微波辅助细菌纤维素酯的制备及对Pb(II)的高效去除
2016-09-13王吟孙凤玲张晓东陶红杨一琼
王吟 孙凤玲 张晓东 陶红 杨一琼
(上海理工大学环境与建筑学院,上海200093)
微波辅助细菌纤维素酯的制备及对Pb(II)的高效去除
王吟*孙凤玲张晓东*陶红杨一琼
(上海理工大学环境与建筑学院,上海200093)
以细菌纤维素(BC)为原料,通过微波辅助酯化改性的方法制得了两种改性细菌纤维素,细菌纤维素黄原酸酯(XMBC)和细菌纤维素硫酸酯(SMBC)。对所制备的样品进行X射线衍射(XRD)、扫描电镜-电子能谱(SEM-EDS)、傅里叶变换红外(FT-IR)光谱和BET比表面积分析,通过续批式实验考察其对Pb(II)的去除效果。研究了pH值、反应时间、温度、污染物初始浓度、离子强度对其吸附能力的影响以及材料再生性能。结果表明,改性细菌纤维素的比表面积和孔容均有上升,其对Pb(II)的吸附量随反应温度和离子强度的增加而降低,最优pH值为5.0。巯基的引入增强了细菌纤维素对Pb(II)的吸附能力,改性后的吸附剂显示出比原始BC更优异的吸附性能,其中XMBC和SMBC的最大吸附量分别为144.93和126.58mg∙g-1,该吸附过程符合准二级速率方程和Langmuir等温吸附模型。材料对Pb(II)的吸附是自发的放热过程,且吸附剂易于再生和重复回收。因此,SMBC和XMBC作为从水中富集分离重金属的新型材料具有及大应用前景。
细菌纤维素;酯化改性;微波辅助;生物吸附;重金属
1 Introduction
Water pollution caused by heavymetal ionshasbecomeamajor issue and perp lexed researchers for a long time.Among these metals,lead ions are significantcontam ination sources ofwater, because they arewidely used in a variety of industrialprocesses such as electronics,batterymanufacture,petroleum refining,and metalm ining1.Lead exposure,even at low concentrations,can cause serious diseases such as renal disturbances,hepatitis,encephalopathy,anem ia,lung failure,bonelesions,and cancer2. Therefore,the removalof Pb(II)from wastewater issignificant for preserving public health and the environment3,4.At present,there are severalmethods for removing heavymetal ions from w astewater,such as chemical precipitation,membrane filtration,ion exchange,electrodialysis,reverseosmosis,electrolysis,and adsorption technique5-9.However,mostof thesemethods havehigh operating cost and the need for disposal of the resulting solid waste.Due to the advantages of econom ical feasibility and environmental friendly behavior,adsorption is regarded as the best technique for removing heavymetal ions10-12.Consequently,many effective adsorbentswith strong affinity and high loading capacity for Pb(II)w ere subsequently prepared,such as alum inum hydroxide13,carbon14,zeolite15,clay16,resin17,and silicagel.Recently theuse of biosorbents to removemetal ionshas been discussed w idely18,19.
As biosynthesized cellulose,bacterial cellulose(BC)is an extracellular cellulose produced by bacteria of the Acetobacter xylinum20.It is identical to plant cellulose with respect to the microfibrous structure,which has a ribbon-like structure,and the thickness is two orders ofmagnitude smaller than thatof plant cellulose.In addition,it has an ultrafine nanofiber network structure and unique properties including high w ater holding capacity,high tensile strength,elasticity,no secondary pollution, high specific surface,pore structure,andmany hydroxyl groups in the chains21,22.Benefit from these unique properties,BC has attracted an increased interest in commercialapplicationsover the past few years,and italso has the potentialasanew adsorbent for effective separation of heavymetal ions recently.
However,BC cannotbe directly used asan adsorbentbecause of its low adsorption capacity and poor selectivity.Therefore,it isnecessary tomodify BCw ith a bettermode in order to increase the adsorption capacity.Specifically,twomain approaches are utilized for themodification of BC:(1)directmodification,involving the cellulose backbone by introducing chelating functionalities,(2)grafting of specific monomers to the cellulose backbone and subsequent functionalization of these grafted polymer chains.Several chemicalmethods tomodify BC have been reported,diethylenetriam ine BC was synthesized by amination with diethylenetriamine on bacterial cellulose,and its adsorption properties for Cu(II)and Pb(II)were investigated by Shen etal.23.Donia etal.24have reported the preparationofnanomagnetic cellulosehybrid obtained from precipitation of cellulose in presenceof Fe(II)/Fe(III)mixture.Thehybridmaterialwas then functionalizedwith amino group through successive treatmentby glycidylmethacrylateand tetraethylenepentamine,and showed fast kinetics for theadsorption of threeheavymetal ions.O-Rak etal.25grafted poly(vinylidene fluoride)(PVDF)onto bacterial cellulose by a binary blend system.Among these modifications,those containing sulfur-bearing groupshavea strong affinity for heavy metals,and enhance the interaction w ith them.Recently,grafting thiolsor xanthate onto variousorganic compounds to obtain new compositematerialshas been explored26-28,which could improve the physicaland chem ical propertiesof organicmatter for removal of variousmetals.However,to our know ledge,therehave been few reports for suchmodification onto bacterial cellulose.
Microwave irradiation isone of the very effectivemethods for activating chem ical reactions in a homogeneous and selective manner,it also hasmany properties such as rapid volumetric heating,short reaction time,energy saving,and high reaction selectivity.Stonica-Guzun et al.22have reported the synthesis of bacterial cellulose-calcium carbonate composites using a rapid method,microwave irradiation.The resultsproved thatmicrowave irradiation can be used to obtain good quality BC-calcium carbonate com posites.Sathvika etal.29have studied them icrow ave assisted preparation of yeast immobilized cellulose for the removalof toxic Cr(VI),which showed thatm icrowave irradiation could promote the incorporation of yeast in the biopolymermatrix. The adsorption of fluoride onto microwave induced Al-Zr impregnated cellulosewasstudied by Barathi etal.30.Asa result,the innovations in themethodologies have evolved with a view to overcome some of the drawbacks of the existingmethodswhen applied to a specific problem.
In thisstudy,we focused on BC asastartingmaterial to prepare tw o new biosorbents,xanthate-bacterial cellulose and sulfatebacterial celluloseby esterificationmodification through a novel microwave assisted method,sincemicrowave assisted methodology could be conceived asan energy efficientand accelerating the etherification process.Our approach w as to offer interesting possibilities to obtain the cross-linkwith various functionalgroups in a dispersiveway on the BC,maximizing subsequentadsorption for Pb(II).Themain objective of the study w as to determ ine the adsorption capacity of thebiosorbents,to establish the applicable isotherm modeland to elucidate the sorptionmechanism.
