天然活性成分雏菊叶龙胆酮的研究进展△
2016-09-25赵维韩华锐赵雅婵尚志强吕丽娟
赵维,韩华锐,赵雅婵,尚志强*,吕丽娟*
(1.天津农学院 基础科学学院,天津 300384;2.包头医学院,内蒙古 包头 014060)
天然活性成分雏菊叶龙胆酮的研究进展△
赵维1,韩华锐2,赵雅婵1,尚志强1*,吕丽娟1*
(1.天津农学院 基础科学学院,天津 300384;2.包头医学院,内蒙古 包头 014060)

雏菊叶龙胆酮;植物分布;药理活性;进展

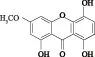
图1 雏菊叶龙胆酮结构式

1 雏菊叶龙胆酮在植物中的分布

表1 雏菊叶龙胆酮在植物中的分布

表1(续)
2 制备方法、结构确证及质量评价方法
虽然雏菊叶龙胆酮在药用植物中分布广泛,但是其在植物中的含量比较低。截止目前,有关雏菊叶龙胆酮的制备方法多为溶剂萃取(或同时辅以超声等手段),再借助多种分离介质,如HP-20树脂、大孔吸附树脂、硅胶、葡聚糖凝胶以及制备液相色谱、高速逆流色谱(HSCCC)等技术[56]来实现其提取分离和纯化。其中HSCCC法作为一种液-液分配色谱技术,无需固体支撑载体,避免了因不可逆吸附而造成的样品损失,有着广阔的应用空间。
目前,该化合物的制备仍然局限于从植物中提取分离而制得,未见有关其生物合成或化学全合成的报道。在现有技术中,刘东锋等[57]提出自己的制备方法,该方法适用于雏菊叶龙胆酮的产业化放大。其具体做法:将尖叶假龙胆(G.acuta)以70%~90%丙酮溶液超声提取,提取液回收试剂经大孔树脂吸附,分别用40%~50%、70%甲醇溶液洗脱,得粗提物,再用高速逆流色谱仪分离。
自上述药用植物中制备得到的雏菊叶龙胆酮,其结构确证方法多用紫外光谱(UV)、红外光谱(IR)、质谱(MS)、氢谱(1HNMR)、碳谱(13CNMR)、二维核磁共振谱(2D-NMR)等技术,仅有个别文献[58]采用了单晶X射线衍射法(SXRD)。其检测及质量评价多采用HPLC法,少量报道采用高效液相色谱-电喷雾电离-多级质谱联用(HPLC-ESI-MSn)技术[52]、基于1H-NMR代谢物组学技术[59]完成化合物的检测分析。
3 药理活性、作用机制及应用
研究表明,雏菊叶龙胆酮具有广泛的生物学活性。
3.1 降血糖作用

Tian等[64]发现,日本獐牙菜(S.japonica)的醇提物具有降血糖作用;紫红獐牙菜(S.punicea)提取物对由各因素引起的高血糖均有一定的抑制作用,其中雏菊叶龙胆素通过抑制G-6-P活性,能明显地降低空腹血糖,改善口服糖耐量,降低空腹血清胰岛素。实验结果表明,雏菊叶龙胆素可降低血清总胆固醇(TC)、低密度脂蛋白胆固醇(LDL)和甘油三脂(TG),升高高密度脂蛋白胆固醇(HDL),通过增强胰岛素信号转导,改善胰岛素抵抗,增加肝糖原,降低葡萄糖激酶活性,增加葡萄糖-6-磷酸酯酶活性。雏菊叶龙胆素不仅能够有效地治疗Ⅱ型糖尿病,而且可以有效地改善机体对胰岛素的耐受性[65]。
文莉[66]考察了湖北麦冬[Liriopespicata(Thunb.)Lour.var.proliferaY.T.Ma]多糖与雏菊叶龙胆酮配伍对STZ小鼠空腹血糖和糖耐量的作用。结果表明,二者均有降血糖活性,但两者间无明显协同作用。同时,采用逆转录-聚合酶链反应(RT-PCR)技术探究了雏菊叶龙胆酮的胰岛素抵抗机理。结果表明,雏菊叶龙胆酮在过氧化物酶体增殖物激活受体γmRNA(PPARγmRNA)表达中未见明显促进PPARγmRNA表达的作用,其降低血糖活性与PPARγ无明显关系。
3.2 抗氧化作用


