鸭坦布苏病毒鸡胚弱化毒株的选育
2016-09-19于可响马秀丽袁小远刘存霞凌红丽李玉峰
于可响,马秀丽,袁小远,刘存霞,胡 峰,凌红丽,李玉峰,黄 兵
(1山东省农业科学院家禽研究所/山东省家禽疫病诊断与免疫重点实验室,济南 250023;2青岛蔚蓝生物股份有限公司,山东青岛 266061)
鸭坦布苏病毒鸡胚弱化毒株的选育
于可响1,马秀丽1,袁小远1,刘存霞1,胡 峰1,凌红丽2,李玉峰1,黄 兵1
(1山东省农业科学院家禽研究所/山东省家禽疫病诊断与免疫重点实验室,济南 250023;2青岛蔚蓝生物股份有限公司,山东青岛 266061)
【目的】将鸭坦布苏病毒BZ_2010株在SPF鸡胚上进行连续传代致弱,旨在选育安全性高、免疫原性好的活疫苗候选毒株。【方法】以SPF鸡胚为增殖宿主,将BZ_2010株连续传代,直至第120代,以1日龄雏鸭和30周龄的产蛋种鸭为试验对象,对第120代次毒株(命名为VC2)的安全性进行评价;以1d雏鸭为试验对象,对VC2株的返强情况进行评价;以18周龄的种鸭为试验对象,对VC2株免疫后的中和抗体进行监测;以25周龄的种鸭为试验对象,对VC2株免疫后的保护效果进行评价;利用RT-PCR方法分别扩增BZ_2010株和VC2株的E基因和NS4A基因,进行测序分析。【结果】传代病毒对鸡胚的平均死亡时间逐渐缩短,而病毒毒价有逐渐提高的趋势,ELD50由第20代的10-5.3/0.1mL提高到第120代的10-5.8/0.1mL,而且第80代之前提高较快,后期基本稳定。将VC2株通过颈部皮下接种1d雏鸭和肌肉接种30周龄产蛋种鸭,接种后试验鸭无异常临床表现,肝脏也无明显病理变化,这说明VC2株具有良好的安全性。将VC2在1d雏鸭进行连续5次传代,未发现试验鸭有任何异常症状,将第5代组织悬液接种1d雏鸭,采集肝脏进行病理切片观察,发现无明显病理变化,这说明VC2株具有良好的稳定性。基因测序分析结果显示,VC2株 E蛋白的第86、157、189、301和312位氨基酸发生改变,而NS4A蛋白只有一个氨基酸发生突变,即第54位氨基酸由F变为L。VC2免疫种鸭后抗体水平上升很快,第4周即可达到高峰,并且维持较长时间;VC2免疫后于第2周和第50周利用强毒株进行攻毒试验,结果VC2免疫组在攻毒后未出现异常症状,粪便正常,产蛋率保持正常,这说明VC2株免疫可对强毒株的攻击产生完全保护。【结论】通过鸡胚连续传代成功获得了一株安全性高、免疫原性好的鸭坦布苏病毒鸡胚弱化毒株。VC2免疫种鸭后抗体水平上升很快,可维持较长时间。攻毒试验结果表明,VC2株免疫可对强毒株的攻击产生完全保护。
鸭坦布苏病毒;鸡胚;弱化;活疫苗
0 引言
【研究意义】鸭坦布苏病毒病(duck Tembusu virus infection),又称鸭黄病毒病,是2010年在中国首先暴发的一种新型传染病。临床上以种鸭、蛋鸭产蛋量骤减,育成鸭、商品肉鸭出现神经症状为主要特征,该病来势迅猛,传播广泛,给中国的种鸭养殖业造成的巨大经济损失[1-10]。目前,该病已经成为一种常见病、多发病。本研究通过将鸭坦布苏病毒在SPF鸡胚上连续传代,筛选出一株符合活疫苗标准的弱毒株,为鸭坦布苏病毒活疫苗的研发奠定基础。【前人研究进展】该病的病原为鸭坦布苏病毒,属于黄病毒科黄病毒属。