Purification,Crystallization and X-ray Diffraction Analysis of a Halotolerant Esterase PE8
2016-09-21JIRuiKUANGSiyunHUOYingyiHUANGJingLANXingyangXUXueweiLIJixi
JI Rui, KUANG Siyun, HUO Yingyi, HUANG Jing, LAN Xingyang , XU Xuewei, LI Jixi
(1. Shanghai Engineering Research Center of IndustrialMicroorganisms, School of Life Sciences, Fudan University, Shanghai 200438, China; 2. State Key Laboratory ofGenetics Engineering, Fudan University, Shanghai 200438, China; 3. Laboratory of Marine Ecosystemand Biogeochemistry, Second Institute of Oceanography, State Oceanic Administration, Hangzhou 310012, China)
纪 锐1,2,匡思运1,2,霍颖异3,黄 静1,2,兰星洋1,2,许学伟3,李继喜1,2
(1. 复旦大学 生命科学学院 上海市工业菌株工程技术研究中心,上海 200438;2. 复旦大学 遗传工程国家重点实验室,上海 200438;3. 国家海洋局第二海洋研究所 海洋生态系统和生物地球化学重点实验室,浙江 杭州 310012)
Purification,Crystallization and X-ray Diffraction Analysis of a Halotolerant Esterase PE8
JI Rui1,2, KUANG Siyun1,2, HUO Yingyi3, HUANG Jing1,2, LAN Xingyang1,2, XU Xuewei3, LI Jixi1,2
(1.ShanghaiEngineeringResearchCenterofIndustrialMicroorganisms,SchoolofLifeSciences,FudanUniversity,Shanghai200438,China; 2.StateKeyLaboratoryofGeneticsEngineering,FudanUniversity,Shanghai200438,China; 3.LaboratoryofMarineEcosystemandBiogeochemistry,SecondInstituteofOceanography,StateOceanicAdministration,Hangzhou310012,China)
The esterase PE8 was firstly identified as a new member of type Ⅵ lipolytic enzyme from the marine bacteriumPelagibacteriumhalotoleransB2T. Due to its halotolerance, hyperthermostability, and high enantioselectivity, PE8 is a potential candidate for the highly efficient production of chiral(R)-3-MFG in industry. Here, PE8 was purified to a high homogeneity by gel filtration chromatography, and then crystallized by hanging drop method. The crystals diffracted to 1.66 Å resolution, and belong to space group P21, with unit-cell parametersa=41.772,b=73.398,c=66.403Å,β=102.37°. An asymmetric unit contains two protein molecules, with a solvent containing at 35%.
Esterase; PE8; Crystallization
Esterases(EC 3.1) belong to the hydrolase enzyme family. Due to their high enzymatic activity and stereospecificity to many esters, esterases are extensively applied in pharmaceutical and chemical synthesis processes[1]. Many esterases are found in marine microbes[2]. As these esterases have specific enzymatic characterizations, which include halotolerance, high enantioselectivity and hyperthermostability, they are promising candidates to become biocatalyst in industry[3].
The esterase PE8 was firstly identified from marine bacteriumPelagibacteriumhalotoleransB2T, and was characterized as a new type Ⅵ lipolytic enzyme[4-5]. PE8 is an alkaline esterase with an optimal temperature of 45℃ for catalyzing short chainp-nirophenyl acetates(C2-C6). Moreover, PE8 has high activity at even pH 11.0 in 1 mol/L phosphate buffer[5].(R)-3-MFG(methyl(R)-3-(4-fluorophenyl) glutarate) is an important precursor to produce antidepressant(-)-paroxetine hydrochloride[6]. Compared to the traditional chemical separation method,(-)-paroxetine hydrochloride can be more effectively and efficiently obtained from prochiral molecular 3-DFG(dimethyl 3-(4-fluorophenyl) glutarate) through enzymatic ways[5-7]. Previous studies have shown that PE8 has properties such as halotolerance, hyperthermostability, barophilicity, cold adaptivity and high enantioselectivity in the synthesis of(R)-3-MFG[5-7](Fig.1).
Here, we reported the purification and crystallization of PE8 in order to obtain the structural information for understanding the potential uses of PE8 as a biocatalyst in industry. PE8 diffracted to 1.66 Å resolution,and belongs to space group P21.