2 Experimental
2.1Materia ls
All reagents used in thiswork were of analytical grade and purchased from Sinopharm Chemical ReagentShanghaiCo.Ltd. Pb(NO3)2wasused assource for Pb(II)ions.Stock solution(1000mg∙L-1)of the studied ionswasprepared in distilledwater.
2.2Preparation and p retreatmento fbac terial ce llu lose
BC was prepared in static culture on amodified HSmedium containing 2%fructose,and extracted from Acetobactersp.which isolated from the traditionally fermented vinegaraccording to the reported method31,32.The gel-like pellicles were washed w ith running tapwater for 24h to removeany residueon their surfaces and stirred in a 0.1mol∙L-1NaOH solution for 24h at70°C followed by extensive washing w ith deionized water until the filtratewasneutral.Thepurified BC gelswere dried under vacuum at80°C and crushed into a 40mesh powder beforebeing stored for futuremodification.
2.3Prepara tion o fm od ified bac te ria l ce llu loses
2.3.1Microwave-assisted xanthatemodified bacteria l ce llu lose
PretreatmentBC(10g),in 10mL of CS2and 100mLof NaOH solution(10%)were taken in a 250m L flask and irradiated in a Panasonic(NN-GF352M,1000W)domesticmicrowaveoven for 180s using only 10%of the total powerw ith interm ittent time durationof 30ssoas to ensure thatcellulosedosenotundergo any degradation.Then 0.25mol∙L-1of MgSO4solution wasadded and stirred at room temperature for24h.
2.3.2Mic row ave-assisted su lfatemodified bac terial ce llu lose
Firstly,the sodium BC(10g)w as m ixed w ith 60m L epichlorohydrin and 125mL 2mol∙L-1NaOH,and keptunder the samem icrowave condition as above to form epoxypropy l-BC. Afterwards,50mLH2SO4and 60mL isoamylolwere added to the particles and them ixture was stirred for 1 h at ambient temperature.
Finally,the obtained products were collected and w ashed thoroughly with deionizedwater to beneutral,dried in vacuum at 60°C.Designed as XMBC(xanthate-modified BC)and SMBC (sulfate-modified BC),respectively.
A ll of the synthesis conditions have been optim ized in the laboratory.
2.4Characterization o f themodified bac terial cellu loses
Surface areasand poroussize distribution of themodified BCs weremeasured by nitrogen adsorption and desorption analysis (ASAP 2020,Micrometrics,USA).Crystalstructures of samples were determ ined by performing X-ray diffraction(XRD)on D8 ADVANCE X-ray diffraction spectrometer(Bruker,German). Surfacemorphologies were exam ined by a scanning electron m icroscope(SEM,HitachiS4700,Japan)w ith theworking distance of 5-12mm and an accelerating voltage of 20keV.The SEMwasequipped w ith an energy dispersion spectrometer(EDS) and itwas used to perform theanalysis of chemical constituents of the biosorbents.Infrared(IR)absorption spectraweremeasured at room temperature on a Fourier transform infrared(FT-IR) spectroscopy(Nicolet Instrument Corporation,USA)using the KBr Pellettechnique.
2.5Adsorp tion experim en t
Adsorption experimentsw ere carried out using a batch w ise method.Allbatch reactorswere placed on a shakerat150r∙min-1under controlled temperature.The solution pH wasadjusted from 1.0to6.0w ith0.1mol∙L-1HClor0.1mol∙L-1NaOH.Theamount of metal ions adsorbed on the adsorbents at adsorption equilibrium,qe(mg∙g-1),was calculated according to the follow ing Eq. (1):

where C0and Ceare the initial and equilibrium metal ion concentrations(mg∙L-1),respectively,V is the volumeof the solution (L),and W is themassof the adsorbentused(g).
Theeffectsof pH and ionic strengthwere studiedwithin the pH rangeof 1.0-6.0and for non-fixed ionic strength,0.1,1 and 10mmol∙L-1NaNO3solution w ith the initial Pb(II)concentration of 20mg∙L-1and the contact time of 24h at25°C.Adsorption kineticsw ere studied using an initial concentration of 20mg∙L-1w ith the sorbent dosage of 2.0g at 25°C.During the kinetics experiment,aliquots of sampleswerewithdrawn at fixed intervals and the concentration of the Pb(II)ions in each of thesampleswas determined.Adsorption isothermswere studied atvarious initial concentrations of Pb(II)ions under three different temperatures (288,298,308K)with the sorbentdosageof 0.05g.
Before analysis,samplesweremeasured immediately after the solution w as filtered through a 0.45μm membrane.The amount of remainingmetal ionswasdeterm ined by an atomic absorption spectrophotometer(AAnalyst600,PerkinElmer company,USA).
2.6Deso rp tion and regeneration experim en t
Reusability of thebiosorbentswereexamined in a0.1mol∙L-1ethylene diam ine tetraacetic acid(EDTA)23,24and 0.5mol∙L-1thioureaacidified with dropsof 0.2mol∙L-1H2SO4solution24as the regenerators.Typically,the biosorbents w ithmetal ions adsorbed was added into 50mL of the desorption solution.The mixtureswere stirred in awaterbath shakerat150r∙m in-1and at 25°C for 1 h,and sampleswere taken from the solution tomonitor the amountofmetal ions desorbed into the solution.A fter the desorption test,thebiosorbentswere separated andwashedw ith deionized water,and reused in the next cycle of adsorption experiment.Theadsorption-desorption experimentswere conducted for five cycles.
3 Results and discussion
3.1Characterization of modified bacteria lcellu loses Fig.1 show s the X-ray diffraction patterns of the biosorbents. According to the powder diffraction file(PDF)based on the XRD database obtained from a jade5software,the profile of BC is cellulose IIXRD pattern(2θ=12.26°,20.16°,21.13°)33,itw as clear that the original BC without pretreated with NaOH hadtypical cellulose Idiffraction anglesataround 14.87°,16.25°,and 22.64°34.SMBC and XMBC show the similar cellulose IIpattern with BC,however,the degreeof crystallinitywassmaller than that of BC and crystalline structurewasweakened for SMBC.These changes indicate that the crystalline regionsof the BC havebeen destroyed during the dissolution and homogeneous sulfating processeswhich formsamore stable cellulose IIpolymorph35.It is beneficial to decrease the degree of crystallinity of BC by etherification because structure of hydrogen bonding is destroyed. At the same time,adsorption efficiency is improved by the introduction of thiolgroup into the structureof BC.