病毒基因组为不分节段的具有感染性的正链单股RNA,由10 990个核苷酸组成,只有一个长的开放阅读框,其中5′端有帽状结构,3′端无polyA尾[11-16]。编码 3个结构蛋白基因:衣壳蛋白(C)、膜蛋白(PrM)、囊膜蛋白(E)和7个非结构蛋白,其中E蛋白与病毒致病力、组织亲嗜性、宿主嗜性、免疫保护等密切相关[17-18]。【本研究切入点】疫苗免疫是用于家禽疫病防控的主要措施,活疫苗的使用省时省力,适于在鸭群中批量免疫,但目前还没有商品化的鸭坦布苏病毒活疫苗,也没有鸭坦布苏病毒自然弱毒株的相关报道。【拟解决的关键问题】将鸭坦布苏病毒BZ_2010株在SPF鸡胚上进行传代致弱,将120代次VC2毒株进行了安全性、遗传稳定性和免疫效力评价,旨在选育一株安全性高、免疫原性好的活疫苗毒株。
1 材料与方法
试验于2011年1月至2015年5月在山东省家禽疫病诊断与免疫重点实验室和山东省农业科学院家禽研究所进行。
1.1病毒、鸡胚、雏鸭、种鸭
鸭坦布苏病毒BZ_2010株于2010年分自山东地区 A种鸭场(在 10d SFP鸭胚上的 ELD50为10-6.2/0.1mL);鸭坦布苏病毒SX-12株于2012年分自山东地区B种鸭场(在10 d SFP鸭胚上的ELD50为10-5.9/0.1mL);SPF鸡胚由山东省昊泰动物繁育有限公司提供;鸭坦布苏病毒抗体阴性的樱桃谷雏鸭和种鸭由山东省德州市某种鸭场提供。
1.2病毒传代与滴定
将鸭坦布苏病毒 BZ_2010株用生理盐水以 1:1 000倍稀释,通过尿囊腔途径接种9d SPF鸡胚,弃掉接种24h内死亡的鸡胚,观察并记录鸡胚死亡时间。收集接种24h后死亡鸡胚的尿囊液,按照前面的接种方法继续进行传代,直至第120代(命名为VC2)。分别取第20、40、60、80、100和120代病毒在9 d SPF鸡胚上测定鸡胚半数致死量(ELD50)。方法如下:将病毒分别作10倍系列稀释,即10-2、10-3…10-7、10-8共7个不同稀释度,分别经尿囊腔接种9 d鸡胚,每个稀释度接种5枚,0.1mL/枚,同时设生理盐水对照组,观察记录接种后24—168 h内鸡胚死亡情况,并按Reed-Muench法计算ELD50。
1.3E蛋白与NS4A蛋白变异分析
参照BZ_2010株基因组序列(GenBank登录号:KC990540)设计两对引物,分别用于扩增VC2的E基因和NS4A基因,引物由上海立菲生物技术有限公司合成。按照RNAiso Reagent(TAKARA,JAPAN)试剂说明提取VC2病毒RNA,按照常规RT-PCR方法扩增E基因和NS4A基因并测序。将VC2的 E蛋白与NS4A蛋白序列同BZ_2010株进行比对分析。
1.4安全性试验
将鸭坦布苏病毒BZ_2010株和VC2株分别通过颈部皮下接种1 d雏鸭,接种剂量为104.0ELD50/只,每组试验鸭接种20只。10只对照鸭用同样体积的生理盐水进行颈部皮下接种。接种后观察试验鸭的精神状态与死亡情况。采集接种后发病或死亡试验鸭的肝脏制备病理切片;若无发病或死亡的情况,则于接种后第7天剖杀试验鸭取肝脏制备病理切片。
将鸭坦布苏病毒BZ_2010株和VC2株分别通过腿肌注射30周龄的产蛋种鸭,接种剂量为106.0ELD50/只,每组试验鸭接种30只。10只对照鸭用同样体积的生理盐水进行腿肌接种。接种后观察试验鸭的精神状态与产蛋情况。
