氧化石墨烯/聚电解质层层自组装制备单价离子选择性膜
2016-09-13赵胜君张伟邓会宁刘伟
赵胜君 张伟 邓会宁,* 刘伟
(1河北工业大学化工学院,天津300130;2河北工业大学海洋科学与工程学院,天津300130)
氧化石墨烯/聚电解质层层自组装制备单价离子选择性膜
赵胜君1张伟2邓会宁2,*刘伟2
(1河北工业大学化工学院,天津300130;2河北工业大学海洋科学与工程学院,天津300130)
采用层层自组装法在改性聚丙烯腈(PAN)膜表面交替沉积聚乙烯亚胺(PEI)和聚丙烯酸-氧化石墨烯(PAA-GO)混合液,制得了单价离子选择性复合膜。X射线衍射(XRD)测试结果表明成功合成了氧化石墨烯(GO)并在复合膜中均匀分散。扫描电镜(SEM)观察结果证实了多层聚电解质PEI/PAA-GO成功地组装在基膜上,并用紫外-可见(UV-Vis)光谱进一步证实了组装过程的均匀性和连续性。接触角和性能测试表明加入GO后,复合膜的亲水性和单价阳离子的选择性明显增大。这种高通量、高选择性的防污复合膜在分离和水的软化方面有很好的应用前景。
多层结构;层层自组装;沉积;氧化石墨烯;选择性
Themembranes for selective separation ofmonovalent ions from multivalentones are of special utility for edible salt from seawater,water softening,and recovery of spentacids from waste water.Since multivalent ions have a larger size than themonovalentones,they could be separated bymembranesbased on a mechanism of sieve effect.The compositemembranes could form tortuouspaths in their skin with GO added,so the selectivity for monovalent ion could be enhanced.Moreover,the functional groupson GO such as carboxyland hydroxyl could also improve the hydrophilic of the surface of themembranes.Thiswould effectively enhance the antifouling ability of the prepared composite membranes.
In thisstudy,we prepared apoly(ethylenimine)/polyacrylicacid-GO(PEI/PAA-GO)compositemembraneby layer-by-layer(LBL) method for themonovalentandmultivalent ion separation.The membrane preparation procedure,the dispersibility of GO in the compositemembranes,and theeffectsof GO on hydrophilcity and monovalentcation selectivity were systematically investigated.
2 Experimental
2.1Materia ls
Natural flake graphite(99.9%)waspurchased from Qingdao Guyu Grapite Co.,Ltd.Polyethylenimine(50%(w,mass fraction), Mw=70000)was procured from A laddin IndustrialCorporation and Polyacrylic acid(25%,Mw=240,000)was purchased from Acros Organics Company.All other chemicals(99%)were obtained from Tianjin Jiangtian Chem ical Technology Co.,Ltd.The polyacrylonitrile(PAN)ultrafiltrationmembranewassupplied by the Sepro Company.
2.2Prepara tion o f laye r-by-laye r assem b led com positememb ranes
GO was prepared via amodified Hummersmethod14-16.Firstly, 2 g of graphite powderand 1 g of NaNO3were dispersed in 100m L of concentrated H2SO4at0°C,and then 6g of KMnO4was added slow ly for 1.5h.Themixturewas stirred at35°C for 30m in,and then 100mL of deionized water was added into the m ixture.To reduce the residual permanganate,200m L of distilled waterand 30mLof 30%H2O2aqueoussolutionwereadded to the m ixture sequentially at95°C2,17.Finally,them ixturewas centrifuged andwashedw ith HCl,deionizedwaterand dried18.
The supporting PANmembranewas immersed in 0.2mol∙L-1sodium hydroxide solution at 60°C for 30min to convert the nitrile(―C≡N)groups of the PAN membrane to carboxylate (―COOH)11,19.The pretreated supporting membrane was immersed in 3 g∙L-1poly(ethylenimine)(PEI)solution for15m in, and then immersed in am ixed solution of 3 g∙L-1PAA and 1 g∙L-1GO for15m in to complete the firstbilayer deposition.This procedure was repeated to obtain the desired number of bilayers. Betw een each layer of deposition,themembrane was rinsed w ith deionized water to remove the residual polyelectrolyte20.A ll the membranes were ended in PEI,because that positive charged surface could increase the selectivity for cation by electrostatic repulsion.A t last,themodified membrane w as chem ical crosslinkedw ith 12%(w)ethanol solution of epichlorohydrin to improve their stability.The poly(ethylenimine)and polyacrylic acid (PEI/PAA)membraneswere fabricatedwith similar procedurebut w ithoutGO in the PAA solution.