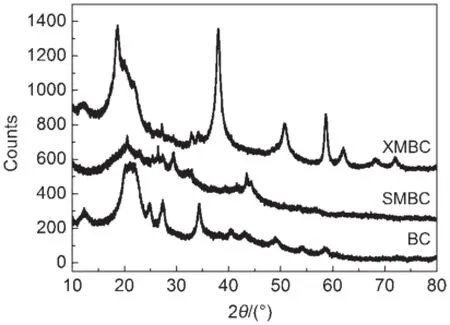
Fig.1 XRD patternsof bacterial cellulose(BC),xanthatem odified bacterial cellulose(XMBC),and su lfate-modified bacterial cellulose(SMBC)

Fig.2 In frared spectra of theb iosorbents
FT-IR spectra of thebiosorbents are presented in Fig.2.In all samples,the spectraexhibit typicalpeaks for functional groups. The broad band centered at3320cm-1is corresponding to the O―H stretching vibration.The C―H asymmetric and symmetric tensile vibrationmode isobserved at2900cm-1.The peak at1636cm-1originates from thebendingmodeof theadsorbedwater,as well asC―O antisymmetric bridge stretching vibration at1160cm-1.The C―O―C mode from the pyranose rings′skeletal vibrationsobserved at1060cm-1,and theβ-glucosidic linkagesat 898 cm-136.Theseabsorption bandsare characteristic absorption bands of cellulose37,38,indicating that the structure of BC is not destroyed by themodification.Moreover,the peak intensity of the modified BCs becomes weaker than BC,indicating that the modification reduced the infrared absorption sensitivity of cellulosemolecules.The spectrum of SMBC showsadditionalnew band at813 cm-1corresponding to S=O stretching vibration,this proved the presence of sulfate groups.For XMBC,there are severalpeaksin the rangeof1300-1500cm-1assigning to theC―S bending vibration,imp lying the existence of―O―CSSH. Moreover,the transmittanceof O―H at3320cm-1decreased after the modification,indicating that the hydroxy l groups play an important role during microwave irradiation.The localized rotationson an almost immobileOH group arising asa resultof the dielectric heating involve energy transfer to surrounding solvent molecules.
The surface physical parameters obtained from the N2adsorption isotherms(Table 1)suggests that the BET surfacearea and totalpore volume change significantly afteretherification.In comparison w ith BC,the BET surface area and total pore volume increase to126m2∙g-1and 0.05cm3∙g-1forXMBC,and to134m2∙g-1and 0.09 cm3∙g-1for SMBC.Meanwhile,the pore size reduces to 2.8 nm for XMBC and 4.3 nm for SMBC.Although the surface areaof XMBC issmaller than SMBC,the pore sizeof XMBC is smaller,which ismore suitable for the adsorption ofmetal ions.
Fig.3 shows the SEMand EDSanalysis ofmodified BC.For SEMimages,the dense network structure can beobserved on the surface of BC.The average diameter of the sample is approximately 50-100nm.Comparedwith BC,XMBC and SMBC have denser netw orksand the ribbon become broader.Itm ight due to that xanthate can adhereon the surfaceof the BC nanofibrilsand incorporates into the ribbon.The result indicates thatesterification treatmentunder a short timemicrowave heating can improve the porosity,structure and crystalof the bacterial cellulosematerial, w hich is consistentw ith the literature result39.
Furthermore,the EDSspectra of selected zoneof the samples are carried out to investigate the chemical constituents in the BC matrix.For XMBC and SMBC,there isa peak corresponding to S elementwhich confirms the success of themodification by xanthate and sulfate.This result is in good agreementw ith the XRD observation.
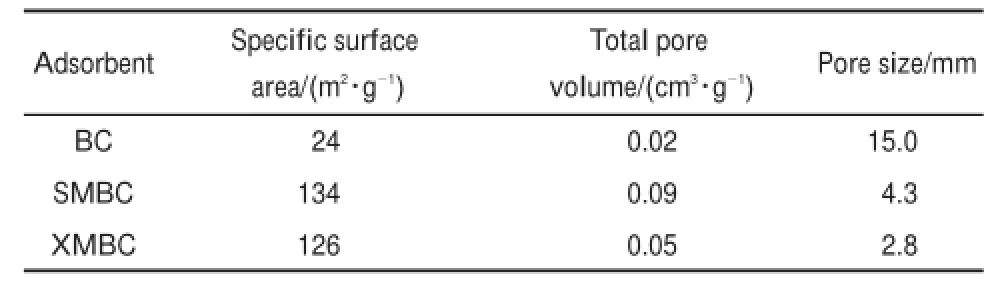
Table1 Physical proper ties of theb iosorben ts
3.2Resu lts o f adsorp tion experim ents
3.2.1EffectofpH on static adsorption
ThepH levelof the solution hasa profound impactonmetal ion adsorption efficiency because it changes both the form of functionalgroupson themodified celluloseand also the form ofmetal ions40.Fig.4shows theeffectof pH on theadsorption behavior. When the pH was adjusted to a higher value of 6.0,lead precipitationwasobserved owning to the increaseof theOH-ions in the adsorptionmedium,as a result,the pH rangew as setat 1.0-6.0to investigate theeffectof solution pH on theadsorption perfor-mance.It is found that the removalefficiency of Pb(II)increases quick ly w ith increasing pH to 5.0.The XMBC shows the best removal efficiency(91.99%),and it exhibited approximately 10%-20%higher Pb(II)removalefficiency than theunmodified BC.Thismay be ascribed to the preferential ofmetal complex formation between thiolgroup and Pb(II)41.SMBC showsa little lower removalefficiency of 86.23%than XMBC,thisobservation can beexplained by the smallpore sizeof XMBC,which ismore suitable for the adsorption ofmetal ions.When the pH valuewas above5.0,the adsorption capacity declined.The reason could be attributed to the very unstable complex on thebiosorbent surface. As for the BC,the system stillshowsdecent removalefficiency, likely due to the charge attraction between negatively charged carboxylategroupson the cellulose surfaceand positively charged Pb(II).With the increaseof pH,more―COOH groups could be dissociated into COO-groups,indicating higher adsorption capacity of Pb(II)ions due to electrostatic attraction42.At the same time,the negative charge density on the carbon surface increased due to depronation of the H+ion-containing binding sites,thereby improving the adsorption capacity toward Pb(II)ions41.And when the pH was higher than 5.0,competition between H+and Pb(II) ions for surface adsorption sites decreased.To increase the adsorption capacity aspossibleand keep Pb(II)asa simple form,i. e.,Pb(II)in the solutions,pH of 5.0is selected for the rest of the batch experiments.