1.5病毒毒力返强试验
将VC2以105.0ELD50/只的剂量通过腿肌注射的方式接种1 d雏鸭10只,5 d后剖杀取肝、脾、脑制成20%悬液,冻融3次后,取上清液再接种1 d雏鸭10只,连续传代5次,观察雏鸭的精神状态。将第5代的组织悬液通过颈部皮下接种1 d雏鸭10只,接种剂量为104.0ELD50/只,观察雏鸭的精神状态。采集第5代组织接种后发病或死亡实验鸭的肝脏制备病理切片;若无发病或死亡的情况,则于接种后第7天剖杀试验鸭取肝脏制备病理切片。
1.6免疫后抗体中和效价的测定
将VC2以103.5ELD50/只的剂量通过胸肌注射的方式免疫18周龄种鸭200只,每隔4周随机采集20份鸭血清通过鸡胚中和试验测定其中和抗体,直至免疫后56周,取中和抗体的平均值绘制抗体消长曲线。
1.7免疫后攻毒保护试验
将VC2以103.5ELD50/只的剂量通过胸肌注射的方式免疫25周龄种鸭2 000只,分别于免疫后第2周和第50周利用鸭坦布苏病毒SX-12株对试验鸭进行攻毒,每组攻毒免疫鸭100只,阴性对照鸭100只,攻毒剂量为105ELD50/只。观察并记录试验鸭发病情况及产蛋情况。
2 结果
2.1病毒传代
将鸭坦布苏病毒BZ_2010在SPF鸡胚上进行连续传代,结果发现鸡胚的平均死亡时间有缩短的趋势,而病毒毒价有逐渐提高的趋势,ELD50由第 20代的10-5.3/0.1mL提高到第 120代的 10-5.8/0.1mL,而且第80代之前提高较快,后期基本稳定(图1)。

图1 不同代次病毒的鸡胚半数致死量Fig.1 ELD50of duck Tembusu virus VC2 strain at different passage times
2.2E蛋白与NS4A蛋白的变异分析
通过测序分析发现,VC2的E基因和NS4A基因与亲本毒BZ_2010相比,分别有11个和3个碱基的差异,同源性分别为99.3%和99.2%。E基因的变异导致第86、157、189、301和312位氨基酸发生变化,而NS4A基因的变异导致第54位氨基酸由F变为L(表1)。
2.3安全性试验
将BZ_2010和VC2分别通过颈部皮下接种1 d雏鸭20只,接种14 d内BZ_2010接种组有7只死亡,大部分试验鸭死亡前有神经症状,而VC2接种组无死亡,且不表现神经症状。肝脏的病理切片显示,BZ_2010组试验鸭的肝脏有明显的脂肪变性,而VC2组与对照组相比无明显变化(图2)。
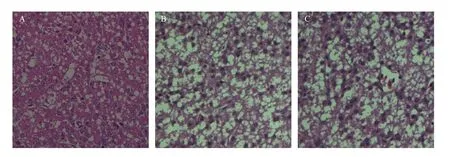
图2 BZ_2010和VC2接种雏鸭后肝脏的病理变化Fig.2 Liver pathological changes of ducklings injected with BZ_2010 or VC2 (400×)
将BZ_2010和VC2分别通过腿肌注射30周龄的产蛋种鸭,结果发现,BZ_2010接种组从接种后第4天开始出现绿色粪便,产蛋同时下降,至第9天产蛋量由接种前的26枚降到5枚。VC2接种组的产蛋量保持稳定,粪便也无变化。
2.4病毒毒力返强试验
将VC2在1 d雏鸭进行连续5次传代,未发现试验鸭有任何异常症状。第5代组织悬液颈部皮下接种1 d雏鸭,也未发现任何异常症状。第5代组织接种后第7天剖杀实验鸭取肝脏制备病理切片,结果显示,VC2组与对照组相比,肝脏无明显病理变化(图3)。