3.3 抗菌作用

Saeed等[28]发现,雏菊叶龙胆酮对5种革兰阳性菌(巨大芽孢杆菌、枯草芽孢杆菌、苏云金芽孢杆菌、藤黄八叠球菌和金黄色葡萄球菌)、4种革兰阴性菌(大肠杆菌、克雷伯肺炎菌、变形杆菌和假单胞菌)以及黑曲霉等8种真菌均有不同程度的抑制作用。
3.4 抗病毒及保肝作用
雏菊叶龙胆酮体外抗乙肝病毒实验表明,其对2215细胞的乙型肝炎表面抗原(HBsAg)表达有抑制作用,半数抑制浓度(IC50)为13 μg·mL-1,选择指数(SI)为6.8,而对2215细胞DNA无明显抑制作用[72-73]。Cao等[74]发现,从川西獐牙菜(S.mussotii)中提取的雏菊叶龙胆酮也具有明显的乙型肝炎表面抗原HBsAg(IC50>0.98 mM)和乙型肝炎e抗原(HBeAg)(IC50=0.35 mM)作用。
体外毒性及保肝活性实验表明,雏菊叶龙胆酮具有保护人肝癌细胞系HepG2细胞活性[75],通过增加谷胱甘肽的含量、减少氢氧自由基[45,47]和明显地抑制谷丙转氨酶(GPT)的活性[76-77],减轻对乙酰氨基酚诱导的药源性HepG2肝细胞损伤。

