Polypyridyl Ligands-Based Double-Decker Triggered by Silver(Ⅰ)Coordination: Crystal Structures and Spectroscopic Analysis
2016-09-18ZHAOQiangLIUXiuMingFENGRuiZHANGYingHuiKONGManManLIUYangCollegeofChemistryandPharmaceuticalEngineeringNanyangNormalUniversityNanyangHenan47306ChinaSchoolofmatrerialsscienceandengineeringNankaiUniversityTianjin30007China
ZHAO QiangLIU Xiu-MingFENG RuiZHANG Ying-Hui*,KONG Man-ManLIU Yang(College of Chemistry and Pharmaceutical Engineering, Nanyang Normal University, Nanyang, Henan 47306, China)(School of matrerials science and engineering, Nankai University, Tianjin 30007, China)
Polypyridyl Ligands-Based Double-Decker Triggered by Silver(Ⅰ)Coordination: Crystal Structures and Spectroscopic Analysis
ZHAO Qiang1LIU Xiu-Ming2FENG Rui2ZHANG Ying-Hui*,2KONG Man-Man1LIU Yang1
(1College of Chemistry and Pharmaceutical Engineering, Nanyang Normal University, Nanyang, Henan 473061, China)
(2School of matrerials science and engineering, Nankai University, Tianjin 300071, China)
Three Agcoordination complexes, namely [Ag(dpq)(NO3)2]n(1), [Ag4(tppq)2(NO3)4](2) and [Ag6(hpdq)2(NO3)6](3) have been synthesized based on three polypyridyl ligands: 2,3-di(pyridin-2-yl)quinoxaline (dpq), 2,3,7,8-tetra(pyridine-2-yl)pyrazino[2,3-g] -quinoxaline (tppq) and 2,3,6,7,10,11-hexakis(2-pyridyl) dipyrazino[2,3-f:2′,3′-h]quinoxaline) (hpdq), respectively. Single crystal X-ray diffraction analyses reveal that complexes 1~3 assembled based on distinct double-decker unit comprising two respective polypyridyl ligands but four, four, and six Agions, respectively. Among them, The double-decker units in 1 and 2 both extended to two dimensional net via the coordination interaction between Agand NO3-anion, while that in 3 to three dimensional net via C-H…O hydrogen bond. Furthermore, the coordintion interaciton of polypyridyl ligands and Agion in solution was also investigated by UV-Vis absorption and fluorescence spectroscopic analyses, which witnesses the much more sensitive spectral response of hpdq to the additon of Agion than other two ligands. CCDC: 868734, 1; 868735, 2; 790268, 3.
polypyridyl ligands; metal-organic coordination complexes; self-assembly; UV-Vis spectrum; fluorescence spectrum
0 Introduction
With the aim to di versify the architecture and properties of metal organic coordination complexes, increasing attentions have been paid on the design of multidentate organic ligands[1-5], due to their versatile coordination modes. Among them N-donor heterocyclic multidentate ligands have been widely used in the construction of coordination complexes with intriguing topologies[6-10]and nanostructure[11-12], because of their versatile coordination behaviors.
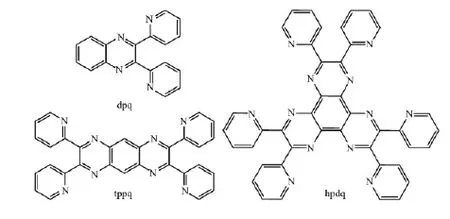
Scheme 1 Structure of three polypyridyl ligands
Recently, a large polypyridyl ligand, 2,3,6,7,10,11-hexakis(2-pyridyl)dipyrazino[2,3-f:2′,3′-h]- quinoxaline (hpdq) was reported to react with Ag (CF3SO3)[20], and the resultant complex comprises a novel double decker unit that is extended into three dimension architecture via the hydrogen bond interaction between CF3SO3-and the C-H bonds of hpdq. However, when reacting with Cdor Znions, hpdq exhibits distinctive coordination modes that facilitate to form a discrete planar structure based on single hpdq ligand[21]. Despite several intrinsic characteristics have been unveiled about the coordination interaction between Ag(Ⅰ) ion and polypyridyl quinoxaline ligands, some questions still remain unsettled about the structural dependence of double decker on the backbone of polypridyl quinoxaline ligands. Meanwhile, the influence of counter anions on the packing mode of double decker units is still not clear.