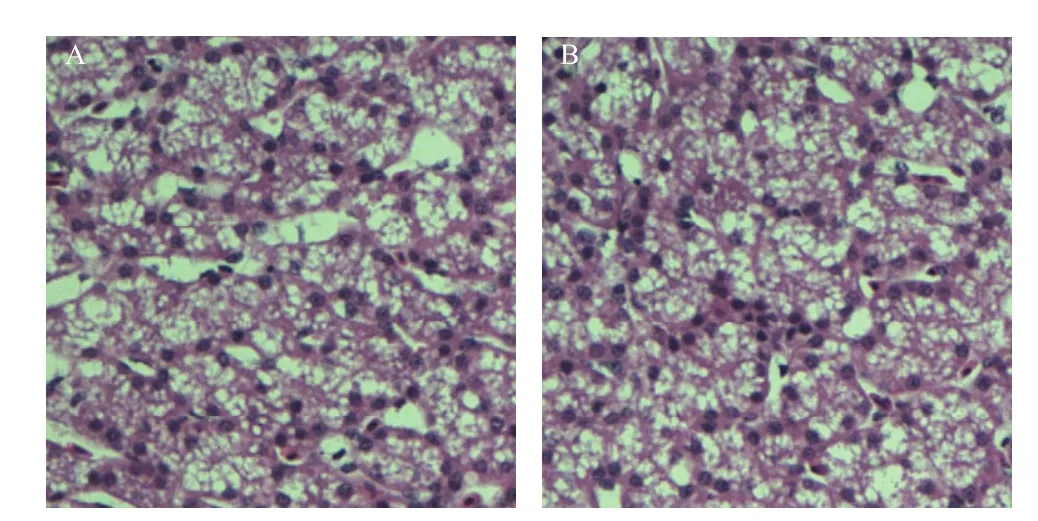
图3 第5代组织接种雏鸭后肝脏的病理变化Fig.3 Liver pathological changes of ducklings injected by fifth tissue (400×)
2.5免疫后抗体中和效价的测定
VC2免疫18周龄种鸭后定期采集血清,利用鸡胚中和试验测定其中和抗体,绘制抗体消长曲线(图4)。结果显示,VC2免疫后前4周中和抗体上升很快,到第4周时基本达到高峰,高峰期维持20周,然后缓慢下降,到56周时中和抗体效价仍能达到1∶22。
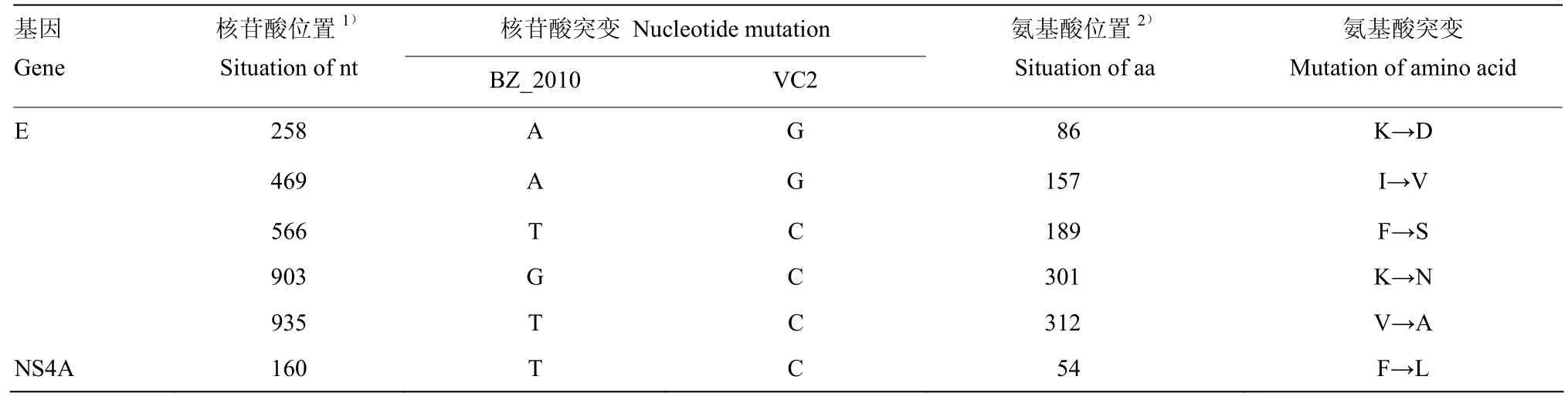
表1 第120代次病毒株的核苷酸与氨基酸突变情况Table 1 Change of nucleotide and amino acid of Tembusu virus at passage 120
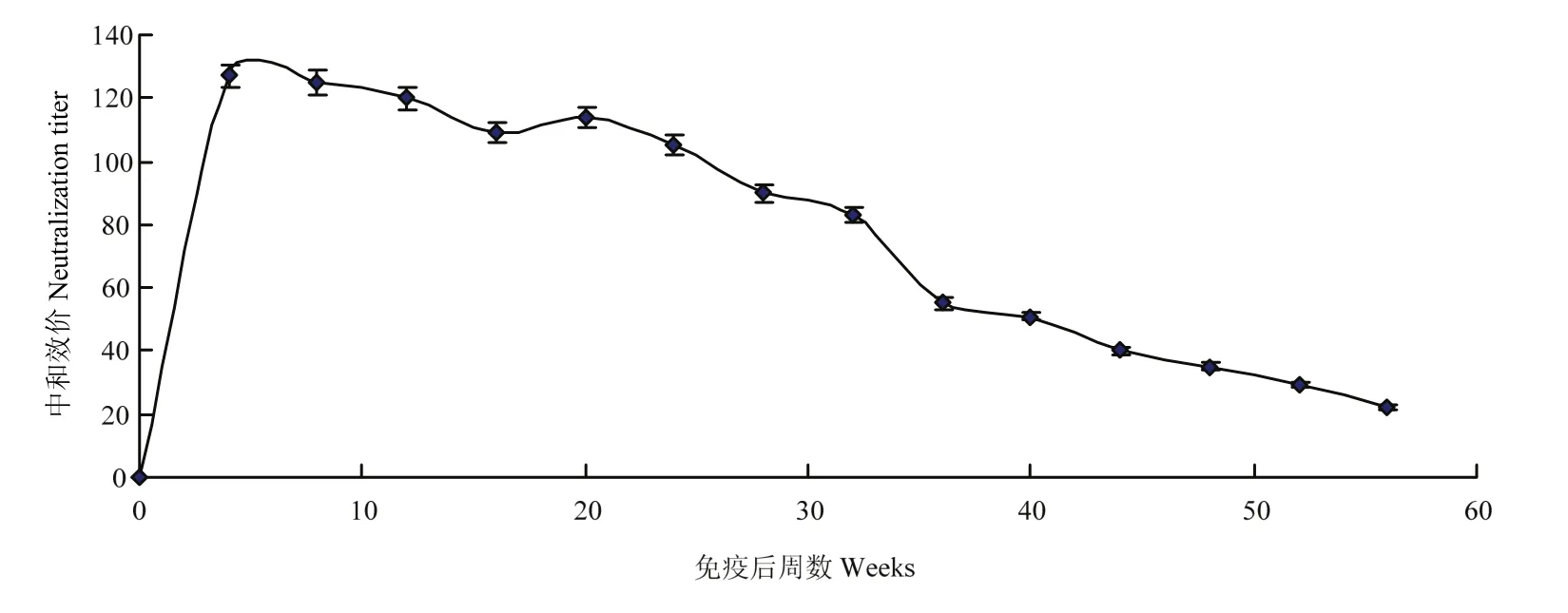
图4 VC2免疫种鸭抗体消长曲线Fig.4 Neutralization antibodies change curve of adult duck after VC2 immunization
2.6免疫后攻毒保护试验
VC2免疫25周龄种鸭,分别于免疫后第2周和第40周对试验鸭进行攻毒,结果显示:免疫后第2周攻毒的对照组从攻毒后第4天开始出现绿色粪便,产蛋率从第3天开始下降,到第15天降至20%,VC2免疫组在攻毒后未出现异常症状,粪便正常,产蛋率保持正常的上升趋势(图5)。免疫后第50周攻毒的对照组从攻毒后第5天开始出现绿色粪便,产蛋率从第5天开始出现明显下降,到第15天降至33%,VC2免疫组在攻毒后未出现异常症状,粪便正常,产蛋率保持稳定(图6)。
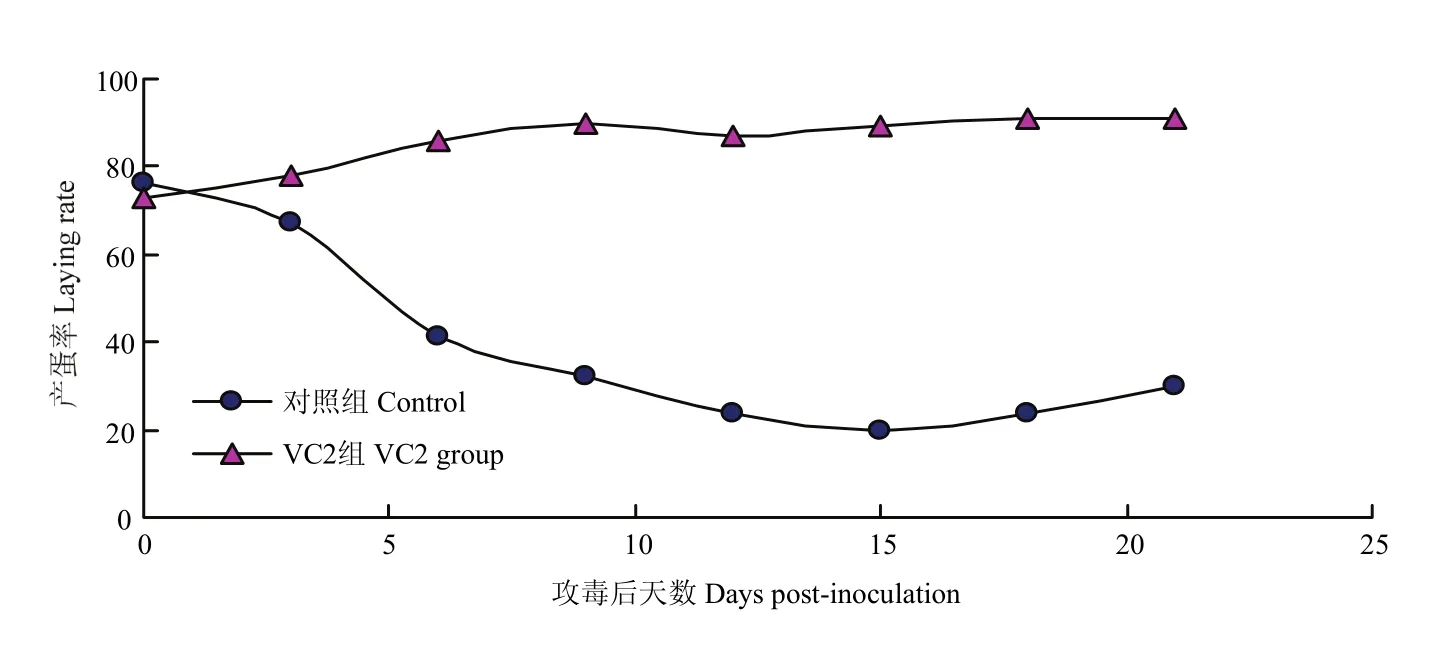
图5 VC2免疫后第2周攻毒种鸭产蛋率的变化Fig.5 Laying rate change of ducks with SX-12 challenging at weeks 2 post-vaccination
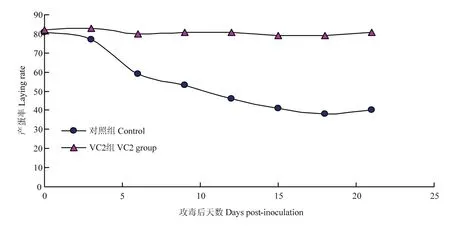
图6 VC2免疫后第50周攻毒种鸭产蛋率的变化Fig.6 Laying rate change of ducks with SX-12 challenging at weeks 50 post-vaccination
3 讨论
鸭坦布苏病毒病自从2010年在中国暴发以来,一直困扰着种鸭业的发展,生产中急需高效疫苗来预防该病。本研究将鸭坦布苏病毒山东分离株BZ_2010在SPF鸡胚进行连续传代,直至第120代,获得的第120代次毒株VC2的ELD50由亲本毒的10-5.3/0.1mL提高到10-5.8/0.1mL,说明病毒在传代过程中毒价在逐步上升。为了确定VC2株是否已经致弱,笔者分别通过颈部皮下接种1d雏鸭和肌肉接种30周龄产蛋种鸭,结果显示,VC2接种后试验鸭无异常症状和死亡,粪便和产蛋率也保持正常。这些都说明VC2毒株已完全丧失了对鸭的致病力。进而又将该致弱株在1 d雏鸭体内进行了毒力返强试验,连续传5代未出现任何返强迹象,这些数据表明,VC2候选疫苗株具有良好的安全性。
