In situ FT-IR spectroscopic studies of curing reaction of HTPB and TDI on solid surface
2016-10-21李翠华张小雪刘剑洪庞爱民池旭辉
李翠华, 罗 涛, 张小雪, 刘剑洪, 庞爱民, 池旭辉
1)深圳大学化学与环境工程学院,广东深圳518060;2)航天科技集团四院42所,湖北襄阳441003
【化学与化工 / Chemistry and Chemical Engineering】

In situ FT-IR spectroscopic studies of curing reaction of HTPB and TDI on solid surface
A diffuse reflectance infrared fourier transform spectroscopy (DRIFTS) was loaded down with extra heating device in order to determine the concentration of isocyanate (NCO) on the solid surface via the band area at 2 270 cm-1in situ. The real-time progress of the curing reaction between toluene diisocyanate (TDI) and hydroxyl-terminated polybutadiene (HTPB) can also be monitored directly with only tiny amounts of the reactant mixtures at elevated temperatures. The corresponding reaction activation energy was obtained by fitting the data of NCO concentration at different temperatures. The experiment results indicate that DRIFTS in situ can determine accurately and rapidly the curing reaction of HTPB and TDI in real time.
analytical chemistry; diffuse reflectance infrared fourier transform spectroscopy (DRIFTS); in situ; isocyanate (NCO); curing reaction; polyurethane
固体表面HTPB和TDI固化反应原位红外光谱分析
李翠华1, 罗涛1, 张小雪1, 刘剑洪1, 庞爱民2, 池旭辉2
1)深圳大学化学与环境工程学院,广东深圳518060;2)航天科技集团四院42所,湖北襄阳441003
Solid rocket motors are insulated by thin-walled container loaded with composite solid propellants in which the most important ingredients are oxidizers and polymeric binders. Hydroxyl-terminated polybutadiene (HTPB) with low viscosity is extensively used as an adhesive and a binder in present solid rocket motors and launch vehicles[1-4]. HTPB reacts with diisocyanate to form polyurethane network which holds solid propellants and the casing insulation together. In the curing reaction, the amount of the functional groups on the surface of the prepolymer affects significantly the polyurethane linear network and thus the rheological and mechanical properties of the propellant[5-6]. Understanding the change in amount of isocyante(NCO) on the solid surface can help to arrive at optimum conditions suitable for rocket application.
A large number of characterization methods have been used to monitor the change of NCO amount and the kinetics of urethane formation in solutions and in the bulk, such as differential scanning calorimetry (DSC), viscosity measurement, conventional chemical analysis and spectroscopic techniques[7-10]. Compared with these methods, spectroscopic techniques have the advantage that they can directly measure extent of reaction[11-14]. Hailu et al[15]explored the curing reaction between HTPB and IPDI at 35 ℃ by simultaneous IR measurement at reaction temperature 35 ℃. Shane et al[16]characterized the polymerization kinetics of an uncatalyzed polyester at 180 ℃ with Raman spectroscopy. However, these studies monitor the concentration change of NCO only in bulk rather than on the surface where the concentration change of the functional group is vital to surface adhesion.
Diffuse reflectance infrared fourier transform spectroscopy (DRIFTS) is one of spectral methods to measure samples on solid surface directly[17]. DRIFTS with a heat stage is capable of monitoring the sample changes on solid surface, tracking the reaction intermediates and products in situ at different reaction temperatures, which provides the direct evidence for the reaction mechanism. Other several advantages of DRIFTS include minimal required sampling volume, the ability to utilize glass and other sample matrix. The objective of this study is to use sensitive and beneficial DRIFTS in situ to characterize the amount of NCO on solid surface, and develop a method to estimate the progress of the curing reaction between toluene diisocyanate (TDI) and HTPB on the surface of the polymer at different temperatures. In addition, the activation energy of the polymerization between HTPB and TDI is calculated.
1 Experiment
HTPB (hydroxy concentration of 0.678 mol/L, the 42nd Institute of the Fourth Academy of CASC), toluene diisocyanate (TDI, Shanghai Chemical Reagent Co.) and triphenylbismuth (TPB, the 42nd Institute of the Fourth Academy of CASC) were used without further purification.