1 Materials and methods
1.1Protein expression and purification
The molecular cloning and expression of PE8 have been described previously[4-5]. In brief, PE8 was expressed as a fusion protein with a cleavable N-terminal 6×His tag from pET-28(b) vector[8].E.coliRosseta(DE3) cells transformed with the pET-28(b)-PE8 fusion plasmid were grown in Luria-Bertani broth at 37℃ to an absorbance(OD600) of 0.8 and then induced with 0.5mmol/L IPTG for 8 h at 25℃. Cells were harvested through centrifugation at 5000 r/min, resuspended in a lysis buffer(50 mol/L Tris-HCl pH8.0, 500mmol/L NaCl, 5% glycerol, 10mmol/L imidazole, 2mmol/Lβ-mercaptoethanol), and passed twice through a high-pressure homogenizer. The crude cell lysate was centrifuged at 17000 r/min for 1 h at 4℃, and the supernatant was loaded on a pre-equilibriumed Ni-NTA column. After washed three times with a washing buffer(50mmol/L Tris-HCl pH8.0, 500mmol/L NaCl, 5% glycerol, 50mmol/L imidazole, 2mmol/Lβ-mercaptoethanol), the PE8 protein was eluted with an elution buffer(50mmol/L Tris-HCl pH8.0, 500mmol/L NaCl, 5% glycerol, 250mmol/L imidazole, 2mmol/L β-mercaptoethanol), and further purified by chromatography through a Superdex200 16/600 gel filtration column in a buffer with 20mmol/L Tris-HCl pH7.4, 100mmol/L NaCl, 2mmol/L DTT. The protein concentration was measured with the Bradford method[9]and its purity was analyzed by SDS-PAGE.
1.2Crystallization
The PE8 protein was concentrated to 20 mg/mL and first screened at 20℃ in sitting drops. The buffer composition of protein solution is 20mmol/L Tris-HCl pH7.4, 100mmol/L NaCl, 2mmol/L DTT. After several rounds optimization, the high quality crystals were grown in hanging drops for 3d with the reservoir buffer: 0.05 mol/L CaCl2, 0.1 mol/L Bis-Tris, pH 6.5, 25%(V/V) PEG MME550. Volume of drop is 2 μL and the ratio of protein and reservoir solution is 1∶1. Volume of reservoir is 0.4mL. All crystals were serially transferred to the well solution with 25%(V/V) glycerol before being flash frozen in liquid nitrogen.
1.3Data collection and processing
All diffraction datasets for PE8 crystals were collected on the BL19U beamline at Shanghai Synchrotron Radiation Facility. Data was processed and scaled using HKL3000[10]. Details of the data collection, processing, and statistics describing the data quality are listed in Tab.1.
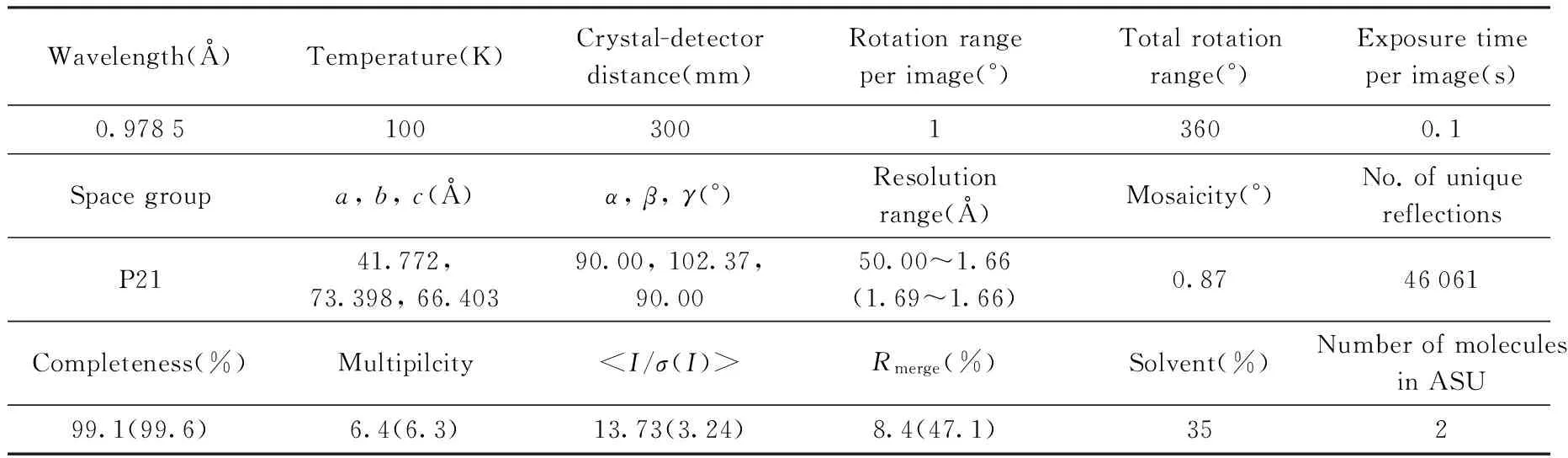
Tab.1 Data collection and processing values in parentheses are for the highest resolution shell
2 Results and discussion
PE8 contains 219 amino acids, and has a theoretical molecular weight of 23.2kD. When purified with size-exclusion chromatography, PE8 was eluted at a peak with an apparent molecular weight of 44 kD, which clearly shows PE8 was dimeric in solution. The SDS-PAGE analysis of protein fractions from gel filtration shows the purity of PE8 protein is more than 95%(Fig.2).