鸭坦布苏病毒的E蛋白与病毒致病力、宿主嗜性等密切相关,而有报道非结构蛋白NS4A影响日本乙型脑炎对小鼠的致病力[19-25]。因此,选择了E和NS4A这两个蛋白进行测序分析,试图在基因水平上找到VC2丧失对鸭的致病力原因。通过与亲本毒BZ_2010比对分析发现,E蛋白的第86、157、189、301和312位氨基酸发生变化,其中第157、189位氨基酸位于结构域I区,这一区域内发生的很多突变都与黄病毒毒力相关而NS4A蛋白只有一个氨基酸的突变,即第54位氨基酸由F变为L。这些突变或者突变组合(包括其他蛋白)可能是导致VC2致病力减弱的关键因素[26-30]。
为了评价弱毒株VC2的免疫效果,笔者进行中和抗体追踪和攻毒保护试验。结果显示,VC2免疫种鸭后抗体水平上升很快,并且维持时间较长,衰减缓慢;VC2免疫后第2周和第50周都可产生完全保护,这说明VC2的免疫期很长,基本可以持续整个产蛋期。
4 结论
通过将鸭坦布苏病毒在 SPF鸡胚上进行连续传代,获得了安全性高、免疫原性好的弱毒株VC2,为鸭坦布苏病活疫苗的研发奠定了基础。
References
[1] 李玉峰,马秀丽,于可响,王友令,高巍,黄兵,徐怀英,吴静,王生雨,王莉莉,秦卓明. 一种从鸭新分离的黄病毒研究初报. 畜牧兽医学报,2011,42(6): 885-891. LI Y F,MA X L,YU K X,WANG Y L,GAO W,HUANG B,XU H Y,WU J,WANG S Y,WANG L L,QIN Z M. A brief report of flaviviruses newly isolated from duck. Acta Veterianaria et Zootechnica Sinaca,2011,42(6): 885-891. (in Chinese)
[2] 滕巧泱,颜丕熙,张旭,闫丽萍,李泽君. 一种新的黄病毒导致蛋鸭产蛋下降及死亡. 中国动物传染病学报,2010,18(6): 1-4. TENG Q Y,YAN P X,ZHANG X,YAN L P,LI Z J. A novel flavivirus causing duck egg drops and death. Chinese Journal of Animal Infectious Desease,2010,18(6): 1-4. (in Chinese)
[3] 万春和,施少华,程龙飞,陈红梅,傅光华,张大丙,林芳,林建生,黄瑜. 一种引起种(蛋)鸭产蛋骤降新病毒的分离与初步鉴定.福建农业学报,2010,25(6): 663-666.Wan C H,Shi S H,Chen L F,Chen H M,Fu G H,Zhang D B,Lin F,Lin J S,Huang Y. A newly identified flavivirus virus causing abrupt egg-laying reduction in ducks. Fujian Journal of Agricultural Sciences,2010,25(6): 663-666. (in Chinese)