In order to promote solving these questions, the coordination interaction between AgNO3and several polypyridyl quinoxaline ligands with different size and pyridine number (Scheme 1), i.e. 2,3-di(pyridin-2-yl) quinoxaline (dpq), 2,3,7,8-tetra(pyridin-2-yl)pyrazino [2,3-g]quinoxaline (tppq) and 2,3,6,7,10,11-hexakis(2-pyridyl) dipyrazino[2,3-f:2′,3′-h]quinoxaline (hpdq) had been studied. Based on these ligands, three coordination complexes [Ag(dpq)(NO3)2]n(1), [Ag4(tppq)2(NO3)4] (2) and [Ag6(hpdq)2(NO3)6] (3) have been synthesized. Single crystal analyses reveal distinctive packing mode of double-decker unit for 1~3, associated with different double decker depending on the used ligands. The packing mode of double decker in 3 differs significantly from that reported in literature[20], due to different counter anions used. Furthermore, both fluorescence and UV-absorption spectra titration were utilized to investigate the assembly process of the organic ligands and Agion in solution. The most obvious spectral variation wasobserved for hpdq, indicative of potential application of hpdq as a molecular modle in sepctral detection of metal ions.
1 Experimental
1.1Materials and method
All the starting materials for the synthesis were of reagent grade and used as received. All the solvents used for titration measurements were puried by standard procedures. Hpdq was synthesized according to literature[21]. Elemental analyses (C, H, and N) were performed on a Perkin-Elmer 240C analyzer. IR spectra in the 4 000~400 cm-1range were measured on a TENSOR 27 OPUS FTIR spectrometer using KBr disks dispersed with sample powders. UV-Vis absorption spectra were measured with a Hitachi U-3010 UV-Vis spectrophotometer. Fluorescence spectra were recorded at room temperature on a Varian Cary Eclipse uorescence spectrometer.
1.2Preparation of complex 1, 2 and 3
Complexes 1, 2 and 3 were obtained through the reaction of AgNO3with respective polypyridyl ligands by solution diffusion method at room temperature.
Complex 1: A buffer layer of dichloromethaneacetonitrile (12 mL, 1∶1, V/V) was carefully layered over a dichloromethane solution (2 mL) of dqp (0.1 mmol). Then a solution of AgNO3(0.2 mmol) in acetonitrile (3 mL) was layered over the buffer layer. After ca. four weeks colourless block crystals were obtained with 38% yield (based on dbq). Anal. Calcd. for C18H12N6O6Ag2(% ): C 34.64, H 1.94, N 13.47; Found (%): C 34.59, H 1.57, N 13.84. FTIR (KBr pellets, cm-1): 1 734w, 1 700w, 1 684m, 1 653m, 1 559s, 1 540m, 1 507w, 1 473m, 1 457m, 1 386s, 419w.
Complex 2: A buffer layer of dichloromethaneacetonitrile (10 mL, 1∶1, V/V) was carefully layered over a dichloromethane solution (3 mL) of tppq (0.1 mmol). Then a solution of AgNO3(0.3 mmol) in acetonitrile (3 mL) was layered over the buffer layer. Orange red block-shaped crystals were obtained after ca. five weeks in 30% yield (based on tppq). Anal. Calcd. for C15.25H10.25N5O3.375Cl0.5Ag (2·CH2Cl2·1.5H2O, %): C 41.33, H 2.33, N 15.80; Found(%): C 41.72, H 2.53, N 16.05. FTIR(KBr pellets, cm-1): 1 684m, 1 653m, 1 559m, 1 540w, 1 457m, 1 340s.
Complex 3: A buffer layer of chloroformacetonitrile (12 mL, 1∶1, V/V) was carefully layered over a chloroform solution (2 mL) of hpdq (0.1 mmol). Then a solution of AgNO3(0.3 mmol) in acetonitrile (3 mL) was layered on the buffer layer. Yellow blockshaped crystals were obtained after ca. five weeks in 35% yield(based on hpdq). Anal. Calcd. for C96.5H60.5N30O18Cl37.5Ag6(3·12.5CHCl3, %): C 29.68, H 1.56, N 10.76; Found (%): C 31.82, H 2.63, N 13.15. FTIR(KBr pellets, cm-1): 1 734w, 1 700w, 1 684w, 1 653w, 1 559m, 1 540w, 1 507w, 1 362s, 419w.