Fig.3 SEMand EDS data of the biosorbents
3.2.2Effectof ionic strength
The presence of salt or co-ions in solution can affect the sorption ofmetal ions.As investigated in Fig.5,Pb(II)adsorptionby the biosorbentsdecreased with increasing ionic strength.The declineof removalefficiency indicates that the cations can alter the surface property of biosorbents and thus can influence the adsorption of Pb(II)on the surface.Actually,there exists a competition force between the hydrated ions(Na+)and Pb(II),thus reducing Pb(II)adsorption43.This indicates that the adsorption interaction between the functionalgroups of thebiosorbents and Pb(II)ions ismainly of ionic interaction nature,which is in agreement with an ion exchangemechanism.Besides,Na+in solutionmay influence the double layer thicknessand interfacepotential44.And for XMBC and SMBC,the impact of ionic strength is relatively smaller than thatof BC,which reveals that the increase of Na+would not inhibit the comp lexion reaction between thiolgroup and Pb(II).

Fig.4Effects of pH on the rem ovalof Pb(II)on the biosorbnets
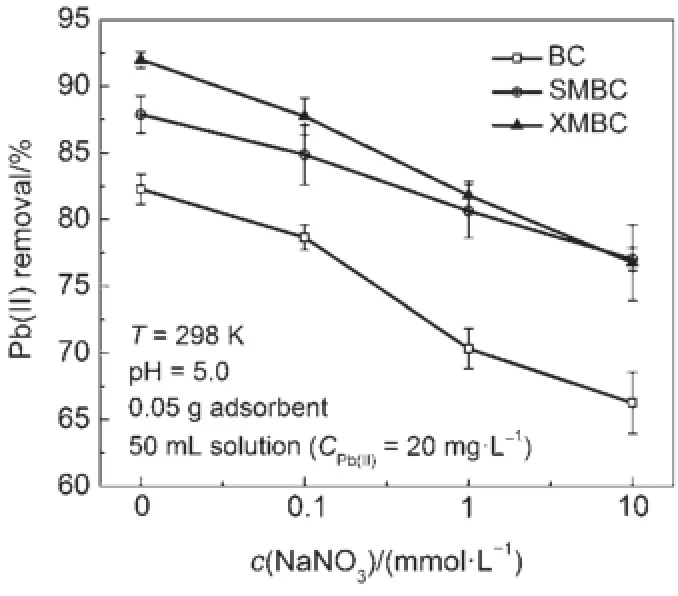
Fig.5Effectsof ionic strength on the removalofPb(II)on thebiosorbents
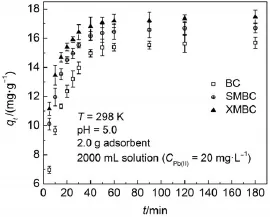
Fig.6Adsorption kineticsof Pb(II)on the biosorbents
3.2.3Adsorp tion kinetics
Fig.6demonstrates the adsorption capacity of Pb(II)by the biosorbentsatdifferent time.As illustrated in Fig.6,theadsorption capacity ofmodified BCs rapidly increases in the first30min and then slow ly augments.The adsorption equilibrium has been achieved aftermore than 40m in for XMBC and SMBC.Compared w ith BC,the adsorbing time ofmodified BCs is clearly shortened and the adsorption capacity are clearly increased.One possibleexplanation is that the formation of Pb-thiolate complex through the chelating reaction of S=O and C―Swith Pb(II)is very fastand strong for SMBC and XMBC,respectively45.
To examine themechanism of theadsorption process,adsorption kineticsare tested w ith the pseudo-firsorder equation(Eq.(2)),the pseudo-second-order equation(Eq.(3)),and particle diffusion equation(Eq.(4))based on adsorption equilibrium capacity46.
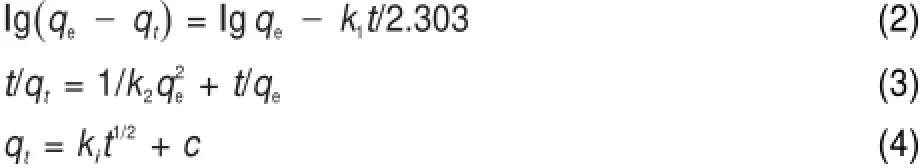
where qe(mg∙g-1)and qt(mg∙g-1)are theadsorption quantity at adsorption equilibrium and theadsorption quantity at time t(min), respectively.k1(min-1),k2(g∙mg-1∙min-1),and ki(mg∙g-1∙min-1/2) are the kinetics rate constants for the pesudo-first-order,pesudosecond-order,and particle diffusion equation,respectively.c(mg∙g-1)is the interceptof intraparticle diffusionmodel.Valuesof c give information about the thickness of the boundary layer,that is,the larger intercept the greater is theboundary layereffect47.
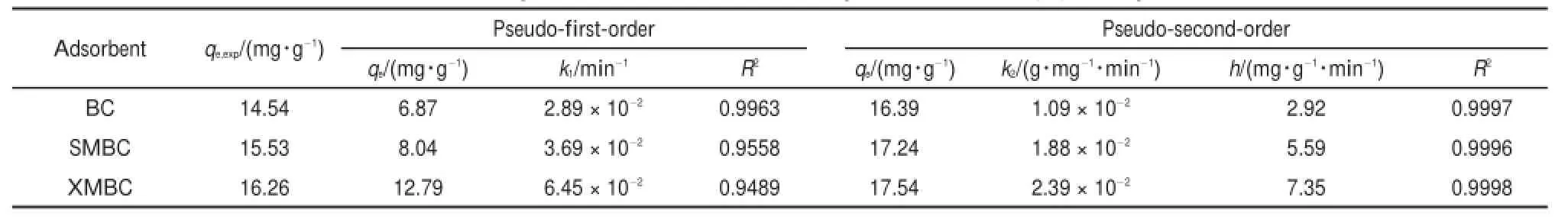
Table2 Pseudo-first-order and pseudo-second-order kineticsparametersof Pb(II)adsorp tion on the biosorbents
The rate constants of adsorption for kineticsmodels are calculated and the results are reported in Table 2.As illustrated in Table2,the valuesof correlation coefficient(R2)for the pseudosecond-order adsorptionmodel are relatively high(>0.9990), moreover,theadsorption capacities calculated by themodel are also close to those determ ined by experiments.However,the values of R2for the pseudo-first-order are not satisfactory. Therefore,itcan be concluded that the pseudo-second-orderadsorption model ismore suitable for describing the adsorption kinetics of Pb(II)on thebiosorbents.It reveals that the chem ical adsorption was themain control process for the biosorbents48.The product k2qe2is the initialsorption rate,represented asaswe can see in Table2,the initialsorption rateofmodified BCsare much higher than that of BC,verified that themodification is favorable for promoting the adsorption rate.The result indicates thatboth physicaland chemicaladsorption existed in adsorption process,butchem icaladsorption wasdom inated.