[4] 曹贞贞,张存,黄瑜,刁有祥,叶伟成,刘月焕,韩婧文,马国明,张冬冬,许丰,王丹,姜甜甜,袁媛,谢小雨,高绪慧,唐熠,施少华,万春和,张晨,何玢,杨梦婕,陆新浩,张冰,张国中,马学军,张大丙. 鸭出血性卵巢炎的初步研究. 中国兽医杂志,2010,46(12): 3-6. CAO Z Z,ZHANG CUN,HUANG Y,DIAO Y X,YE W C,LIU Y H,HAN J W,MA G M,ZHANG D D,XU F,WANG D,JIANG T T,YUAN Y,XIE X Y,GAO X H,TANG Y,SHI S H,WANG C H,ZHANG C,HE F,YANG M J,LU X H,ZHANG B,ZHANG G Z,MA X J,ZHANG D B. Preliminary studies on duck hemorrhagic ovaritis. Chinese Journal of Veterinary Medicine,2010,46(12): 3-6. (in Chinese)
[5] 刘志刚,孙青松,姚蓉,刘冰心,晁行周,邹 忠,刘立峰,吴 彦,郑爱芳,赵苏红,金梅林. 鸭坦布苏病毒研究进展. 中国动物传染病学报,2013,21(1): 81-86. LIU Z G,SUN Q S,YAO R,LIU B X,CHAO X Z,ZOU Z,LIU L F,WU Y,ZHONG A F,ZHAO S H,JIN M L. Research progress on duck tembusu virus. Chinese Journal of Animal Infectious Diseases,2013,21(1): 81-86. (in Chinese)
[6] 朱丽萍,颜世敢. 鸭坦布苏病毒研究进展. 中国预防兽医学报,2012,34(1): 79-82. ZHU L P,YAN S G. Research progress on duck tembusu virus. Chinese Journal of Preventive Veterinary Medicine,2012,34(1): 79-82. (in Chinese)
[7] JIANG T,LIU J,DENG Y Q,SU J L,XU L J,LIU Z H,LI X F,YU X D,ZHU S Y,GAO G F,QIN E D,QIN C F. Development of RT-LAMP and real-time RT-PCR assays for the rapid detection of the new duck Tembusu-like BYD virus. Archives of Virology,2012,157(12): 2273-2280.
[8] SU J,LI S,HU X,YU X,WANG Y,LIU P,LU X,ZHANG G,HU X,LIU D,LI X,SU W,LU H,MOK N S,WANG P,WANG M,TIAN K,GAO G F. Duck egg-drop syndrome caused by BYD virus,a new Tembusu-related flavivirus. PLoS ONE,2011,6(3): e18106.