Note: The crystal of complex 3 is not stable at room temperature, especially upon the easily losing of the guest solvent molecules(CHCl3) during its collection from solvents, which leads to the deviation of experimental Element. Anal. data from the calculated values.
1.3X-ray data collection and structure determinations
Single-crystal X-ray diffraction measurements for 1 and 2 were carried out on a Rigaku SCX-mini diffractometer at 293(2) K with Mo Kα radiation (λ= 0.071 073 nm) by ω scan mode. The structures were solved by direct methods using the SHELXS program of the SHELXTL package and refined with SHELXL[22](semi-empirical absorption corrections were applied by using the SADABS program). The non-hydrogen atoms were located in successive dierence Fourier syntheses and refined using anisotropic thermal para-meters on F2. All hydrogen atoms of ligands were generated theoretically at the specific atoms and refined isotropically using fixed thermal factors. Single-crystal X-ray diffraction measurements for complex 3 was carried out on a Bruker Smart 1000 CCD diffractometer at 113 K with Mo Kα radiation (λ= 0.071 073 nm) . The structure was solved by direct methods with the SHELXS-97 program[23]. Refinements were done by full-matrix least-squares techniques on F2with SHELXL-97[24]. The hydrogen atoms bound tocarbon were located by geometrically calculations. All non-hydrogen atoms were refined by full-matrix leastsquares techniques. The electron density of the disordered guest molecules in complex 2 and 3 was treated using the SQUEEZE routine of PLATON[25-27]. The results were appended to the bottom of the CIF file. Detailed crystallographic data of 1, 2 and 3 (after the SQUEEZE) are summarized in Table 1.
CCDC: 868734, 1; 868735, 2; 790268, 3.
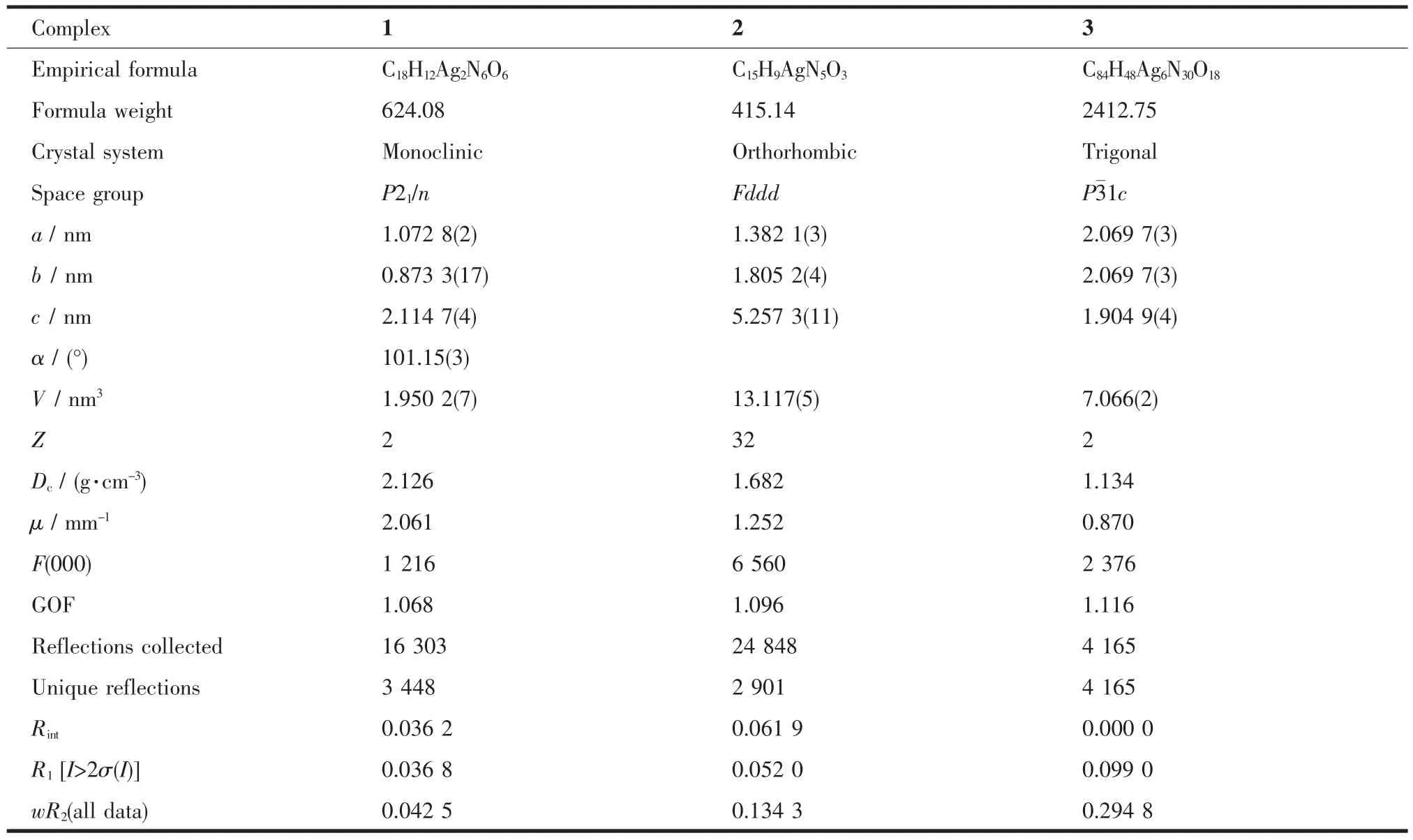
Table 1 Crystal data and structure renement parameters for complexes 1~3
2 Results and discussion
2.1Crystal structure analysis of complexes 1~3