Equivalent NCO/OH molar ration of HTPB (2.664 g) and TDI (0.1594 g) were mixed with 0.53% (mass fraction) triphenylbismuth (TPB) at room temperature and air humidity 30%. The resulting mixture contains 640.3 mmol·L-1of c(NCO) and 639.7 mmol·L-1of c(OH). The reactant mixture was degassed under vacuum before one drop of the sample was smeared on an aluminum slip. The thickness of mixture sample after smearing was approximately 0.016 mm.
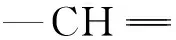
2 Results and discussion
2.1NCO Conversion from IR Spectroscopy
The polymerization reaction between equimolar HTPB and TDI was monitored in situ via the changes in absorption peak area of NCO at 2 272 cm-1. Although the two isocyanate groups in TDI are not equivalent in reactivity, the two isocyanate groups give only one IR absorption band at 2 272 cm-1and the peak position remains practically unaltered throughout the reaction, only the total amount of unreacted isocyanate was determined.
Fig.1 shows partial IR spectra (2 100 - 2 500 cm-1) of a reaction mixture at 75 ℃. The band at 2 272 cm-1for NCO is very strong and interferes a little with the asymmetric stretching vibration band of CO2at around 2 360 and 2 334 cm-1in the beginning of the polymerization reaction. To monitor accurately the concentration of NCO and the reaction degree, the interference from CO2absorption bands at 2 360 and 2 334 cm-1was eliminated with Gaussian fitting using Origin software. The fitting result of the FT-IR peak is shown in Fig.2. The black original line is deconvoluted to three peaks, which are ascribed to 2 272 cm-1for NCO, 2 360 and 2 334 cm-1for CO2individually. The red cumulative line of these three peaks is in good accordance with the original spectrum, indicating that the concentration of NCO can be calculated exactly with the fit peak area at 2 272 cm-1.
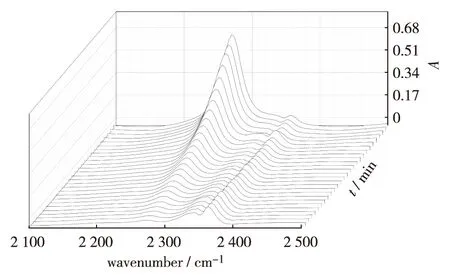
Fig.1 Partial IR spectra of HTPB and TDI reactant mixture in situ at 75 ℃ with 3 min interval图1 75 ℃时HTPB和TDI反应混合物的部分红外谱图(每图间隔3 min)
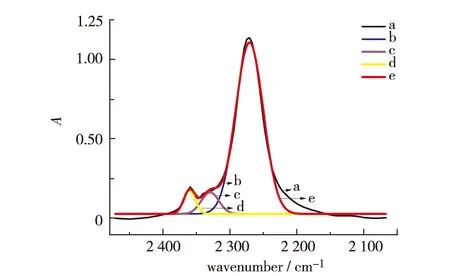
a: the original line; b: the deconvoluted peak owing to NCO; c and d: the deconvoluted peak owing to CO2; e: the recumulative line.Fig.2 The fit peak from the original FT-IR spectrum图2 原始谱图与拟合谱图的对比
The characteristic FT-IR peaks can be quantified on the basis of Beer’s law[18]:
A=log(I/I0)=εcl
(1)
where A is the absorbance, I is the intensity of the radiation, I0is the intensity of the incident light, ε(kg·mol-1·cm-1) is the molar absorptivity of the sample at a given wave number, c is the corresponding concentration of NCO, l is the path length of the radiation within the sample.
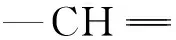
ct(NCO) is the concentration of NCO at t, mmol·L-1, and it was calculated by the following equation:
ct(NCO)=c0(NCO)At/A0
(2)
where c0(NCO) is the concentration of NCO in the beginning of the polymerization reaction, mmol·L-1, Atand A0are the ratio of the absorbance area of 2 272 cm-1divided by that of 910 cm-1at t and t0, respectively.
The reacting degrees(α) can be calculated according to the Eq.(3).
(3)
Fig.3 and Fig.4 demonstrate the time-dependent behavior of concentrations with reaction degree in the course of the polymerization at several reaction temperatures. Both NCO concentration and reaction degree exhibit straight-line change in the initial stage, followed by slower progress. With increasing the temperature, the straight-line part gets longer, being indicative of that the temperature promotes greatly the curing reaction of HTPB and TDI.