3.5 胆碱酯酶抑制作用

3.6 单胺氧化酶抑制作用
单胺氧化酶(MAO)是一种与神经系统中生物单胺的代谢有关的酶。体外实验发现,雏菊叶龙胆酮是MAO的有效抑制剂,有望在治疗抑郁、精神分裂等精神疾病中发挥作用[5,80-85]。
3.7 心血管系统的作用
武海军等[86]研究了雏菊叶龙胆酮对正常大鼠心率及心电图的影响。结果表明,大鼠心率随雏菊叶龙胆酮剂量增加而减慢;P波时间和P波电压略有抬高,心房到心室传导所需时间(P-R)间期明显缩短,心室除极和复极的全过程所需时间(Q-T)间期在大剂量组(0.024 mg·kg-1)、中剂量组(0.012 mg·kg-1)延长,而小剂量组(0.006 mg·kg-1)无明显变化,心室除极(QRS)时限随剂量增加而延长。
李旻辉等[87]采用维拉帕米作为阳性对照组,研究雏菊叶龙胆酮对氯化钡致大鼠心律失常的防治作用。结果表明,与对照组相比,3个剂量组(0.05、0.025、0.0125 mg·kg-1)均使大鼠心律失常发生时间推迟,严重程度减轻,并且0.05、0.025 mg·kg-1剂量组心律失常持续时间明显缩短。
3.8 对缺血性脑损伤的保护作用
宋慧君等[88]研究雏菊叶龙胆酮对小鼠永久性、完全性全脑缺血损伤和大鼠弥散性不完全性全脑缺血损伤的保护作用。结果表明,雏菊叶龙胆酮可减轻大鼠脑缺血后的脑损伤程度;延长小鼠断头后的张口喘息时间;降低大鼠脑缺血后的脑指数,说明雏菊叶龙胆酮对缺血性脑损伤具有显著的脑保护作用。同时发现,大鼠脑缺血后,脑内MDA含量增加,超氧化物歧化酶(SOD)活力下降,脑内谷氨酸(GLu)及γ-氨基丁酸(GABA)含量明显增加。给予一定剂量雏菊叶龙胆酮后,可显著升高大鼠脑内SOD活力,降低MDA及Glu含量,说明雏菊叶龙胆酮的脑保护作用有抗氧化及减轻兴奋毒性损伤机制的介入。而给药后使GABA含量显著降低的确切机制还有待进一步探讨。
另外,张剑辉等[89]利用电凝法制作大鼠右侧大脑中动脉阻塞(MCAO)模型,证明了口服给药雏菊叶龙胆酮能改善MCAO缺血后神经功能障碍,并缩小脑梗塞面积,减轻相关脑区神经元损伤程度,显著抑制大鼠局灶性脑缺血损伤细胞间黏附分子-1(ICAM-1)表达,上调缺血周边区神经元B淋巴细胞瘤-2(Bcl-2)抗凋亡蛋白的表达。说明雏菊叶龙胆酮对大鼠局灶性脑缺血损伤有保护作用,其作用机制可能与抑制ICAM-1表达和促进Bcl-2表达有关。
4 毒性研究
4.1 急性毒性
丁莉等[90]以小鼠为研究对象,经口灌胃方式给药,通过急性毒性实验证明,在实验期间小鼠未出现明显中毒症状和死亡;小鼠对雏菊叶龙胆酮最大耐受量(MTD)为15 g·kg-1。表明该化合物安全可靠,可为其制剂开发提供一定的理论依据。
4.2 遗传毒性
文莉等[91]以0.9%氯化钠溶液作为阴性对照,环磷酰胺作为阳性对照,通过小鼠骨髓嗜多染红细胞(PCE)微核实验证明,雏菊叶龙胆酮各组小鼠骨髓嗜多染红细胞细胞微核发生率明显低于阳性对照组,且差异有统计学意义,而与阴性对照组比较差异无统计学意义,说明雏菊叶龙胆酮对体细胞无明显的致突变作用。另外,选用已知致突变物二氨基芴、1,8-二羟基蒽醌、敌克松、叠氮化钠作为阳性对照,二甲基亚砜为阴性对照,通过Ames实验证明,无论是否加入诱导剂,0.5~5000 μg·mL-1的雏菊叶龙胆酮对实验用4种菌株(鼠伤寒沙门菌组氨酸营养缺陷型TA97、TA98、TA100、TA102)所诱发的回变菌落数均与自发回变和阴性对照相近,与超过阴性对照组的2倍才判断为能导致突变的评价标准还有较大距离。
5 展望
综上所述,雏菊叶龙胆酮主要分布于龙胆科獐牙菜属(33种)、假龙胆属(20种)、龙胆属(7种)植物中。此外,其在龙胆科扁蕾属(1种)、腺鳞草属(1种)、萝藦科娃儿藤属(1种)和鸢尾科鸢尾属(1种)植物中也有少量分布。雏菊叶龙胆酮在降血糖、抗氧化、抗菌、抗病毒、抑制胆碱酯酶及单胺氧化酶、保护心血管系统及缺血性脑损伤等方面具有一定的疗效。同时毒性研究证实,该化合物安全,且没有表现出明显的致突变作用。