The intra-particle diffusion constants are computed from the plotof qtvs t1/2(Fig.7).A ll plots in Fig.7 presentmulti-linear and therewere three differentportionswith differentslopes,indicating that several processes affected the Pb(II)adsorption on allmaterials.The p lots for the initial linear did not pass through origin (c≠0)(Table 3),implying that intraparticle diffusion isnot the only rate-controlling step and boundary layer controlmay affect theadsorption.
3.2.4Adsorp tion isotherm s
Theadsorption quantity of Pb(II)by thebiosorbentsatdifferent initial concentration of Pb(II)are presented in Fig.8.The adsorption capacity of Pb(II)increasesw ith increasing initial concentration.In the early stage,the adsorption capacity increases sharply,after that,the slop of curve decreasesobviously and the curve flattens out.Furthermore,the adsorbed amounts all decreaseswith the increasing of temperature,which indicates an exotherm ic process in nature.
Theadsorption dataareanalyzedwith Langmuir(Eq.(5))49and Freundlich(Eq.(6))50isotherm models:

where Ce(mg∙L-1)is theequilibrium concentration ofmetal ions, qe(mg∙g-1)is the amountofmetal ionsadsorbed,qm(mg∙g-1)is themaximum adsorption capacity ofmetal ions,and b(L∙mg-1) is the Langmuir adsorption equilibrium constant related to the affinity of the binding sites.Kf(mg∙g-1)and n are the Freundlich constants.
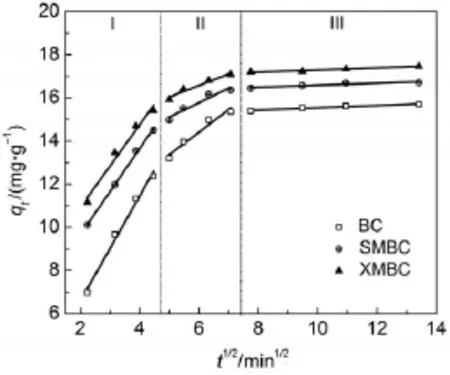
Fig.7 Intraparticlediffusion kinetics for adsorp tion of Pb(II)on thebiosorbents

Fig.8 Adsorp tion isotherm sof Pb(II)on thebiosorbents

Table3 Intraparticle diffusionm odel constan ts and correlation coefficients for adsorption of Pb(II)on thebiosorben ts
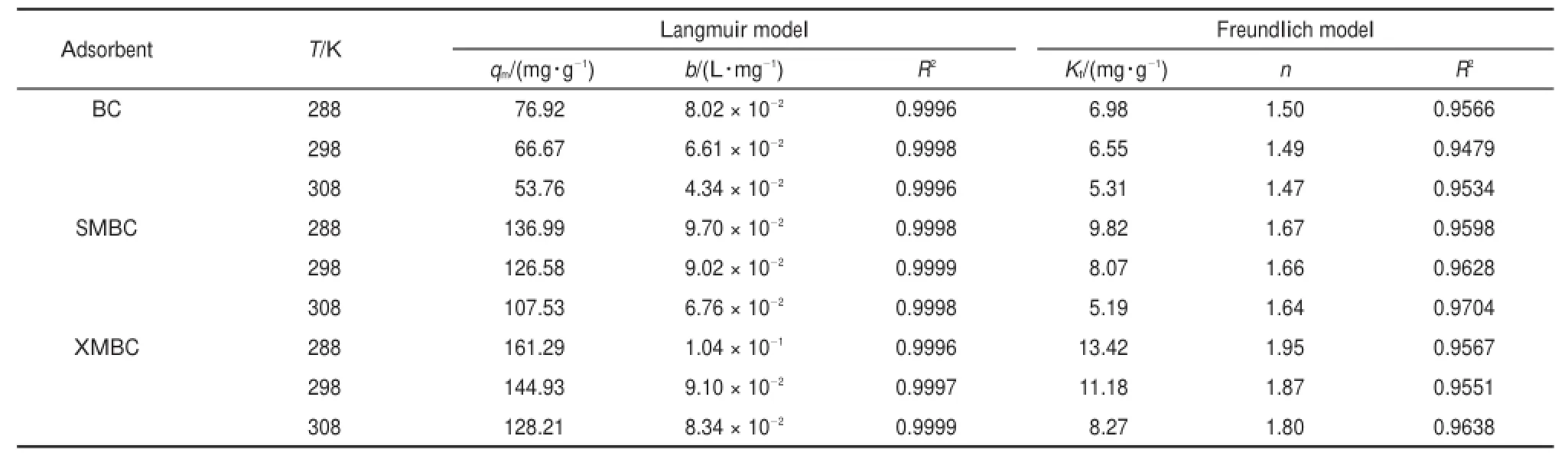
Table 4Langmuir and Freundlich isotherm parameters for adsorption of Pb(II)on thebiosorbentsat different temperatures
The valuesof the constantsand the correlation coefficientsare listed in Table 4.Based on the coefficient obtained,it can be concluded that the Langmuir equation gives a better fit(R2> 0.9990)to the experimental data than that of the Freundlich equation,which indicatesamonolayermolecule adsorption during theadsorption process.A steady decrease in KL(Langmuir constant(L∙mol-1))valuesw ith increasing temperaturein Table 4proves that theaffinity of Pb(II)forall thebiosorbentsdecreasesw ith rising temperature from 288 to 308 K,also illustrating that the process is exothermic in nature.It can be seen that themaximum adsorption capacity of Pb(II)followed the orderas XMBC (144.93mg∙g-1)>SMBC(126.58mg∙g-1)>BC(66.67mg∙g-1), show ing a favorable Pb(II)removal w ith the modification. Moreover,Freundlich isotherm data in Table 4disp lays that the valuesof n weremore than 1 for the two biosorbentsatdifferent temperatures,suggesting thatPb(II)adsorptionwas favorable.
Theaboveadsorption resultsare comparedwith othermodified cellulose-based adsorbents(Table5).Under similar testconditions in terms of optimum pH,room temperature,the XMBC and SMBC demonstrate higher adsorption capacity than the chosen adsorbents.Taking into account of its considerable adsorption capacity andcost-effective sources,the XMBC and SMBC develop in presentstudy havegreatpotential forapplication in Pb(II)removal from aqueous solution.
