intensities vary linearly with the scan rate and the linear equation isIpc= -1.70×10-7ν-8.72×10-5with the correlation coefficient of 0.992 (Fig.4d), declaring that the electrochemical process is surface controlled[30].

Fig.4 (a) Cyclic voltammogram of 1-CPE in pH = 3.23, 0.5 mol·L-1 Na2SO4+H2SO4 aqueous solution at 20 mV·s-1. (b) Cyclic voltammograms of 1-CPE at different scan rates. (c) Variation of cathodic peak currents of the MoVI-based wave (I/I′) with the square root of the scan rates (20, 50, 80, 110 mV·s-1) for 1-CPE. (d) Variation of cathodic peak currents of the MoVI-based wave (I/I′) with the scan rates (140, 170, 200, 230, 260, 290 mV·s-1) for1-CPE
3 Conclusion
In summary, a new Ag-organic-complex containing Keggin-type germanomolybdate hybrid [NH4]2[Ag(H2O)(H2PDCA)]2[α-GeMo12O40]·12H2O (1) was synthesized in the conventional aqueous solution and its structure was characterized. The most remarkable structural feature of 1 is that each [α-GeMo12O40]4-polyanion is connected with adjacent four [α-GeMo12O40]4-polyanions through four [Ag(H2O)(H2PDCA)]+bridges, giving rise to a 2-D sheet structure. In the future time, rare earth cations and other polycarboxylic acids will be introduced to this system to construct novel organic-inorganic hybrid 4d-4f heterometallic germanomolybdates.
[1] RHULE J T, HILL C L, JUDD D A. Polyoxometalates in medicine [J]. Chem Rev, 1998, 98(1): 327-358.
[2] LONG D L, BURKHOLDER E, CRONIN L. Polyoxometalate clusters, nanostructures and materials: From self-assembly to designer materials and devices [J]. Chem Soc Rev, 2007, 36(1): 105-121.
[3] PROUST A, THOUVENOT R, GOUZERH P. Functionalization of polyoxometalates: towards advanced applications in catalysis and materials science [J]. Chem Commun, 2008(16): 1837-1852.
[4] DOLBECQ A, MIALANE P, SÉCHERESSE F, et al. Functionalized polyoxometalates with covalently linked bisphosphonate, N-donor or carboxylate ligands: from electrocatalytic to optical properties [J]. Chem Commun, 2012, 48(67): 8299-8316.
[5] KOZHEVNIKOV I V. Catalysis by heteropoly acids and multicomponent polyoxometalates in liquid-phase reactions [J]. Chem Rev, 1998, 98(1): 171-198.
[6] LONG D L, BURKHOLDER E, CRONIN L. Polyoxometalate clusters, nanostructures and materials: from self assembly to designer materials and devices [J]. Chem Soc Rev, 2007, 36(1): 105-121.
[7] ZHENG S T, YANG G Y. Recent advances in paramagnetic-TM-substituted polyoxometalates (TM = Mn, Fe, Co, Ni, Cu) [J]. Chem Soc Rev, 2012, 41(22): 7623-7646.
[8] MA X, LI H L, ZHAO J W, et al. The main progress over the past decade and future outlook on high-nuclear transition-metal substituted polyoxotungstates: from synthetic strategies, structural features to functional properties [J]. Dalton Trans, 2016, 45(12): 4935-4960.
[9] PICKERING A L, LONG D L, CRONIN L. Coordination networks through the dimensions: from discrete clusters to 1D, 2D, and 3D silver(I) coordination polymers with rigid aliphatic amino ligands [J]. Inorg Chem, 2004, 43(16): 4953-4961.
[10] VILLANNEAU R, PROUST A, ROBERTS F. Synthesis and characterization of [NBu4]4[Ag2{Mo5O13(OMe)4(NO)}2], a novel polyoxomolybdate complex with a short AgI…AgIdistance [J]. Chem Commun, 1998(14): 1491-1492.
[11] PANG H J, CHEN J, PENG J, et al. Synthesis of a Ag-bridged V-centred tungstovanadate dimer capped by [Ag(phen)] units [J]. Solid State Sci, 2009, 11(4): 824-828.
[12] ZHANG C J, CHEN Y G, PANG H J, et al. Synthesis and characterization of the highest connected 3Dα-metatungstate POM/TMC hybrid with AgI…AgIinteractions [J]. Inorg Chem Commun, 2008, 11(7): 765-768.
[13] HU Y, LUO F, DONG F F. Design synthesis and photocatalytic activity of a novel lilac-like silver-vanadate hybrid solid based on dicyclic rings of [V4O12]4-with {Ag7}7+cluster [J]. Chem Commun, 2011, 47(2): 761-763.
[14] HU Y, AN H Y, YIN J Q, et al. pH-controlled assembly of hybrid architectures based on Anderson-type polyoxometalates and silver coordination units [J]. Dalton Trans, 2014, 43(6): 2488-2498.
[15] SHI Z Y, PENG J, LI Y G, et al. Assembly of multinuclear Ag complexes and Keggin polyoxometalates adjusted by organic ligands: syntheses, structures and luminescence [J]. CrystEngComm, 2013, 15(37): 7583-7588.
[16] WANG X, PENG J, ALIMAJE K, et al. Keggin POM-based 3D framework tuned by silver polymeric motifs: structural influences of tetrazolate functional groups [J]. CrystEngComm, 2012, 14(24): 8509-8514.