Complex 1 crystallizes in the P21/n space group with an asymmetric unit consisting of one dpq ligand and two crystallographically independent Agions (Fig.1a). Ag1 ion is five coordinated by three N atoms (N1, N2, N4A) from two dpq ligands and two O atoms (O1 and O3) from one nitrate ion, while Ag2 ion is five coordinated by one N atom (N3) from dpq and four O atoms (O2C, O3B, O4 and O5) from three nitrate ions. All of the N-Ag-N angles (69.90(11)°~134.64(12)°), O-Ag-N angles (91.66(12)°to 125.23(11)°), Ag-N (0.222 4(3)~0.258 6(4) nm) and Ag-O (0.235 2(3)~0.258 6(4) nm) bond lengths are within the normal range[20,26]. It is worth noticing that two dpq ligands are linked to form a double decker unit via N-Ag1 bonds (Fig.1b). Furthermore, each double decker is linked with other four neighboring one through Ag2-NO3coordination interaction, leading to a two-dimensional (2D) packing net (Fig.1c). Simplifying the double decker unit as a four-connecting node generates a (4,4) topologic structure for complex 1 (Fig.1(d)).
Complex 2 crystallizes in the Fddd space group, and the asymmetric unit consists of one quarter of a tppq ligand, one Agion and one nitrate ion, as shown in Fig.2a. The Agion is four coordinated by three N atoms (N1, N2, N3) from tppq and one O atom(O3) from nitrate ion. Meanwhile, a double decker unit was also observed as in complex 2, but with four coordinated Agions (Fig.2b). Inspection of the double decker in 2 reveals a centroid-to-centroid distance of 0.342 nm between the central benzene ring of two tppq ligands, indicative of π-π interaction existing between two ligands. Furthermore, each double decker unit is further linked with four neighboring units through a weak Ag-O coordination interaction (0.280 51 nm), leading to a 2D network (Fig.2c). All of the N-Ag-N angles (68.63(14)°~152.08(16)°), O-Ag-N angles (90.43(16)°~130.40(15)°), the bond length of Ag-N (0.226 8(5)~0.245 5(4) nm) and Ag-O (0.259 5(5) nm) bond length are within normal range[28]. Simplifying the double decker as a fourconnecting node generates a (4,4) topology structure for complex 2 (Fig.2(d)).