3.2.5Adsorp tion the rm odynam ics
The thermodynam ic parameters,such as change in standard freeenergy(ΔG0,kJ∙mol-1),enthalpy(ΔH0,kJ∙mol-1),andentropy (ΔS0,J∙mol-1∙K-1)were determ ined by using the following equations48:

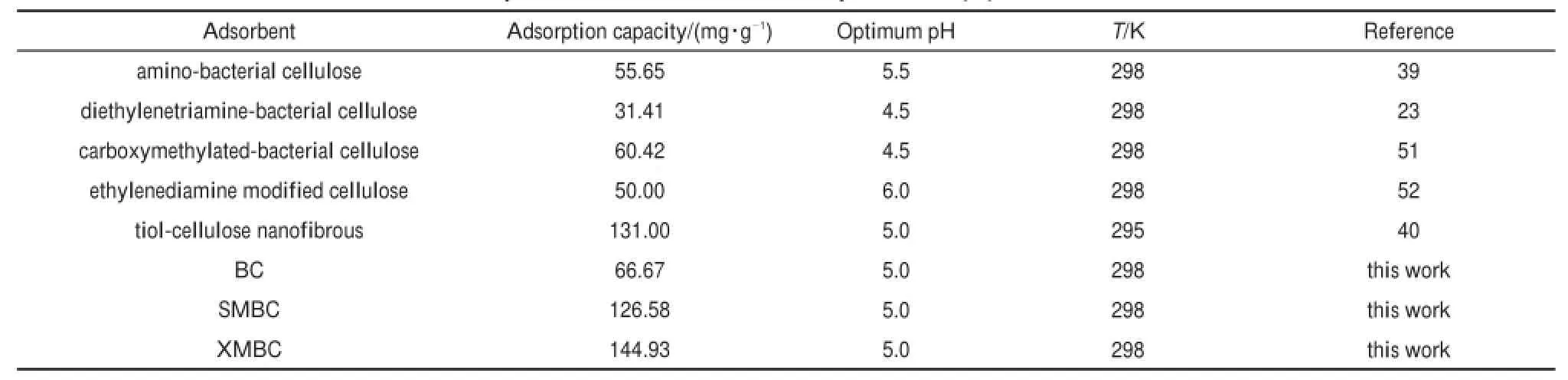
Table5Com parison of them axim um adsorption of Pb(II)on various celluloses

Table6Valuesof thermodynam ic parameters for adsorption of Pb(II)onto thebiosorbents

Table7 Desorption and regeneration efficienciesof Pb(II)on the biosorbents
3.3Regene ration o fm od ified bac te rial cellu lose
In practicalapplication,it isvery important to investigate the ability of an adsorbent to be regenerated and reused57.The effect of five adsorption-desorption consecutive cycleson the efficiency of the individualadsorption of Pb(II)on thebiosorbents isstudied. Table 7 shows the corresponding desorption efficienciesobtained at EDTA and acidified thiourea.For desorption conducted w ithEDTA solution,itwas found that the Pb(II)adsorbed on allbiosorbentswaseasily desorbed.The desorption efficiency reached about 98%after first cycle,and the biosorbents could retain greater than 92%of their initial adsorption capacity after five cycles,which indicates that thereare no irreversible siteson the surfaceof thebiosorbent.Moreover,The desorbedmaterialswere highly effective for the readsorption of Pb(II),and the adsorption abilitywaskeptconstantafter several repetitionsof theadsorptiondesorption cycles.For desorption conducted with the acidified thiourea solution,the desorption efficiency reached about 94% after the firstcycle,which is lower than the EDTA solution.And the adsorption capacity reduced significantly after the third cycle. Thismay be attributed to the differentmechanisms of the two eluents.EDTA is a better regenerator for desorption of heavy metal ions due to its high affinity to metal ions.W hen using acidified thiourea,theabundanthydrogen ions in the solution,a dom inant protonation reaction takes place between hydrogen ions and active sites(COO-groups).Thus,the complexation between theactive sites andmetal ions is destroyed and the adsorbent is regenerated.How ever,the structure ofmodified BCs and their adsorption active sitesareeasily destroyed by the acid solution, leading to lower adsorption capacities after each regeneration cycle.While the EDTA solution is am ild desorption solution which does notdestroy the active sites.Therefore,theadsorption onmodified BCs remained athigh value after several recycling procedures.
4Conclusions
In this work,two different modified bacterial celluloses (SMBC,XMBC)were synthesized by introducing xanthateand sulfate asmodification agents under a short timem icrowave heating.The characteristics analysis revealed thatmodification brought the network structure of the bacterial cellulosemuch denser and improved its surface area.The experimental data showed that thebestadsorption performanceof thebiosorbents for Pb(II)were obtained in solution at pH 5.0.The adsorption equilibrium time of themodified BCswere shortened compared w ith BC,which was 40m in for XMBC and SMBC.The adsorption kinetics closely followed the pseudo-second-order kineticsmodel. The adsorption isotherm data were well fitted with Langmuir model and the maximum adsorption capacity of Pb(II)were followed the order of XMBC(144.93mg∙g-1)>SMBC(126.58 mg∙g-1)>BC(66.67mg∙g-1)at298 K.Theadsorption process of Pb(II)was controlled by both boundary layer control and intraparticle diffusion,and wasan exotherm ic reaction,indicating that the adsorbing rate could beaccelerated with the decrease of reaction temperature.The spent biosorbent could be readily regenerated for reuseby EDTA solution.The presentstudy provided the relatively comprehensive data for the XMBC and SMBC application to the removalofmetal ion in thew astewater.