[1] Lesch B,Bräse S.A short,atom-economical entry to tetrahydroxanthenones[J].Angew Chem Int Ed Engl,2004,43(1):115-118.
[2] Tozhiboev M M,Botirov E K,Usmanova G A.Xanthones and flavonoids fromGentianaalgidaPall[J].Russ J Bioorg Chem,2011,37(7):866-870.
[3] Hostettmann M,Hostettmann K,Sticher O.Xanthones,flavones,and secoiridoids of AmericanGentianaspecies[J].Phytochemistry,1981,20(3):443-446.
[4] Isakovic A,Jankovic T,Harhaji L,et al.Antiglioma action of xanthones fromGentianakochiana:Mechanistic and structure-activity requirements[J].Bioorg Med Chem,2008,16(10):5683-5694.
[5] Schaufelberger D,Hostettmann K.Chemistry and pharmacology ofGentianalactea[J].Planta Med,1988,54(3):219-221.
[6] Wolfender J L,Rodriguez S,Hostettmann K,et al.Liquid chromatography/ultra violet/mass spectrometric and liquid chromatography/nuclear magnetic resonance spectroscopic analysis of crude extracts of Gentianaceae species[J].Phytochem Anal,1997,8(3):97-104.
[7] Nadinic E L,Saavedra C L,Lira P D L,et al.Tetraoxygenated xanthones fromGentianellaflorida[J].Pharm Biol,1997,35(5):379-381.
[8] Rivaille P,Raulais D.Xanthones and other constituents of grentiana and swertia.presence of a new triterpene in gentiana verna[J].Comptes Rendus des Seances de l’Academie des Sciences,Serie D:Sciences Naturelles,1969,269(12):1121-1124.
[9] Nadinic E,Penna C,Saavedra C.Isolation of antimicrobial compounds fromGentianellaachalensis(Gilg) Ho & Liu(Gentianaceae) extracts[J].Lat Am J Pharm,2002,21(2):123-130.
[10] Lv L J,Li M H.Terpenoids,flavonoids and xanthones fromGentianellaacuta(Gentianaceae)[J].Biochem Syst Ecol,2009,37(4):497-500.
[11] Jankovic T,Krstic D,Aljancic I,et al.Xanthones and C-glucosides from the aerial parts of four species ofGentianellafrom Serbia and Montenegro[J].Biochem Syst Ecol,2005,33(7):729-735.
[12] Urbain A,Marston A,Grilo L S,et al.Xanthones fromGentianellaamarellassp.acuta with acetylcholinesterase and monoamine oxidase inhibitory activities[J].J Nat Prod,2008,71(5):895-897.
[13] Benn M H,Joyce N I,Lorimer S D,et al.Xanthones and bisxanthones in five New Zealand and subantarctic Gentianella species[J].Biochem Syst Ecol,2009,37(4):531-534.
[14] Zhang Y J,Yang C R.Chemical studies onGentianellaazurea,a Tibetan medicinal plant[J].Acta Bot Yunnan,1994,16(4):401-406.
[15] Markham R.Gentian pigments.II.Xanthones fromGentianabellidifolia[J].Tetrahedron,1965,21(6):1449-1452.
[16] Urbain A,Marston A,Marsden-Edwards E,et al.Ultra-performance liquid chromatography/time-of-flight mass spectrometry as a chemotaxonomic tool for the analysis of Gentianaceae species[J].Phytochem Anal,2009,20(2):134-138.
[17] Rosella M A,Etile E D,Spegazzini S L,et al.97-Parametros micrograficosy fitoquimicos para el reconocimiento de dos especies de Gentianella(Gentianaceae)[J].Bol Latinoam Caribe Plant Med Aromát,2007,6(6):384-385.
[18] Báez D H,Quintero M,Nieves B.Toxicity,antimicrobial activity and detection of xanthones inGentianellanevadensis[J].Ciencia,1999,7(2):111-117.
[19] Hostettmann-Kaldas M,Jacot-Guillarmod A.Contribution to the phytochemistry of the genus Gentiana.Part XXIII.Xanthones and flavone C-glucosides of the genus Gentiana(subgenus Gentianella)[J].Phytochemistry,1978,17(12):2083-2086.
[20] Massias M,Carbonnier J,Molho D.Chemotaxonomy of Gentianopsis:Xanthones,C-glycosylflavonoids and carbohydrates[J].Biochem Syst and Ecol,1982,10(4):319-327.
[21] Tomas G E,Lock O,Jurupe H.Chemical study and hypoglycemic and hypolipemic activity ofGentianellathyrsoideaHooker Fabris[J].Boletin de la Sociedad Quimica del Peru,1999,65(4):231-238.
[22] KhanT A,HaqqanM H,NisarN M.Chemical investigation ofSwertiaalata[J].Planta Med,1979,37(2):180-181.
[23] 杨俊美.固公果根和美丽獐牙菜的化学成分研究[D].昆明:云南中医学院,2014.
[24] 李兆云,王聪,张桢,等.宾川獐牙菜化学成分研究[J].时珍国医国药,2011,22(5):1086-1087.
[25] 田峦鸢,陈家春,黄风娇,等.高效液相法同时测定獐牙菜属植物中四种活性成分(英文)[J].中国天然药物,2008,6(6):444-449.