[9] LI G,GAO X,XIAO Y,LIU S,PENG S,LI X,SHI Y,ZHANG Y,YU L,WU X,YAN P,YAN L,TENG Q,TONG G,LI Z. Development of a live attenuated vaccine candidate against duck Tembusu viral disease. Virology,2014,450: 233-242.
[10] LIU M,CHEN S,CHEN Y,LIU C,CHEN S,YIN X,LI G,ZHANG Y. Adapted Tembusu-like virus in chickens and geese in China. Journal of Clinical Microbiology,2012,50(8): 2807-2809.
[11] YU K,SHENG Z Z,HUANG B,MA X,LI Y,YUAN X,QIN Z,WANG D,CHAKRAVARTY S,LI F,SONG M,SUN H. Structural,antigenic,and evolutionary characterizations of the envelope protein of newly emerging duck Tembusu virus. PLoS ONE,2013,8(8): e71319.
[12] 张琳,逯茂洋,胡北侠,蒋一男,许传田,杨少华,张贝,张秀美. 4株鸭坦布苏病毒包膜蛋白基因的分子进化分析及表达. 中国兽医学报,2013,33(2): 175-180. ZHANG L,LU M Y,HU B X,JIANG Y N,XU C T,YANG S H,ZHANG B,ZHANG X M. Molecular evolution and expression of envelop protein of duck Tembusu virus isolated from China,Chinese Journal of Veterinary Science,2013,33(2): 175-180. (in Chinese)
[13] LI S,ZHANG L,WANG Y,WANG S,SUN H,SU W,HE W,HAN B,SU J. An infection full-length cDNA clone of duck Tembusu virus,a newly emerging flavivirus causing duck egg drop syndrome in China. Virus research,2013,171(1): 238-241.
[14] LI L,AN H,SUN M,DONG J,YUAN J,HU Q. Identification and genomic analysis of two duck-origin Tembusu virus strains in southern China. Virus Genes,2012,45(1): 105-112.
[15] LI X,LI G,TENG Q,YU L,WU X,LI Z. Development of a blocking ELISA for detection of serum neutralizing antibodies against newly emerged duck Tembusu virus. PLoS ONE,2012,7(12): e53026.
[16] MULLER D A,YOUNG P R. The flavivirus NS1 protein: molecular and structural biology,immunology,role in pathogenesis and application as a diagnostic biomarker. Antiviral Rescearch,2013,98(2): 192-208.
[17] HUANG Q,LI Q,JOY J,CHEN A S,RUIZ-CARRILLO D,HILL J,LESCAR J,KANG C. Lyso-myristoyl phosphatidylcholine micelles sustain the activity of Dengue non-structural (NS) protein 3 protease domain fused with the full-length NS2B. Protein Expression and Purification,2013,92(2): 156-162.
[18] MODIS Y,OGATA S,CLEMENTS D,HARRISON S C. A ligand-binding pocket in the dengue virus envelop eglycoprotein. Proceedings of the National Academy of Sciences of the United States of America,2003,100 (12): 6986-6991.
[19] YAMAGUCHI Y,NUKUI Y,TAJIMA S,NEROME R,KATO F,WATANABE H,TAKASAKI T,KURANE I. An amino acid substitution (V3I) in the Japanese encephalitis virus NS4A protein increases its virulence in mice,but not its growth rate in vitro. Journal of General Virology,2011,92(7): 1601-1606.
[20] REY F A,HEINZ F X,MANDL C,KUNZ C,HARRISON S C. The envelope glycoprotein from tick-borne encephalitis virus at 2 Aresolution. Nature,1995,375: 291-298.
[21] Murray C L,Jones C T,Rice C M. Architects of assembly: roles of Flaviviridae non-structural proteins in virion morphogenesis. Nature reviews microbiology,2008,6(9): 699-708.
[22] MARKOFF L,PANG X,HOUNG H S H S,FALGOUT B,OLSEN R,JONES E,POLO S. Derivation and characterization of a dengue type 1 host range-restricted mutant virus that is attenuated and highly immunogenic in monkeys. Journal of Virology,2002,76: 3318-3328.
[23] Potapova U V,Feranchuk S I,Potapov V V,Kulakova N V,Kondratov I G,Leonova G N,Belikov S I. NS2B/NS3 protease: allosteric effect of mutations associated with the pathogenicity of tick-borne encephalitis virus. Journal of Biomolecular Structure and Dynamics,2012,30(6): 638-651.
[24] LINDENBACH B D,RICE C M. Genetic interaction of flavivirus nonstructural proteins NS1 and NS4A as a determinant of replicase flmction. Journal of Virology,1999,73(6): 4611-4621.
[25] ARROYO J,GUIRAKHOO F,FENNER S,ZHANG Z X,MONATH T P,CHAMBERS T J. Molecular basis for attenuation of neurovirulence of a yellow fever Virus/Japanese encephalitis virus chimera vaccine (ChimeriVax-JE). Journal of Virology,2001,75: 934-942.