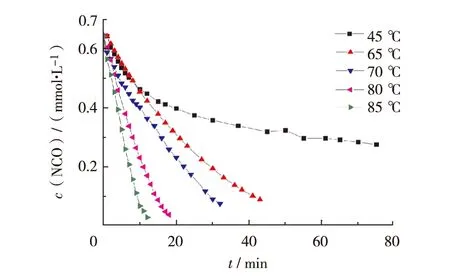
Fig.3 Concentration of NCO during curing reaction versus time图3 聚合反应中NCO 浓度随时间的变化
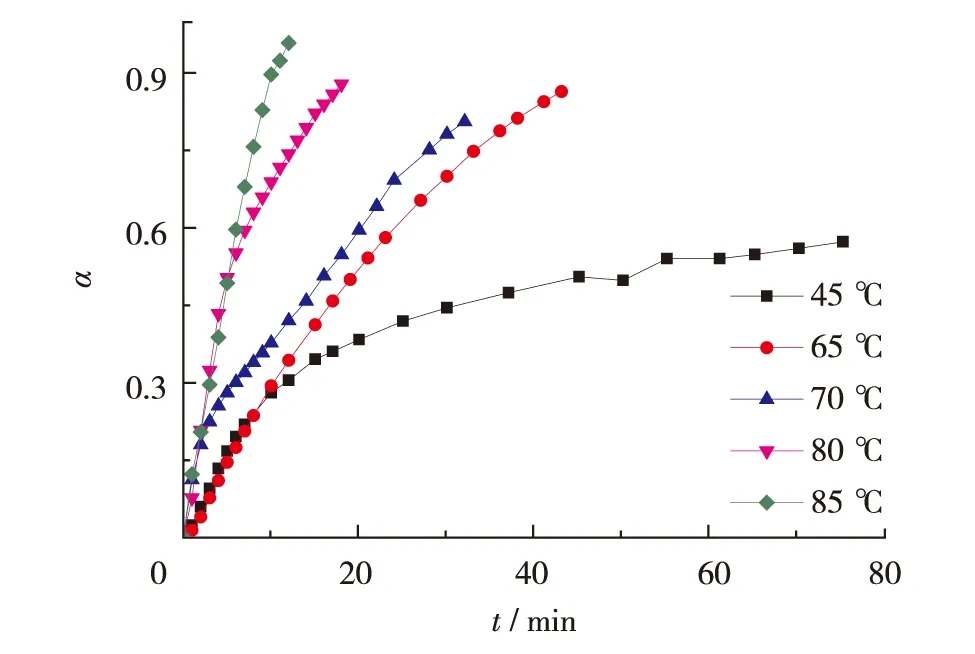
Fig.4 Curing degree (α) of NCO as a function of reaction time图4 聚合反应中NCO 聚合度随时间的变化
2.2Kinetic parameter determination
The initial stage of the curing reaction between HTPB and TDI can be described by a second-order reaction as the two NCO groups of the diisocyanates exhibit different reactivity[18,21]. The rate law of the second-order reaction is given as
rate=kct(NCO)ct(OH)
(4)
where ct(NCO) and ct(OH) are the concentrations of NCO and OH in the reaction mixture, respectively. k stands for the rate constant.
If we assume that the initial concentrations of NCO and OH are taken to be equal in the curing reaction (i.e.c0(NCO)=c0(OH)), the rate of Eq.(4) can be integrated to retrieve the concentration-time relationship.
-dct(NCO)/dt=k[ct(NCO)]2
(5)
(6)
Eq.(6) can be rewritten as the Eq.(7) in combination with Eq.(3).