[26] Ya B Q,Gen X P.Four xanthones fromSwertiacalycinaFranch[J].Pharm Pharmacol Commun,1998,4(12):595-596.
[27] Shi G F,Wang G Y,Chen X F.Screening of radical-scavengine natural neuroprotective antioxidants fromSwertiachirayita[J].Acta Biol Hun,2013,64(3):267-278.
[28] Saeed M A,Khan Z U D,Ford M R.Antimicrobial potential of some xanthones fromSwertiaciliataBuch et Ham[J].Acta Pharm Turcica,1998,40(4):175-184.
[29] 徐康平,申健,李福双,等.多波长HPLC同时测定3种獐牙菜属植物中6种活性成分[J].中国中药杂志,2009,34(11):1384-1389.
[30] Tomimori T,Yoshizaki M,Namba T.Nepalese crude drugs.II.Xanthone constituents of the plants of Swertia species[J].Yakugaku Zasshi,1974,94(5):647-651.
[31] 肖晶.北方獐牙菜的化学成分研究[D].大连:大连理工大学,2010.
[32] Hu B L,Hong S F,ShuH F,et al.The xanthones ofSwertiaerythrostictaMaxim[J].Acta Bot Sin,1992,34(11):886-886.
[33] Liu Y,Xu Q,Xue X,et al.Two-dimensional LC-MS analysis of components inSwertiafranchetianaSmith[J].J Sep Sci,2008,31(6/7):935-944.
[34] Wang H L,Cao T W,Jiang F Q,et al.Swerpunilactones A and B,the first example of xanthone and secoiridoid heterodimers fromSwertiapunicea,S.hispidicalyx,andS.yunnanensis[J].Tetrahedron Lett,2013,54(21):2710-2712.
[35] Ghosal S,Biswas K,Jaiswal D K.Chemical constituents of Gentianaceae.Part 27.Xanthone and flavonol constituents ofSwertiahookeri[J].Phytochemistry,1980,19(1):123-126.
[36] Denisova A,Solov’eva V,Glyzin I,et al.Xanthone glycosides from the aboveground part ofSwertiaiberica[J].Khim Prir Soedin,1980(5):724-5
[37] Wang Z,Ma C,Tang S,et al.Qualitative and quantitative analysis of Swertia herbs by high performance liquid chromatography-diode array detector-mass spectrometry(HPLC-DAD-MS)[J].Chem Pharm Bull,2008,56(4):485-490.
[38] 周青.贵州獐牙菜化学成分研究[D].武汉:湖北中医学院,2003.
[39] Zhou H M,Liu Y L,Blasko G,et al.Swertia bisxanthone-I fromSwertiamacrosperma[J].Phytochemitry,2008,28(12):3569-3571.
[40] Fan G,Luo W Z,Luo S H,et al.Metabolic discrimination ofSwertiamussotiiandSwertiachirayitaknown as “Zangyinche” in traditional Tibetan medicine by 1H NMR-based metabolomics[J].J Pharm Biomed Anal,2014,98:364-370.
[41] 郭志威.显脉獐牙菜活性成分及其含量测定[D].长沙:中南大学,2007.
[42] Prakash A,Basumatary P C,Ghosal S,et al.Chemical constituents ofSwertiapaniculata[J].Planta Med,1982,45(5):61-62.
[43] Hostettmann K,Jacot-Guillarmod A.Identification of xanthones and new arabinosides of flavone C-glucosides fromSwertiaperennisL[J].Helv Chim Acta,1976,59(5):1584-1591.
[44] Baslas R K,Kumar P.Isolation and characterization of biflavanone and xanthones in the fruits ofGarciniaxanthochymus[J].Acta Cienc Indica Chem,1981,7(1/4):31-34.
[45] Li J C,Feng L,Sun B H,et al.Hepatoprotective activity of the constituents inSwertiapseudochinensis[J].Biol Pharm Bull,2005,28(3):534-537.
[46] Menkovic N,Savikin-Fodulovic K,Bulatovic V,et al.Xanthones fromSwertiapunctate[J].Phytochemistry,2002,61(4):415-420.
[47] Zheng X Y,Yang Y F,Li W,et al.Two xanthones fromSwertiapuniceawith hepatoprotective activities in vitro and in vivo[J].J Ethnopharmacol,2014,153(3):854-863.
[48] Ghosal S,Sharma P V,Chaudhuri R K,et al.Chemical constituents of gentianaceae.XIV.Tetraoxygenated and pentaoxygenated xanthones ofSwertiapurpurascens[J].J Pharm Sci-US,1975,64(1):80-83.
[49] Hirakawa K,Yoshida M,Nagatsu A,et al.Chemopreventive action of xanthone derivatives on photosensitized DNA damage[J].Photochem Photobiol,2005,81(2):314-319.
[50] 廖志新,胡伯林,纪兰菊.轮叶獐牙菜的化学成分研究[J].植物学报,1991,33(12):968-970.
[51] 于莹,王世盛,丁凤娟,等.云南獐牙菜化学成分的分离与鉴定[J].中国药物化学杂志,2010,20(2):125-128.
[52] 罗洲飞,刘妮娜,徐彦军,等.扁蕾不同部位的化学成分研究[J].湖南农业科学,2012(9):99-102.
[53] Olennikov D N.Chemical investigation ofAnagallidiumdichotomumand anticholinesterase activity of its constituents[J].Chem Nat Compd,2014,49(6):1137-1139.
[54] Suleiman K,Hideki T,Munekazu I.A xanthone C-glycoside fromIrisnigricans[J].Phytochemistry,1995,38(3):729-731.
[55] Yao S,Tang C P,Ye Y.Secoiridoids and xanthones fromTylophorasecamonoidesTsiang[J].J Asian Nat Prod Res,2008,10(5/6):591-596.