[26] CHEN W J,WU H R,CHIOU S S. E/NS1 modifications of dengue 2 virus after serial passages inmammalian and/or mosquito cells. Intervirology,2003,46(5): 289-295.
[27] ZHANG S,LI L,WOODSON S E,HUANG C Y,KINNEY R M,BARRETT A D,BEASLEY D W. A mutation in the envelope protein fusion loop attenuates mouse neuroinvasiveness of the NY99 strain of West Nile virus. Virology,2006,353: 35-40.
[28] NI H,BURNS N J,CHANG G J,ZHANG M J,WILLS M R,TRENT D W,SANDERS P G,BARRETT A D. Comparison of nucleotide and deduced amino acid sequence of the 5’ non-coding region and structural protein genes of the wild-type Japanese encephalitis virus strain SA14 and its attenuated vaccine derivatives. Journal of General Virology,1994,75(6): 1505-1510.
[29] NI H,CHANG G J,XIE H,TRENT D W,BARRETT A D. Molecular basis of attenuation of neurovirulence of wild-type Japanese encephalitis virus strain SA14. Journal of General Virology,1995,76(2): 409-413.
[30] AIHARA S,RAO C M,YU Y X,LEE T,WATANABE K,KOMIYA T,SUMIYOSHI H,HASHIMOTO H,NOMOTO A. Identification of mutations that occurred on the genome of Japanese encephalitis virus during the attenuation process. Virus Genes,1991,5(2): 95-109.
(责任编辑 林鉴非)
Selection of a Live Chicken Embryo Attenuated Duck Tembusu Virus Vaccine
YU Ke-xiang1,MA Xiu-li1,YUAN Xiao-yuan1,LIU Cun-xia1,HU Feng1,LING Hong-li2,LI Yu-feng1,HUANG Bing1
(1Key Laboratory of Poultry Disease Diagnose and Immune of Shandong Province/Institute of Poultry,Shandong Academy of Agricultural Sciences,Jinan 250023;2Qingdao Vland Biotech Inc.,Qingdao 266061,Shandong)
【Objective】 Tembusu virus BZ-2010 strain was continuously passaged in specific-pathogen-free embryonic (SPF)eggs in order to select a live attenuated vaccine candidate of good safety and immunogenicity properties. 【Method】 Tembusu virus BZ-2010 strain was cultured for 120 passages in SPF eggs. The safety of the 120th passage viral strain was evaluated with 1-day-old SPF ducklings and 30-week-old egg-laying ducks. The property of virulent return of VC2 viral strain was evaluated with 1-day-old SPFducklings. The neutralizing antibodies were detected after the 18-week-old breeding ducks were immunized with VC2 strain. The protective effects were evaluated after the 25-week-old breeding ducks were immunized with VC2 strain. E gene and NS4A gene of BZ_2010 and VC2 strains were amplified by RT-PCR and sequenced. 【Result】 The average death time of SPF eggs was shortened by passage virus and viral titer was increased with the escalation of passage times in SPF chicken embryonic eggs. ELD50of the 20th virus was 10-5.3/0.1mL and ELD50of the 120th virus was 10-5.8/0.1mL.The viral titer reached the plateau at passage 80 and remained unchanged further passages. The experimental ducks showed no clinical symptoms after 1-day-old ducklings and 30-week-old breeding ducks were immunized with VC2 strain by subcutaneous injection and by intramuscular injection,respectively. The results showed that VC2 strain had a good safety. No symptoms appeared in 1-day-old ducklings in which VC2 strain were cultured for 5 passages. 1-day-old ducklings were infected with the 5th tissue suspension and no symptoms were observed in liver pathological section by microscope. The results showed that VC2 strain had a good stability. Sequence analysis revealed that the E protein of Tembusu VC2 evolved amino acid changes in positions 86,157,189,301,and 302,respectively. The NS4A protein of Tembusu VC2 only had one amino acid change in position 54 in that phenylalanine was replaced by Leucine. The level of antibodies rose very quickly,reached the plateau at the 4th week and remained a long time. Ducks were challenged by TMUV virulent strain at 2 and 50 weeks after immunization with VC2 strain in the experimental group. There was no symptom,normal stool,and regular egg production in the vaccinated group after challenge of virulent strain. The results showed that VC2 strain could provide complete protection for the challenge of TMUV virulent strain.【Conclusions】 An attenuated strain of TMUV with good immunogenicity and high safety was acquired through serial passages of SPF chicken embryos. The level of antibodies rose very quickly and remained a long time after immunization of the VC2 attenuated strain. The toxicity attack experiments showed that VC2 could provide complete protection for the challenge of TMUV virulent strain.
duck Tembusu virus; chicken embryo; attenuation; live vaccine
2015-12-21;接受日期:2016-05-04
山东省自然科学基金面上项目(ZR2013CM037)、山东省现代农业产业技术体系家禽创新团队计划(SDAIT-13-011-01)、山东省海外高层次人才(泰山学者)引进计划
联系方式:于可响,Tel:0531-85975851;E-mail:yukx1979@163.com。通信作者李玉峰,Tel:0531-85971556;E-mail:dicpd@163.com。通信作者黄兵,Tel:0531-85975851;E-mail:hbind@163.com