2.3Characterization
X-ray powder diffraction(XRD,BrukerAXSCo.,Ltd.,Germany)patternsof the sampleswere performed using a Bruker D8 Focus diffractometerwith a Cu Kαradiation(λ=0.15406nm). Scanning electronmicroscopy(SEM,FEI,Nova Nano SEM450, America)imageswere taken using an FEI/NovaNano SEM450operating at200kV.Hydrophilic of themembrane surfacewas analyzed by measuring the water contact angle using Kruss/ DAS30(Germany)contactanglemeter.To characterize the layerby-layerassembly process,aboiling piranhasolution(30:70,V/ V,H2O2/H2SO4)treated quartz slide was exposed to the PEIsolution and them ixture of PAA and GO alternatively and then testedwith a UV-Vis spectrophotometer(Beijing PurkinjeGeneral InstrumentCo.,Ltd,TU-1810,China).
The selectivity of compositemembraneswith an effectivearea of 16cm2was evaluated w ith a homemade two-com partment diffusion device.The tw ice concentration of seawater and distilled w ater w as selected as feed solution and draw solution,respectively.The volume of feed and draw solutionswere both 100m L. The prepared GO com positemembrane was fixed between the compartmentswithmodified surface facing the feed solution.The concentration of Cland bivalent cationswas titrated after90min of diffusion.Then the selective coefficient S was calculated as:

where S was the selective coefficientbetweenmonovalent ions and divalent ions,C0and CPwere the ion concentrationsof initial and infiltrated solution,respectively.The subscripts Iand II referred to themonovalentand bivalentcations.
3 Results and discussion
3.1SEMana lysis
Fig.1(a)showed the surfacemorphology of support substrate, which appearedmany poreswith diameterof about100nm,while the surfaceofmembranemodified by PEI/PAA-GO(Fig.1(b))wasdense and smooth.Thiswas due to the uniquemonolayer sheet structure of GO and the electrostatic interaction betw een polycation electrolyte PEIand polyanion electrolyte PAA-GO.In addition,the chemical crosslinking also couldmake themembrane dense and uniform21.The SEMimages indicated a successful assembly of PEI/PAA-GO by the LBL technology.
3.2XRD analys is
Fig.2 presented the XRD results of graphite,GO,PEI/PAA compositemembrane,and PEI/PAA-GO compositemembrane. The diffraction peaksat2θ=26.7°(d002=0.34nm)and 2θ=11.4° (d001=0.83 nm)in Fig.2(a)and(b)were the characteristic peaks of graphite and GO,respectively11.The increase of layer spacing indicated that the stacked layers of graphite were laterally expanded by the incorporation of oxygenated functionalgroups,and the graphitewas successfully converted into GO2,22.However,in Fig.2(d),there was no GO peak appeared in the PEI/PAA-GO compositemembrane.The characteristic peak of itw as sim ilar with the peak of PEI/PAA composite(Fig.2(c))butweakened.The decreaseof diffraction intensitywas likely due to the polar-polar interaction between PAA and GO12.
3.3UV-Visib le abso rp tion ana lysis
Fig.3 show ed the UV-visible absorption spectra of quartz slide with varying deposition numbersof PEIand PAA-GO.The peak at214nm wasattributed to theΠ-Π*transition of C=C bonds in the PAA-GO.The smaller shoulder around 300nm corresponded to the n-Π*transition of carbonylgroups23.The intensity of adsorption peak at 214nm exhibited exponentialgrow th w ith the deposition cycles(Fig.3,inset),which indicated that the compositemembrane exhibited uniform and continuousenhanced w ith the increasing numberof deposition cycles.
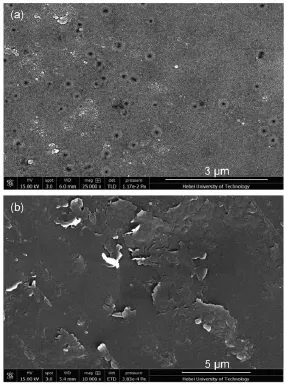
Fig.1 SEMimagesof the PANmembrane support(a)and PEI/PAA-GOmodifiedmembrane(b)

Fig.2 XRD patterns of graphite(a),GO(b),PEI/PAA membrane(c),and PEI/PAA-GOmembrane(d)

Fig.3 UV-Vis spectra of PEI/PAA-GO compositem emb rane The inset show s the absorbance increasing linear relationship at214.0nm.