[57] 刘东锋,杨成东.一种雏菊叶龙胆酮的制备方法:CN103275054A[P].2013-09-04.
[58] Shi G F,Lu R H,Yang Y S.Isolation and crystal structure of xanthones fromSwertiaChirayita[J].Chin J Struc Chem,2004,23(10):1164-1168.
[59] 范刚.基于1H-NMR代谢物组学技术的多基源中藏药品种质量评价研究[D].成都:成都中医药大学,2012.
[60] 万落生.贵州獐牙菜抗糖尿病活性和物质基础研究[D].武汉:华中科技大学,2013.
[61] Basnet P,Kadota S,Shimizu M,et al.Bellidifolin:a potent hypoglycemic agent in streptozotocin(STZ)-induced diabetic rats fromSwertiajaponica[J].Planta Med,1994,60(6):507-511.
[62] Basnet P,Kadota S,Shimizu M,et al.Bellidifolin stimulates glucose uptake in rat 1 fibroblasts and ameliorates hyperglycemia in streptozotocin(STZ)-induced diabetic rats[J].Planta Med,1995,61(5):402-405.
[63] Yamakaral M,Matsuda H.Antieholinergieaetion ofSwertiajaponieaand anaetiveeon stituent[J].J Ethnopharmacol,1991,33(1):31-35.
[64] Tian L Y,Bai X,Chen X H,et al.Anti-diabetic effect of methylswertianin and bellidifolin fromSwertiapuniceaHemsl.and its potential mechanism[J].Phytomedicine,2010,17(7):533-539.
[65] Satendra S,Asthana R K,Gupta R C.Assessment of systemic interaction betweenSwertiachirataextract and its bioactive constituents in rabbits[J].Phytother Res,2009,23(7):1036-1038.
[66] 文莉.紫红獐牙菜和湖北麦冬降血糖活性及遗传毒性研究[D].武汉:湖北中医学院,2008.
[67] 王世盛.藏茵陈活性组分的制备分离和化学表征[D].大连:中国科学院大连化学物理研究所,2004.
[68] 张媛嫒.藏药大籽獐牙菜化学成分及生物活性研究[D].成都:西南交通大学,2006.
[69] Ashida S,Noguchi S F,Suzuki T.Antioxidative components,xanthone derivatives,inSwertiajaponicaMakino[J].J Am Oil Chem Soc,1994,71(10):1095-1099.
[70] Rana V S,Rawat M S M.A new xanthone glycoside and antioxidant constituents from the rhizomes of Swertia speciose[J].Chem Biodivers,2005,2(10):1310-1315.