(7)
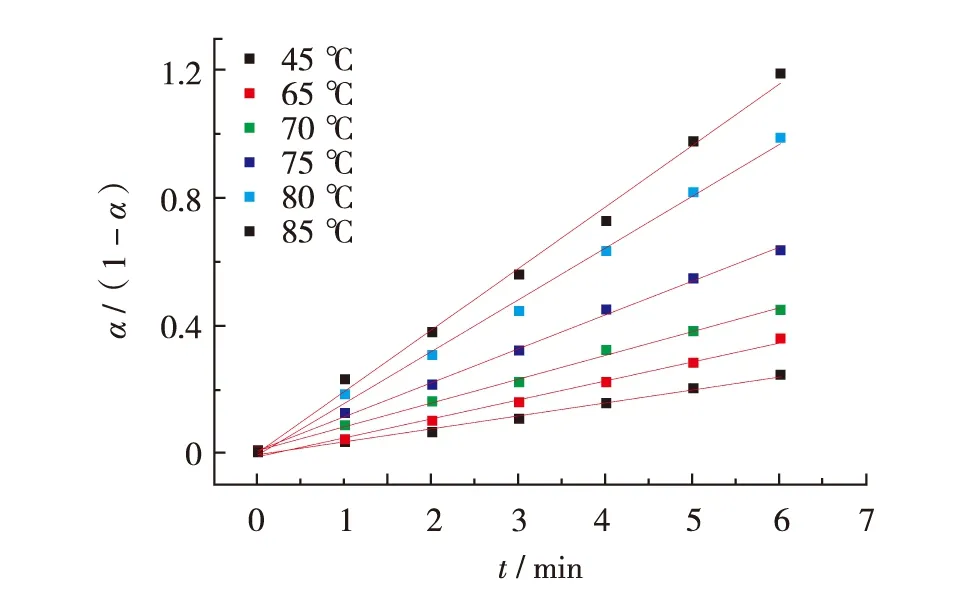
Fig.5 Plot of α/(1-α) of NCO versus time in the initial stage of HTPB-TDI curing reactionFig.5 反应初始阶段NCO的α/(1-α) 随时间的变化
The plots of α/(1-α) as a function of reaction time at varying temperatures are shown in Fig.5, which is indicative of that the curing reaction in the initial stage obeys a second-order rate law. The rate constant k at different temperatures can be obtained by the slopes of the plots.
From the Arrhenius equation:
k=A′e-Ea /(RT)
(8)
where A′ is a frequency factor, Eais activation energy, R is the universal gas constant, and T is temperature in Kelvin. The Eq.(8) can be rewritten as the Eq.(9)
lnk=lnA′-Ea/(RT)
(9)
Arrhenius plot was obtained by plotting ln k vs.1 000/T (Fig.6). The activation energy equals to the slope of the straight, -Ea/R. The activation energy, Eais calculated to be 42.70 kJ·mol-1, which is near to the values in the literature. For example, Hailu et al.[15]estimated Eato be 45.25 kJ·mol-1with near infrared spectroscopy (NIR) and 41.47 kJ·mol-1with 1H nuclear magnetic resonance (NMR) relaxometry. Shane et al.[16]calculated Eato be approximately 38.7 kJ·mol-1with Raman spectroscopy and Sekkar et al.[7]calculated Eato be 41.0 kJ·mol-1with rheo-kinetic evaluation. This indicates that diffuse reflectance infrared fourier transform spectroscopy, DRIFTS is another effective method for monitoring the reaction progress on solid surface and exploring the reaction kinetics.