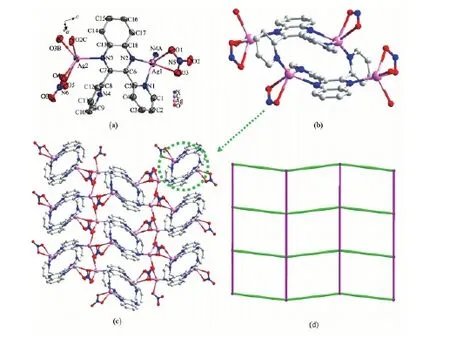
Fig.1 (a) Coordination geometry of Agin 1; (b) The double-decker unit in 1; (c) The 2D network of 1; (d) Schematic representation of (4,4)-connected topology of 1
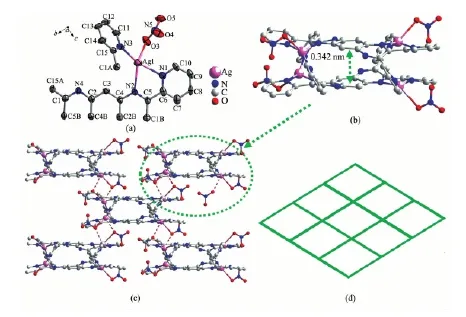
Fig.2 (a) The coordination geometry of Agin 2; (b) The double-decker unit in 2; (c) The 2D network of 2 constructed by weak O-Ag bond (0.280 51 nm, in dotted bond); (d) Schematic representation of (4,4)-connected topology of 2
The asymmetric unit of 3 consists of one third of hpdq ligand and one Agion (Fig.3a). Differing from the case in 1 and 2, the Ag(I) ion in 3 is six coordinated by four N atoms (N1A, N2, N3, N4B) from hpdq and two O atoms (O1, O2) from a nitrate ion, leading to a deformed octahedral geometry. As shown in Fig.3b and c, 3 assembled to 3D structure based on a similar double decker as in 1 and 2, but with six Agions. The planes of two ligands within one double decker are nearly parallel with each other, with a centroid-to-centroid distance of 0.351 nm between two central benzene rings, indicative of the presence of π-π interaction between the rings.
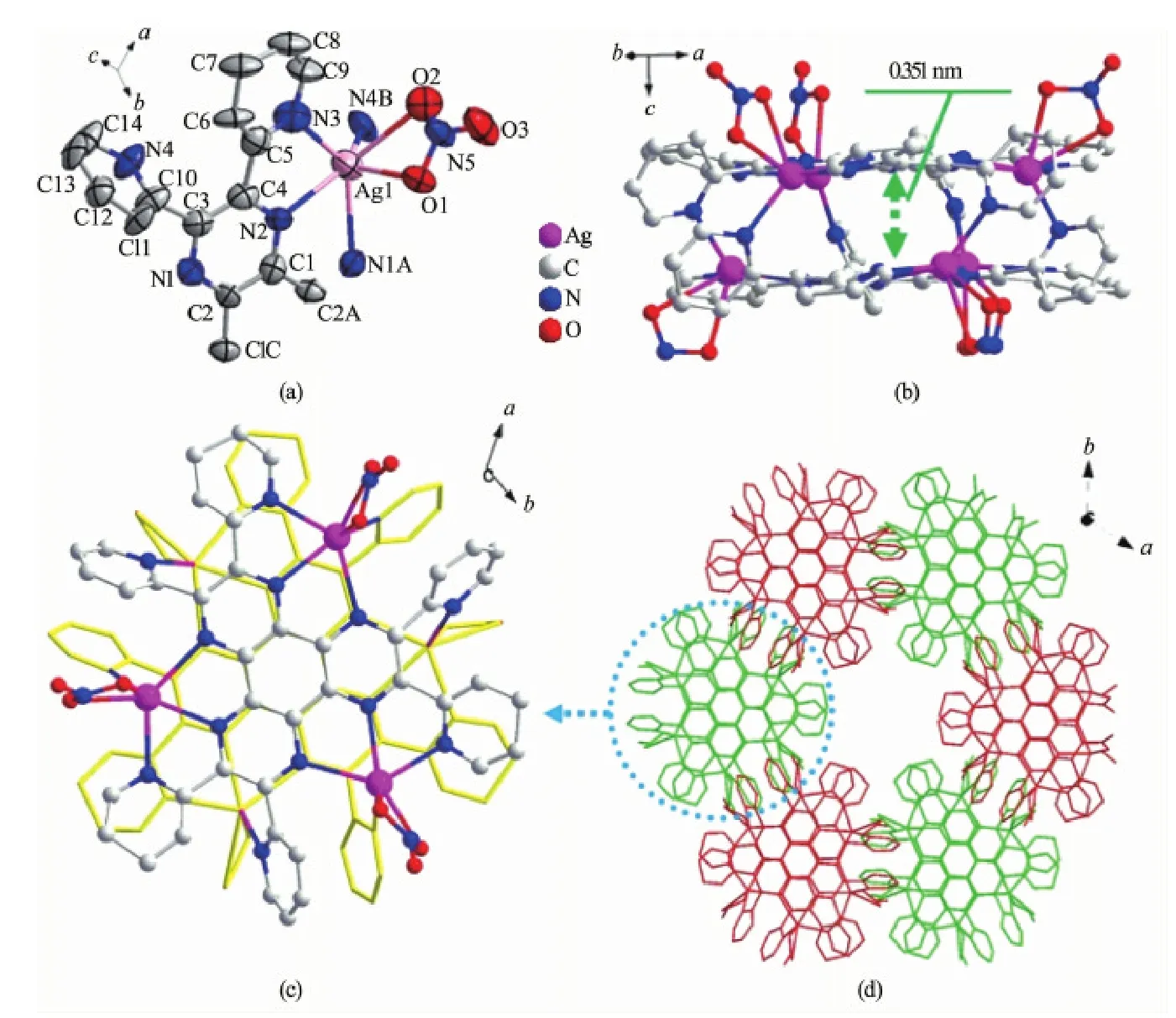
Fig.3 (a) The coordination geometry of Agin 3; (b) View of the double-decker in 3 along the direction normal to c axis; (c) View of the double-decker in 3 from ab plane; (d) The packing diagram of double deckers
Despite similar structure of the double decker in 3 to that in literature[20], several vital differences were revealed via detailed inspection of crystal structure:
(1) The complex reported in literature[20]consists of two kinds of cyrstallographically independent Agthat are both five coordinated. Whereas the Agions in 3 is crystallographically equivalent and six coordinated. In addition, the distance between two hpdq ligands within one double decker of 3 (0.351 nm) is slight smaller than that reported in literature (0.364 nm), due probably to different coordination environment of Agions as well as different hydrogen bond pattern between two hpdq plane (Fig. S1).