[72] 张秀桥.复方紫金肝泰抗HBV物质基础的研究[D].武汉:湖北中医学院,2006.
[73] 黄凤娇.紫红獐牙菜化学成分的含量测定及体外抗HBV活性物质的筛选[D].武汉:湖北中医学院,2006.
[74] Cao T W,Geng C A,Ma Y B,et al.Xanthones with anti-hepatitis B virus activity fromSwertiamussotii[J].Planta Med,2013,79(8):697-700.

[76] Koji H,Li J X,Basnet P,et al.Hepatoprotective principles ofSwertiajaponicaMakino on D-galactosamine/lipopolysaccharide-induced liver injury in mice[J].Chem Pharm Bull,1997,45(11):1823-1827.
[77] Koji H,Kadota T,Basnet P,et al.Tetrahydroswertianolin:a potent hepatoprotective agent fromSwertiajaponicaMakino[J].Chem Pharm Bull,1997,45(3):567-569.
[78] 潘锡平,林艳和,李彪.一种治疗肝炎和糖尿病的药物组合物:CN1686141[P].2005-10-26.
[79] Urbain A,Marston A,Fererira Q E,et al.Xanthones fromGentianacampestrisas new acetylcholinesterase inhibitors[J].Planta Med,2004,70(10):1011-1014.
[80] Masand V H,Patil K N,Mahajan D T,et al.3D-QSAR studies on xanthone derivatives to understand pharmacological activities as MAO inhibitors[J].Pharma Chemica,2010,2(5):22-32.
[81] Masand V H,Patil K N,Mahajan D T,et al.3D-QSAR studies on xanthone derivatives to understand pharmacological activities as MAO inhibitors[J].Pharma Chemica,2010,2(4):298-308.
[82] Deeb O,Alfalah S,Clare B W.QSAR of aromatic substances:MAO inhibitory activity of xanthone derivatives[J].J Enzym Inhib Med Ch,2007,22(3):277-286.
[83] Nunez M B,Maguna F P,Okulik N B,et al.QSAR modeling of the MAO inhibitory activity of xanthones derivatives[J].Bioorg Med Chem Lett,2004,14(22):5611-5617.
[84] Fowler C J,Ross S B.Selective inhibitors of monoamine oxidase A and B:biochemical,pharmacological,and clinical properties[J].Med Res Rev,1984,4(3):323-58.
[85] Ohishi N,Suzuki T,Ogasawara T,et al.Xanthone derivatives as inhibitors for monoamine oxidase[J].J Mol Catal B-Enzym,2000,10(1/3):291-294.
[86] 武海军,李旻辉,张海涛,等.Bellidifolin对麻醉大鼠心电图的影响[J].包头医学院学报,2011,27(1):22.

[88] 宋慧君,张建辉,闫平,等.雏菊叶龙胆酮对缺血性脑损伤保护作用的实验研究[J].大连医科大学学报,2007,29(2):110-112.
[89] 张建辉,宋慧君,李淑媛.雏菊叶龙胆酮对局灶性脑缺血损伤的保护作用及机制探讨[J].中国药理学通报,2005,21(2):220-224.
[90] 丁莉,文莉,汪晖,等.Bellidifolin对小鼠的急性毒性及对小鼠骨髓细胞的微核效应[J].内科,2006,1(2):145-146.
[91] 文莉,陈家春.降血糖活性成分Bellidifolin遗传毒性研究[J].医药导报,2008,27(11):1317-1319.
ResearchProgressonBellidifolin
ZHAO Wei1,HANHuarui2,ZHAOYachan1,SHANGZhiqiang1,LYULijuan1
(1.DepartmentofBasicScience,TianjinAgriculturalUniversity,Tianjin300384,China;2.BaotouMedicalCollege,Baotou,InnerMongolia014060,China)
Bellidifolin,a kinds of natural tetraoxygenated xanthone,was mainly distributed among genusSwertia,Gentianella,andGentianain family Gentianaceae and possesses multifarious pharmacological activities,including hypoglycemic,anti-oxidant,anti-bacterial,anti-viral,protecting cardiovascular and cerebral is chemia injuries,etc.In order to provide scientific references for the in-depth study,development and utilization of the compound,this review comprehensively summarizes the distribution,preparation,quality evaluation,pharmacological activities and toxicity studies of bellidifolin.
Bellidifolin;distribution;pharmacological activities;progress
2015-04-25)
国家自然科学基金项目(81303306,81160504);天津市应用基础与前沿技术研究计划(15JCQNJC13400);天津市大学生创新创业训练计划项目(201410061052,201410061159)
*
尚志强,博士,讲师,研究方向:药物合成化学,E-mail:shangzq1978@sina.com;吕丽娟,博士,讲师,研究方向:药物分析与晶型药物研究,E-mail:lv_lijuan@aliyun.com
10.13313/j.issn.1673-4890.2016.5.029