The initial crystallization screening produced crystals at only micrometer scale. After several rounds optimization, the PE8 crystals grew up to around 2 mm length in the condition of 0.05 mol/L CaCl2, 0.1 mol/L Bis-Tris, pH 6.5, 25%(V/V) PEG MME550 at 293 K(Fig.3). Crystals from the cluster were harvested and flash-cooled in liquid nitrogen. One dataset was collected on the BL19U beamline with wavelength 0.9785 Å at the Shanghai Synchrotron Radiation Facility. 360 diffraction images(oscillation angle 1.0°) were collected at 100K from the best crystal, which diffracted to 1.66Å resolution(Fig.4). The dataset was integrated into the monoclinic space group P21. The unit-cell parameters area=41.772,b=73.398,c=66.403Å,β=102.37°. According to the Matthews coefficient, the asymmetric unit contained two molecules with 35% solvent content.
References:
[1]BOTTCHER D, BORNSCHEUER U T. High-throughput screening of activity and enantioselectivity of esterases [J].NatProtoc, 2006,1(5): 2340-2343.
[2]ARPIGNY J L, JAEGER K E. Bacterial lipolytic enzymes: Classification and properties [J].Biochem, 1999,343(1): 177-183.
[3]TRINCONE A. Some enzymes in marine environment: Prospective applications found in patent literature [J].RecentPatBiotechnol, 2012,6(2): 134-48.
[4]HUO Y Y, CHENG H, HAN X F,etal. Complete genome sequence ofPelagibacteriumhalotoleransB2T[J].Bacteriol, 2012,194(1): 197-198.
[5]WEI X L, JIANG X W, YE L D,etal. Cloning, expression and characterization of a new enantioselective esterase from a marine bacteriumPelagibacteriumhalotoleransB2T[J].JournalofMolecularCatalysisB-Enzymatic, 2013,97: 270-277.
[6]CARMELA D R, GIULIA F, GIAN P P,etal. Recent advances in the stereoselective synthesis of trans-3,4-disubstituted-piperidines: Applications to(-)-paroxetine [J].Tetrahedron-Asymmetry, 2008,19(2): 131-155.
[7]IVAN L, PENG Z Q, YU J,etal. Asymmetric synthesis of(-)-paroxetine using PLE hydrolysis [J].TetrahedronLetters, 2000,41(30): 5647-5651.
[8]LEE J H, YI L, LI J,etal. Crystal structure and versatile functional roles of the COP9 signalosome subunit 1 [J].ProcNatilAcadSciUSA, 2013,110(29): 11845-11850.
[9]BRADFORD M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding [J].AnalBiochem, 1976,72: 248-254.
[10]MINOR W, CYMBOROWSKI M, OTWINOWSKI Z,etal. HKL-3000: The integration of data reduction and structure solution—from diffraction images to an initial model in minutes [J].ActaCrystallogrDBiolCrystallogr, 2006,62(8): 859-866.
从深海耐盐杆菌PelagibacteriumhalotoleransB2T中分离鉴定的酯酶PE8隶属于酯酶第六家族.因具有耐盐、较高的热稳定性、高手性选择性,PE8是工业上高效生产手性药物前体(R)-3-(4-氟苯基)戊二酸单甲酯((R)-3-MFG)很有开发潜力的催化剂,但其分子催化机理并不清楚.PE8在大肠杆菌中体外表达,并通过镍柱亲和层析,以及凝胶过滤层析纯化,最终通过悬滴结晶方法获得了PE8蛋白晶体.晶体经过X-ray衍射分析获得1.66 Å的分辨率,属于空间群P21,晶胞参数为a=41.772,b=73.398,c=66.403 Å,β=102.37°.一个异构单元包含2个蛋白分子,溶剂(水)含量为35%.
酯酶; PE8; 结晶
Q 518.3
A
深海耐盐杆菌来源酯酶PE8的表达纯化、结晶和晶体X-ray衍射研究
纪锐1,2,匡思运1,2,霍颖异3,黄静1,2,兰星洋1,2,许学伟3,李继喜1,2
(1. 复旦大学 生命科学学院 上海市工业菌株工程技术研究中心,上海 200438;2. 复旦大学 遗传工程国家重点实验室,上海 200438;3. 国家海洋局第二海洋研究所 海洋生态系统和生物地球化学重点实验室,浙江 杭州 310012)
date: 2015-11-20
the Program for Professor of Special Appointment(Eastern Scholar) at Shanghai Institutions of Higher Learning(No. TP2014010) to LI Jixi, China Ocean Mineral Resources R & D Association(COMRA) Special Foundation(DY125-14-E-02) to XU Xuewei
Q 518.3Document code: A
Article ID: 0427-7104(2016)04-0534-04
Research Notes
Biography: JI Rui(1987—), Male, Bachelor; KUANG Siyun(1992—), Female, Bachelor; The first two authors contributed equally to this paper. LI Jixi, Male, Professor; XU Xuewei, Male, Professor; Correspondence E-mail: xuxw@sio.org.cn; lijixi@fudan.edu.cn.