[17] ZHAO X F, HAN Z G, ZHAI X L et al. Two new polyoxometalate-based hybrids consisting of Keggin-type cluster modified by {Ag4} group [J]. J Solid State Chem, 2013, 207: 178-183.
[18] MENG C L, ZHANG P P, PENG J, et al. Keggin-type polyoxometalate cluster functionalized with dinuclear M-H2biim (M = Cu, Ag) metal-organic segments [J]. J Clust Sci, 2012, 23(2): 567-576.
[19] YANG H X, GAO S Y, CAO R, et al. pH-Dependent syntheses and crystal structures of a series of organic-inorganic hybrids constructed from Keggin or Wells-Dawson polyoxometalates and silver coordination compounds [J]. Inorg Chem, 2010, 49(2): 736-744.
[20] SHELDRICK G M. SHELXTL-97, Program for crystal structure solution [CP]. Göttingen: University of Göttingen, 1997.
[21] LUO J, CHANG S Z, CHEN L J. A saturated Kegging silicotungstate-based transition metal derivative [Cu(biim)2]4[α-SiW12O40]2·4H2O [J]. Chem Res, 2015, 26(3): 251-257.
[22] TIAN A X, PENG J, SU Z M, et al. Assemblies of copper bis(triazole) coordination polymers using the same Keggin polyoxometalate template [J]. Inorg Chem, 2009, 48(1): 100-110.
[23] ZHANG C X, CHEN Y G, ZHANG Z C, et al. Synthesis and properties of two new 3D organic-inorganic hybrid compounds constructed from Keggin clusters, pyridine-2,3-dicarboxylate and Ag(I) ions [J]. Solid State Sci, 2012, 14(9): 1289-1294.
[24] ZHANG H, DUAN L Y, LAN Y, et al. Synthesis, crystal structure, and photochromism of novel two-dimensional supramolecular networks based on Keggin-type polyoxoanion and lanthanide coordination cations [J]. Inorg Chem, 2003, 42(24): 8053-8058.
[25] RITCHIE C, BASLON V, MOORE E G, et al. Sensitization of lanthanoid luminescence by organic and inorganic ligands in lanthanoid-organic-polyoxometalates [J]. Inorg Chem, 2012, 51(2): 1142-1151.
[26] ZOLIN V F, PUNTUS L N, TSARYUK V I, et al. Spectroscopy of europium and terbium pyridinecarboxylates [J]. J Alloy Compd, 2004, 380(1/2): 279-284.
[27] TOTH J, ANSON F. Electrocatalytic reduction of nitrite and nitric oxide to ammonia with iron-substituted polyoxotungstates [J]. J Am Chem Soc, 1989, 111(7): 2444-2451.
[28] WANG C J, WANG T T, LAN Q, et al. Polyoxometalate-based supramolecular architecture constructed from a purely inorganic 1D chain and a metal-organic layer with efficient catalytic activity [J]. RSC Adv, 2016, 6(19): 15513-15517.
[29] TIAN A X, NING Y L, YANG Y, et al. Three new POM-based compounds constructed by rigid thiabendazole and flexible bis(pyrazole) ligands: structures and properties for Hg2+recognition [J]. Dalton Trans, 2015, 44(37): 16486-16493.
[30] HAN Z G, ZHAO Y L, PENG J, et al. Inorganic-organic hybrid polyoxometalate containing supramolecular helical chains: Preparation, characterization and application in chemically bulk-modified electrode [J]. Electrochim Acta, 2005, 51(2): 218-224.
[责任编辑:吴文鹏]
包含银的Keggin型锗钼酸盐[NH4]2[Ag(H2O)(H2PDCA)]2[α-GeMo12O40]·12H2O
李岩岩,晋光凤,巩培军,罗婕,柴云*
(河南大学 化学化工学院,河南 开封 475004)
利用常规水溶液方法合成了一种有机-无机杂化的包含银的Keggin型锗钼酸盐[NH4]2[Ag(H2O)(H2PDCA)]2[α-GeMo12O40]·12H2O (1),并通过元素分析、红外光谱和单晶X射线衍射等对其进行了表征. X射线晶体学研究表明化合物1的分子结构由1个典型的Keggin结构[α-GeMo12O40]4-聚阴离子、2个[Ag(H2O)(H2PDCA)]+配离子、2个NH4+离子和12个结晶水分子构成. 值得注意的是在[α-GeMo12O40]4-单元中,锗原子呈现出立方体几何配位构型,这归结于与锗原子相连的每个氧原子都无序地排列在两个位置. 有趣的是,每个[α-GeMo12O40]4-多阴离子通过4个[Ag(H2O)(H2PDCA)]+桥配离子与4个[α-GeMo12O40]4-多阴离子相连构筑了二维层状结构. 此外,我们还在室温下研究了化合物1的电化学性质.
锗钼酸盐; Keggin结构; 电化学
date: 2016-06-16.
Supported by the Foundation of Education Department of Henan Province (16A150027) and the Students Innovative Pilot Plan of Henan University (15NA002).
, E-mail: chaiyun@henu.edu.cn.
O614.3 Document code: A
Biography: LI Yanyan (1995-), female, majoring in molecule-based functional materials.*