References
(1)Freitas,O.M.M.;Martins,R.J.E.;Delerue-Matos,C.M.; Boaventura,R.A.R.J.Hazard.Mater.2008,153(1-2),493. doi:10.1016/j.jhazmat.2007.08.081
(2)Cheung,M.R.Asian Pac.J.Cancer Prev.2013,14(5),3105. doi:10.7314/APJCP.2013.14.5.3105
(3)Reddad,Z.;Gerente,C.;Andres,Y.;Cloirec,P.L.Environ. Sci.Technol.2002,36(9),2067.doi:10.1021/es0102989
(4)Crini,G.Prog.Polym.Sci.2005,30(1),38.doi:10.1016/j. progpolymsci.2004.11.002
(5)Ngah,W.S.W.;Hanafiah,M.A.K.M.Bioresour.Technol. 2008,99(10),3935.doi:10.1016/j.biortech.2007.06.011
(6)D jedidi,Z.;Bouda,M.;Souissi,M.A.;Cheikh,R.B.;Mercier, G.;Tyagi,R.D.;Blais,J.F.J.Hazard.Mater.2009,172(2-3),1372.doi:10.1016/j.jhazmat.2009.07.144
(7)Abou-Shady,A.;Peng,C.;Bi,J.;Xu,H.;Juan,A.O. Desalination 2012,286(1),304.doi:10.1016/j. desal.2011.11.041
(8)Chen,T.;Wang,T.;Wang,D.J.;Zhao,J.Q.;Ding,X.C.;Wu, S.C.;Xue,H.R.;He,J.P.Acta Phys.-Chim.Sin.2010,26, 3249.[陈田,王涛,王道军,赵建庆,丁晓春,吴士超,薛海荣,何建平.物理化学学报,2010,26,3249.]doi:10.3866/ PKU.WHXB20101134
(9)Say,R.;Birlik,E.;Erdemgil,Z.;Denizli,A.;Ersöz,A. J.Hazard.Mater.2008,150(3),560.doi:10.1016/j. jhazmat.2007.03.089
(10)Kul,A.R.;Koyuncu,H.J.Hazard.Mater.2010,179(1-3), 332.doi:10.1016/j.jhazmat.2010.03.009
(11)Niu,Y.;Feng,S.;Qu,R.;Ding,Y.;Wang,D.;Wang,Y.Int.J. Quantum Chem.2011,111(5),991.doi:10.1002/qua.v111.5
(12)Anwar,J.;Shafique,U.;Salman,M.;Dar,A.;Anwar,S. Bioresour.Technol.2010,101(6),1752.doi:10.1016/j. biortech.2009.10.021
(13)Wang,W.;Zhang,X.;Wang,H.;Wang,X.;Zhou,L.;Liu,R.; Liang,Y.WaterRes.2012,46(13),4063.doi:10.1016/j. watres.2012.05.017
(14)Ren,X.;Shao,D.;Yang,S.;Hu,J.;Sheng,G.;Tan,X.;Wang, X.Chem.Eng.J.2011,170(1),170.doi:10.1016/j. cej.2011.03.050
(15)Hamidpour,M.;Kalbasi,M.;Afyuni,M.;Shariatmadari,H.; Holm,P.E.;Hansen,H.C.B.J.Hazard.Mater.2010,181(1-3),686.doi:10.1016/j.jhazmat.2010.05.067
(16)Jiang,M.Q.;Jin,X.Y.;Lu,X.Q.;Chen,Z.L.Desalination 2010,252(1-3),33.doi:10.1016/j.desal.2009.11.005
(17)Wang,L.;Yang,L.;Li,Y.;Zhang,Y.;Ma,X.;Ye,Z.Chem. Eng.J.2010,163(3),364.doi:10.1016/j.cej.2010.08.017
(18)Chen,H.;Zhao,J.;Dai,G.;Wu,J.;Yan,H.Desalination 2010, 262(1),174.doi:10.1016/j.desal.2010.06.006
(19)Wang,X.S.;Lu,Z.P.;Miao,H.H.;He,W.;Shen,H.L.Chem. Eng.J.2011,166(3),986.doi:10.1016/j.cej.2010.11.089
(20)Klemm,D.;Schumann,D.;Udhardt,U.;Marsch,S.Prog. Polym.Sci.2001,26(9),1561.doi:10.1016/S0079-6700(01) 00021-1
(21)Shinsuke,I.;Manami,T.;Minoru,M.;Hiroyuki,S.;Hiroyuki, Y.Biomacromolecules 2009,10(9),2714.doi:10.1021/ bm9006979
(22)Stoica-Guzun,A.;Stroescu,M.;Jinga,S.I.;Jipa,I.M.;Dobre, T.Ind.Crop.Prod.2013,50(10),414.doi:10.1016/j. indcrop.2013.07.063
(23)Shen,W.;Chen,S.Y.;Shi,S.K.;Li,X.;Zhang,X.;Hu,W.L.; Wang,H.P.Carbohyd.Polym.2009,75(1),110.doi:10.1016/ j.carbpol.2008.07.006
(24)Donia,A.M.;A tia,A.A.;Abouzayed,F.I.Chem.Eng.J. 2012,191(19),22.doi:10.1016/j.cej.2011.08.034
(25)O-Rak,K.;Phakdeepataraphan,E.;Bunnak,N.;Ummartyotin, S.;Sain,M.;Manuspiya,H.Chem.Eng.J.2014,237(1),396. doi:10.1016/j.cej.2013.10.032
(26)Zhu,H.Y.;Jiang,R.;Xiao,L.;Li,W.J.Hazard.Mater.2010, 179(1-3),251.doi:10.1016/j.jhazmat.2010.02.087
(27)Xia,L.;Hu,Y.X.;Zhang,B.H.Trans.NonferrousMet.Soc. China 2014,24(3),868.doi:10.1016/S1003-6326(14)63137-X
(28)Chand,P.;Bafana,A.;Pakade,Y.B.Int.Biodeter.Biodegr. 2015,97,60.doi:10.1016/j.ibiod.2014.10.015
(29)Sathvika,T.;Manasi;Rajesh,V.;Rajesh,N.Chem.Eng.J. 2015,279,38.doi:10.1016/j.cej.2015.04.132
(30)Barathi,M.;Santhana Krishna Kuma,A.;Rajesh,N. J.Environ.Chem.Eng.2013,1(4),1325.doi:10.1016/j. jece.2013.09.026
(31)Pedro,C.;Joana,A.S.,M.;Eliane,T.;Luísa,S.,S.;Carmen,S. R.,F.;Armando,J.D.,S.;Carlos,P.N.Bioresour.Technol. 2011,102(15),7354.doi:10.1016/j.biortech.2011.04.081
(32)Wang,J.Q.;Lu,X.K.;Ng,P.F.;Lee,K.I.;Fei,B.;Xin,J.H.; Wu,J.Y.J.Colloid Interface Sci.2015,440,32.doi:10.1016/j. jcis.2014.10.035
(33)Zhang,J.;Li,D.;Zhang,X.;Shi,Y.J.Appl.Polym.Sci.1993, 49,741.doi:10.1002/app.1993.070490420
(34)Tokoh,C.;Takabe,K.;Fujita,M.;Saiki,H.Cellulose 1998,5(4),249.doi:10.1023/A:1009211927183
(35)Qin,Z.Y.;Ji,L.;Yin,X.Q.;Zhu,L.;Lin,Q.;Qin,J.M. Carbohyd.Poly.2014,101(101),947.doi:10.1016/j. carbpol.2013.09.068
(36)Yu,X.L.;Tong,S.R.;Ge,M.F.;Wu,L.Y.;Zou,J.C.;Cao,C. Y.;Song,W.G.J.Environ.Sci-China 2013,25(5),933.doi: 10.1016/S1001-0742(12)60145-4
(37)Heinze,T.;Liebert,T.;Koschella,A.Esterification of Polysaccharide,1sted.;Springer:Berlin,2006.