(2) The most greatest difference lies in the packing pattern of double deckers. The double deckers in literature were linked with each other through the O…H-C and F…H-C hydrogen bonds between CF3SO3-anion and the C-H of hpdq, whereas that in 3 was completed only through O…H-C hydrogen bond(Fig.S2). The difference results in distinctive packing pattern of double decker (Fig.S3) and thereby different cell parameters and space group between 3 (a=2.069 7(3) nm, c=1.904 9(4) nm, V= 7.066(2) nm3, P31c group) and the complex reported in literature (a=3.031 9(2) nm, c=1.011 9(1) nm, V= 8.055 6(11) nm3, R3 group)[20]. Different packing mode of double decker in 3 from that of literature validatesthe vital role of counter anions in controlling the assembly architecture of Ag-based coordination complexes.
Detailed Comparison among 1~3 reveals some notable differences in their double decker units as well as the networks. Firstly, the Agions exhibit different coordination number in the double decker of 1, 2 and 3, that is 5 4 and 6, respectively. Secondly, the double deckers are linked with each other by strong O-Ag bond, weak O-Ag bond (>0.28 nm) and O …H-C hydrogen bond in 1, 2 and 3, respectively. These differences can be reasonably interpreted in terms of different backbone of used polypyridyl ligands. The strong O-Ag linkage between adjacent double deckers in 1 is repressed in 2 and 3, due probably to increasing steric hindrance of larger ligands, which therefore brings about elongated O-Ag linking in 2, and even more facilitates the hydrogen bond interaction between counter anions and Agions in 3. This underlines the important role of counter anions in the assembly of Agions and hpdq ligands.