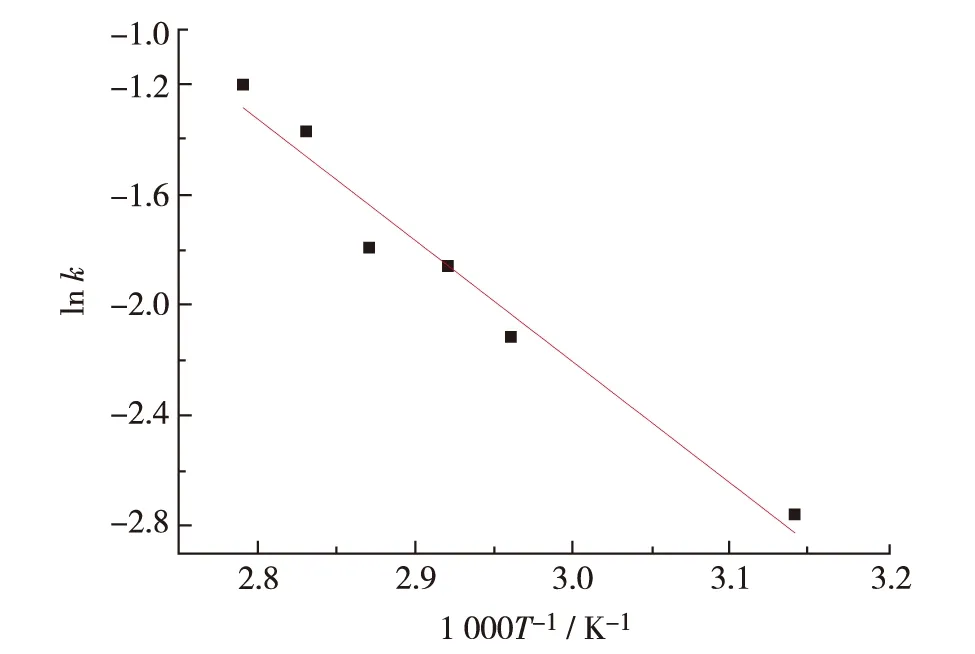
Fig.6 k as a function of temperature of HTPB-TDI curing reaction图 6 HTPB-TDI 聚合反应k随温度的变化
Conclusions

[1] Mehmood Z, Khan M B. Synergistic enhancement in mechanical and ballistic properties of HTPB based energetic polymer composites[J]. Plastics Rubber and Compounds, 2013, 42(10): 446-452.
[2] Han Bo, Ju Yutao, Zhou Changsheng. Simulation of crack propagation in HTPB propellant using cohesive zone model[J]. Engineering Failure Analysis, 2012, 26(12): 304-317.
[3] Krishnan P S G, Ayyaswamy K, Nayak S K. Hydroxyl terminated polybutadiene: chemical modifications and applications[J]. Journal of Macromolecular Science Part A, 2013, 50(1): 128-138.
[4] Zhang Zeyi, Li Xiaojiang, Wang Han, et al. Curing reaction kinetics of polyethylene glycol with isophorone diisocyanate and toluene diisocyanate[J]. Chinese Journal of Energetic Materials, 2007, 15(4): 320-323.
[5] Zia K M, Zuber M, Saif M J, et al. Chitin based polyurethanes using hydroxyl terminated polybutadiene, part I: molecular engineering[J]. International of Biological Macromolecules, 2013, 59(4): 320-327.
[6] Wen Qingzhen, Zhou Qichao, Zhu Jinhua, et al. FT-IR study on the curing behavior of polyurethanes[J]. Polymeric Materials Science and Engineering, 2003, 19(1): 108-111.
[7] Sekkar V, Ambika D K, Ninan K N. Rheo-kinetic evaluation on the formation of urethane networks based on hydroxyl-terminated polybutadiene[J]. Journal of Applied Polymer Science, 2001, 79(10): 1869-1876.
[8] Zia K M, Zuber M, Saif M J, et al. Chitin based polyurethanes using hydroxyl terminated polybutadiene, part III: surface characteristics[J]. International Journal of Biological Macromolecules, 2013, 62(4): 670-676.
[9] Wu Wenming, Zhang Wei, Chen Minbo, et al. Theoretical investigation of the bond dissociation of hydroxyl terminated polybutadiene binder and effect on mechanical properties[J]. Acta Physico-Chimica Sinica, 2012, 70(10): 1145-1152.
[10] Zhao Jinchao, Zhang Aiqing, Luo Zhihua, et al. On-line monitoring the curing behaviors of hydroxyl-terminated polybutadiene/toluene diisocyanate system via ultrasonic wave[J]. Journal of Functional Materials, 2011, 42(9): 1649-1652.
[11] Kincal D, Özkar S. Kinetic study of the reaction between hydroxyl-terminated polybutadiene and isophorone diisocyanante in bulk by quantitative FT-IR spectroscopy[J]. Journal of Applied Polymer Science, 1997, 66(10): 1979-1983.
[12] Sarkar S, Adhikari B. Synthesis and characterization of ligin-HTPB copolyurethane[J]. European Polymer Journal, 2001, 37(7): 1391-1401.
[13] Mishra A K, Chattopadhyay D K, Sreedhar B, et al. FT-IR and XPS studies of polyurethane-urea-imide coatings[J]. Progress in Organic Coatings, 2006, 55(3): 231-243.