(38)Ivanova,N.V.;Korolenko,E.A.;Korolik,E.V.;Zhbankov,R. G.J.Appl.Spectrosc+.1989,51(2),847.doi:10.1007/ BF00659967
(39)Lu,M.;Zhang,Y.M.;Guan,X.H.;Xu,X.H.;Gao,T.T. T.Nonferr.Metal.Soc.2014,24(6),1912. doi:10.1016/S1003-6326(14)63271-4
(40)Yang,R.;Aubrecht,K.B.;Ma,H.;Wang,R.;Grubbs,R.B.; Hsiao,B.S.;Chu,B.Polymer 2014,55(5),1167.doi: 10.1016/j.polymer.2014.01.043
(41)Wu,Z.;Cheng,Z.;Ma,W.Bioresour.Technol.2012,104(1), 807.doi:10.1016/j.biortech.2011.10.100
(42)Fan,L.;Chen,Y.;Wang,L.;Jiang,W.Adsorpt.Sci.Technol. 2011,29(5),495.doi:10.1260/0263-6174.29.5.495
(43)Zhang,J.;Cai,D.Q.;Zhang,G.L.;Cai,C.J.;Zhang,C.L.; Qiu,G.N.;Zheng,K.;Wu,Z.Y.Appl.Clay Sci.2013,83-84, 137.doi:10.1016/j.clay.2013.08.033
(44)Chen,C.L.;Wang,X.K.Appl.Geochem.2007,22(2),436. doi:10.1016/j.apgeochem.2006.11.010
(45)Lu,M.;Guan,X.H.;Xu,X.H.;Wei,D.Z.Chin.Chem.Lett. 2013,24,253.doi:10.1016/j.cclet.2013.01.034
(46)Fu,X.C.;Shen,W.X.;Yao,T.Y.;Hou,W.H.Physical Chemistry,5th ed.;Higher Education Press:Beijing,2009.[傅献彩,沈文霞,姚天扬,侯文华.物理化学.第五版.北京:高等教育出版社,2009.]
(47)Zhu,H.Y.;Jiang,R.;Xiao,L.;Li,W.J.Hazard.Mater.2010, 179(1-3),251.doi:10.1016/j.jhazmat.2010.02.087
(48)Wang,Z.;Yin,P.;Qu,R.;Chen,H.;Wang,C.;Ren,S.Food Chem.2013,136(136),1508.doi:10.1016/j. foodchem.2012.09.090
(49)Kong,J.;Huang,L.;Yue,Q.;Gao,B.Desalin.Water Treat. 2014,52(13-15),2440.doi:10.1080/19443994.2013.794713 (50)Foo,K.Y.;Hameed,B.H.Bioresour.Technol.2012,111(5), 425.doi:10.1016/j.biortech.2012.01.141
(51)Chen,S.Y.;Zou,Y.;Yan,Z.Y.;Shen,W.;Shi,S.K.;Zhang, X.;Wang,H.P.J.Hazard.Mater.2009,161,1355.doi: 10.1016/j.jhazmat.2008.04.098
(52)Musyoka,S.M.;Ngila,J.C.;Moodley,B.;Petrik,L.; Kindness,A.Anal.Lett.2011,44(11),1925.doi:10.1080/ 00032719.2010.539736
(53)Liu,Y.J.Chem.Eng.Data 2009,54(7),1981.doi:10.1021/ je800661q
(54)Kong,J.;Yue,Q.;Sun,S.;Gao,B.;Kan,Y.;Li,Q.;Wang,Y. Chem.Eng.J.2014,241(4),393.doi:10.1016/j. cej.2013.10.070
(55)Albadarin,A.B.;Mangwand,C.;Ala′H,A.;Walker,G.M.; A llen,S.J.;Ahmad,M.N.M.Chem.Eng.J.2012,179,193. doi:10.1016/j.cej.2011.10.080
(56)Sag,Y.;Kutsal,T.Biochem.Eng.J.2000,35(8),801.doi: 10.1016/S0032-9592(99)00154-5
(57)Liu,C.X.;Bai,R.B.J.Membrane Sci.2006,284(1-2),313. doi:10.1016/j.memsci.2006.07.045
Microwave-Assisted Synthesis of Esterified Bacterial Celluloses to Effectively Remove Pb(II)
WANG Yin*SUN Feng-Ling ZHANG Xiao-Dong*TAO Hong YANG Yi-Qiong
(SchoolofEnvironmentand Architecture,University ofShanghaifor Science and Technology,Shanghai200093,P.R.China)
Twomodified bacterialcelluloses,xanthate-modified bacterialcellulose(XMBC)and sulfate-modified bacteria lcellulose(SMBC),were prepared from bacterialcellu lose(BC)esterified w ith xanthate and sulfate, respective ly,usingm ic row ave irrad iation.The as-p repared sam p les we re cha racte rized by X-ray d iffraction (XRD),scanning electronm icroscopy-energy-dispersive spectroscopy(SEM-EDS),Fourier transform infrared (FT-IR)spectroscopy,and Brunauer-Emmett-Teller(BET)surface ana lysis.Batch experimentswere carried out to determ ine the ability o f XMBC and SMBC to remove Pb(II)from solution.The effects ofpH,contact time, tem perature,initialadsorp tion concentration,and ionic strength on Pb(II)removalwere investigated a long with regene ration perform ance.Bo th the specific su rface area and tota lpo re volume of the modified biosorben ts were higher than those ofunmodified bacterialcellu lose.The adsorption o f Pb(II)decreased with increasing temperature and ionic strength,and the optimalpH was 5.0.The introduction of thiolgroups on bacterialcellulose increased its adsorp tion capacity for Pb(II);themodified biosorbents exhibited adsorption capacities of144.93 mg∙g-1for XMBC and 126.58mg∙g-1for SMBC.The adsorption rate closely followed a pseudo-second order modeland the adso rption isotherm data were consistentw ith the Langm uirmodel.The adso rption of Pb(II)wasexotherm ic,and the spentadsorbents could be readily regenerated for reuse.As a result,SMBC and XMBC are prom isingmaterials for the preconcentration and separation ofheavymetals from large volumes ofaqueous solutions.
October20,2015;Revised:December24,2015;Published onWeb:December29,2015.
Bacterialcellulose;Etherificationmodification;Microwave assistance;Biosorption;Heavymetal
O647
10.3866/PKU.WHXB201512294
*Corresponding authors.WANG Yin,Email:625xiaogui@163.com.ZHANG Xiao-Dong,Email:fatzhxd@126.com;Tel:+86-13917013840.
The projectwas supported by the Foundation of Key Laboratory of Yangtze RiverWaterEnvironment,Ministry of Education(TongjiUniversity), China(YRWEF201503),Program of Ability Construction in Shanghai LocalCollege,China(13230502300),Program of Supporting Young Teachers in ShanghaiCollege,China(ZZSLG14015),and ShanghaiSailing Program,China(14YF1409900).
长江水环境教育部重点实验室开放课题(YRWEF201503),上海地方能力建设项目(13230502300),上海高校青年教师培养资助计划(ZZSLG14015)及上海市青年科技英才杨帆计划(14YF1409900)资助©Editorialofficeof Acta Physico-Chim ica Sinica