2.2Monitoring the coordination interaction by spectral titration
dpq, tppq and hpdq all are soluble in CH3CN and are luminescent owing to their conjugating system, which encourages us to investigate and compare their coordination interaction with Agions in solution by spectral analysis method. All of polypyridine ligands were prepared with a concentration of 5×10-5mol·L-1using CH3CN as solvent, while AgNO3solution (0.015 mol·L-1) was prepared using acetonitrile as solvent. During titration, the volume of the polypryridyl ligand solution (3 mL) can be regarded as constant because of negligible added volume of metal ions solution. For fluorescence spectra measurements, excitation and emission slit width were both set as 5 nm.
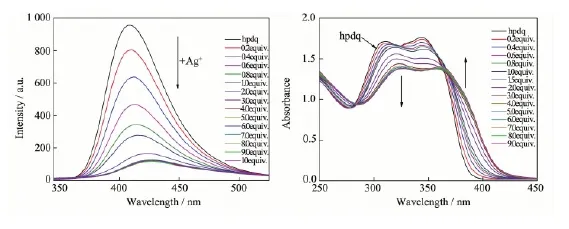
Fig.4 (a) Fluorescence emission spectra of hpdq (λex=300 nm) (5×10-5mol·L-1) in CH3CN (3 mL) upon the addition of Ag; (b) UV-Vis absorption spectra of hpdq (2.5×10-5mol·L-1) in the presence of different concentrations of Agin CH3CN (3 mL)
The Fluorescence variations of hpdq upon the addition of Agions were summarized in Fig.4(a). At beginning of the addition of Agion, the strongest emission peak of hpdq at 409 nm was quenched sharply along with moderate red shift of emission wavelength. The variation becomes stagnant when the addition quantity of Agion approximately approaches to 4 equiv. of hpdq. Finally, the emission peak shifts to 427 nm with intensity less than 15% of the initial peak. The UV-Vis spectra of hpdq were collected in Fig.4(b). The addition of Agresults in the sequential redshift of absorption peaks at 308 and 343 nm, along with the decreasing of the absorbance. The appearance of several isosbestic points between 360~375 nm at different molar ratio of Agto ligands indicates the multistep assembly of hpdq ligand upon interacting with Ag, which is consistent with the coordination of three Agions with one hpdq as observed in the crystal analysis of 3. On the other hand, the variation of Fluorescence and UV-Vis spectra almost has the same equilibrium point with a molar ratio of nhpdq∶nAgequal to 1∶3, which isconsistent with the ratio of nhpdq∶nAgobserved in 3. In addition, the spectral variations of tppq and dpq upon the addition of Agion were also investigated (Fig. S4 and S5), and much more dull variations were observed in comparison with that of hpdq system, due probably to the less π-π interaction of ligands within the double decker in 1, 2 than in 3. This indicates that hpdq is a much sensitive spectral sensor for metal ion detection than tppq and dpq.
3 Conclusions
In summary, three polypyridyl ligands, dpq, tppq and hpdq, were investigated in their coordination interaction with Agion by single crystal analysis and spectral analysis. Single crystal structure analysis unveiled different double decker unit as well as different assembly framework of the resultant complexes 1~3, which was attributed to the cooperation of counter anions and different polypyridyl ligands. Meanwhile, Spectral titration was utilized to study the coordination interaction of polypyridyl ligands with Agin solution, and it was observed that the spectral response of hpdq to the addition of Agions is much more sensitive than the other two ligands, which can be used to guide the design of new organic spectral sensor for Agions detection.