[14] Li Cuihua, Liu Jianhong, Zhang Qianling, et al. Investigation on the absorption and diffusion of water and methanol in the sulfontaed poly(ethersulfone) membrane[J]. Journal of Shenzhen University Science and Engineering, 2008, 25(1): 19-23. (in Chinese)
[15] Hailu K, Guthausen G, Becker W. In-situ characterization of the cure reaction of HTPB and IPDI by simultaneous NMR and IR measurements[J]. Polymer Testing, 2010, 29(4): 513-519.
[16] Shane P, Min K, Cakmk M. Kinetic studies of polyuretheane polymerization with Raman spectroscopy[J]. Polymer, 2003, 44(18): 5137-5144.
[17] Nikolova D, Edreva-Kardjieva R, Giurginca M. The effect of potassium addition on the state of the components in the oxide precursor of the (Ni)(Mo)/γ-Al2O3water-gas shift catalysts: FT-IR, diffuse reflectance and Raman spectroscopic studies[J]. Vibrational Spectroscopy. 2007, 44(2): 343-350.
[18] Yin Huali, Wang Yu, Li Dongfeng. Reaction mechanism at interface of HTPB/TDI liner-NEPE propellant[J]. Journal of Solid Rocket Technology, 2010, 33(1): 63-67.
[19] Sankar G, Nasar A S. Cure reaction kinetics of amine-blocked polyisocyanates with alcohol using hot-stage fourier transform infrared spectroscopy[J]. Journal of Applied Polymer Science, 2008, 109(2): 1168-1176.
[20] Vargas M A,Sachsenheimer K,Guthausen G. In-situ investigations of the curing of a polyester resin[J]. Polymer Testing, 2012, 31(1):127-135.
[21] Wang Wenping,Pan Caiyuan.Synthesis and characterization of poly(ethylene oxide) methyl ether grafted on the expanded graphite with isocyanate groups[J]. European Polymer Journal, 2004, 40(3):543-548.
【中文责编:晨兮;英文责编:新谷】
Li Cuihua, Luo Tao, Zhang Xiaoxue, et al. In situ FT-IR spectroscopic studies of curing reaction of HTPB and TDI on solid surface[J]. Journal of Shenzhen University Science and Engineering, 2016, 33(5): 452-456.
Li Cuihua1†, Luo Tao1, Zhang Xiaoxue1, Liu Jianhong1,Pang Aimin2, and Chi Xuhui2
1)College of Chemistry and Environmental Engineering, Shenzhen University, Shenzhen 518060, Guangdong Province, P.R.China 2)The 42nd Institute of the Fourth Academy, CASC, Xiangyang 441003, Hubei Province, P.R.China
红外光谱采用漫反射模式(diffuse reflectance infrared fourier transform spectroscopy, DRIFTS)可直接对固体表面化学基团进行定性和定量分析. 通过在DRIFTS上安装热台,实时监测不同反应温度时异氰酸酯基的含量及其变化,可实现在固体表面原位表征端羟基聚丁二烯(hydroxyl-terminated polybutadiene, HTPB)与甲苯二异氰酸酯(toluene diisocyanate, TDI)的聚合反应. 通过不同温度时异氰酸酯基(—NCO)含量随反应时间的变化计算了活化能. 实验结果表明,原位漫反射红外光谱可准确监测HTPB与TDI的聚合反应,且实时快捷.
分析化学;漫反射红外光谱;原位;异氰酸酯基;聚合反应;聚氨酯
国防重点基础研究发展计划资助项目(613142020201);深圳市科学技术研究与发展基金资助项目(JCYJ20140418193546111)
李翠华(1968—),女,深圳大学教授. 研究方向:高分子化学与物理. E-mail:licuihua@szu.edu.cn
/
2016-04-14;Accepted:2016-06-07
O 631.3Document code: Adoi:10.3724/SP.J.1249.2016.05452
Foundation:National Defense Basic Research Program of China (613142020201); Scientific and Technological Research and Development Foundation of Shenzhen City (JCYJ20140418193546111)
† Corresponding author:Professor Li Cuihua. E-mail: licuihua@szu.edu.cn
引文:李翠华, 罗涛, 张小雪, 等. 固体表面HTPB和TDI固化反应原位红外光谱分析[J]. 深圳大学学报理工版,2016,33(5):452-456.(英文版)