Supporting information is available at http://www.wjhxxb.cn
References:
[1] Ma L, Mihalcik D J, Lin W. J. Am. Chem. Soc., 2009,131: 4610-4612
[2] Li Y P, Yang H R, Zhao Q, et al. Inorg. Chem., 2012,51: 9642-9648
[3] Yang X L, Wu C D. Inorg. Chem., 2014,53:4797-4799
[4] Zhang Y B, Furukawa H, Ko N, et al. J. Am. Chem. Soc., 2015,137:2641-2650
[5] Xue D X, Cairns A J, Belmabkhout Y, et al. J. Am. Chem. Soc., 2013,135:7660-7667
[6] Tian D, Chen Q, Li Y, et al. Angew. Chem. Int. Ed., 2014, 53:837-841
[7] Wang Y T, Fan H H, Wang H Z, et al. Inorg. Chem., 2005, 44:4148-4150
[8] Harris K, Sun Q F, Sato S, et al. J. Am. Chem. Soc., 2013, 135:12497-12499
[9] Zhao Q, Liu X M, Song W C, et al. Dalton Trans., 2012,41: 6683-6688
[10]Li X H, Chen Z, Zhao Q, et al. Inorg. Chem., 2007,46:5518 -5527
[11]Osuga T, Murase T, Fujita M. Angew. Chem. Int. Ed., 2012, 51:12199-12201
[12]Cui Y, Lee S J, Lin W. J. Am. Chem. Soc., 2003,125:6014 -6015
[13]Lubick N. Environ. Sci. Technol., 2008,42:8617-8617
[14]Hamasaki Y, Nakashima N, Niidome Y. J. Phys. Chem. C, 2013,117:2521-2530
[15]Njua E Y, Steiner A, Stahl L. Inorg. Chem., 2010,49:2163-2172
[16]Zhang X H, Zhao Q, Liu X M, et al. Talanta, 2013,108:150-156
[17]Nakamura T, Ube H, Shionoya M. Angew. Chem. Int. Ed., 2013,52:12096-12100
[18]Li X, Xu H, Kong F, et al. Angew. Chem. Int. Ed., 2013,52: 13769-13773
[19]Liu C S, Chen P Q, Yang E C, et al. Inorg. Chem., 2006,45: 5812-5821
[20]Xiao Z Y, Zhao X, Jiang X K, et al. Chem. Mater., 2011,23: 1505-1511
[21]Zhao Q, Li R F, Xing S K, et al. Inorg. Chem., 2011,50: 10041-10046
[22]Sheldrick G M. SHELXTL Ver6.1, Program for Solution and Refinement of Crystal Structures, University of Göttingen, Germany, 1998.
[23]Sheldrick G M. SHELXS-97, Program for the Solution of Crystal Structures. University of Göttingen, Germany, 1997. [24]Sheldrick G M. SHELXL-97, Program for Crystal Structure Refinement. University of Göttingen, Germany, 1997.
[25]Spek A L. PLATON, Utrecht University, Utrecht, The Netherlands, 2008.
[26]Tan C H, Yang S H, Champness N R, et al. Chem. Commun., 2011,47:4487-4489
[27]Yang W B, Greenaway A, Lin X, et al. J. Am. Chem. Soc., 2010,132:14457-14469
[28]Zhang Z Y, Deng Z P, Huo L H, et al. Inorg. Chem., 2013,52:5914-5923
多吡啶配体和银(Ⅰ)三明治配合物的构筑、结构及光谱学分析
赵强1刘秀明2冯睿2章应辉*,2孔曼曼1刘洋1
(1南阳师范学院化学与制药工程学院,南阳473061)
(2南开大学材料科学与工程学院,天津300071)
三个不同的多吡啶配体:2,3-二(2-吡啶基)喹喔啉(dpq)、2,3,7,8-四(2-吡啶基)喹喔啉(tppq)和2,3,6,7,10,11-六(2-吡啶基)喹喔啉(hpdq)分别和银反应得到3个银的配合物:[Ag(dpq)(NO3)2]n(1)、[Ag4(tppq)2(NO3)4](2)和[Ag6(hpdq)2(NO3)6] (3)。结果显示,3个配体分别和银形成了四核(1)、四核(2)以及六核(3)的三明治单晶结构,1和2的四核结构依靠Ag和NO3-之间的相互作用进一步延伸为二维面,而3的六核结构依靠C-H…O的氢键作用形成三维网。此外,在研究3个配体和金属银在溶液中的光谱学性质时发现,配体hpdq和银反应的紫外吸收光谱和荧光发射光谱都要比其它2个配体灵敏度高。
多吡啶配体;金属有机配合物;自主装;紫外光谱;荧光光谱
O614.122
A
1001-4861(2016)03-0537-08
10.11862/CJIC.2016.060
2015-09-05。收修改稿日期:2016-01-06。
国家自然科学基金(No.21301100)、河南省教育厅基金(No.13A150819)和南阳师范学院专项基金(No.ZX2013029)资助项目。*通信联系人。E-mail:zhangyhi@nankai.edu.cn;会员登记号:S06N8293M1405。