3.4Hyd roph ilic tes t
Thewater contactangles ofmultilayer compositemembranes with andw ithoutGOmodifiedwerecompared in Fig.4.The PEI/ PAA-GO compositemembranes exhibited notable lower contact value than the PEI/PAA membranes aftermore than 3 bilayers deposited.Itshould pointed that smallamountof PAA-GO would not improve the hydrophobic of compositemembrane.The PEI-terminated membranes had an average contact angle value of 75°,which was in agreementwith other researcher24,25.The PEI-terminatedmembranesshowed higher contactangle than the PAA-term inatedmembranes because PEIwasmore hydrophobic thanPAA(Fig.4).A ll these test results confirmed the increase in hydrophilic character of the PAN basement upon GO treatment26. Therefore,the anti-pollution ofmembraneswas improved and further identified the sequentialdeposition of PEIand PAA-GO. 3.5Separation performance evaluation
Fig.5showed the separation property of themultilayer compositemembraneswith andwithoutGOmodified.With thebilayer number increased,the selectivity of the PEI/PAA-GO and PEI/ PAA compositemembraneswas both increased,while the flux of them was reduced.With 3.5bilayersdeposited,the selectivity of the PEI/PAA-GO compositemembranewas1.6timeshigher than that of the PEI/PAA compositemembrane without GO.The lamellar structureand theaggregation tendency of GO sheetswould hinder the ion transportand increase the tortuous diffusion path length,in such above conditions the ionsshould follow a long and tortuous path10.Then themonovalent cation with a smaller diameter could transportmuch faster than the bivalent cations.
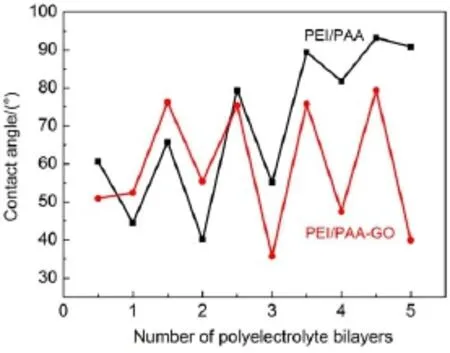
Fig.4Contactanglevaluesof PEI/PAA and PEI/PAA-GO com positem em branesw ith differen tnum ber of bilayers

Fig.5Effectof the assembly numberson theselectivity and flux
4Conclusions
In this study,we introduced themixing of GO and PAA to fabricate PEI/PAA-GO compositemembrane via layer-by-layer method.The results indicated that the GO in the composite membrane could effectively enhance the antifouling ability and im prove the selectivity formonovalent ions.Therefore,the GO multilayer composite membrane could be well suited for the applicationssuch asseawater softening andwater treatment.
References
(1)Xu,Y.X.;Hong,W.J.;Bai,H.;Li,C.;Shi,G.Q.Carbon 2009,47,3538.doi:10.1016/j.carbon.2009.08.022
(2)Lee,D.C.;Yang,H.N.;Park,S.H.;Kim,W.J.J.Membr.Sci. 2014,452,20.doi:10.1016/j.memsci.2013.10.018
(3)Cao,C.H.;Daly,M.;Singh,C.V.;Sun,Y.;Filleter,T.Carbon 2015,81,497.doi:10.1016/j.carbon.2014.09.082
(4)Liang,L.;Lu,Y.;Yao,W.S.;Zhang,X.T.Acta Phys.-Chim. Sin.2015,31,1180.[梁祎,卢赟,姚维尚,张学同.物理化学学报,2015,31,1180.]doi:10.3866/PKU. WHXB201504146
(5)Ji,T.H.;Sun,M.;Zou,L.F.;Ma,N.Mater.Lett.2014,120, 30.doi:10.1016/j.matlet.2014.01.035
(6)Dreyer,D.R.;Park,S.J.;Bielawski,C.W.;Ruoff,R.S. Chem.Soc.Rev.2010,39,228.doi:10.1039/B917103G
(7)Nair,R.R.;Wu,H.A.;Jayaram,P.N.;Grigorieva,I.V.;Geim, A.K.Science 2012,335,442.doi:10.1126/science.1211694
(8)Mi,B.X.Science 2014,343,740.doi:10.1126/ science.1250247
(9)Valentini,L.Mater.Lett.2015,157,266.doi:10.1016/j. matlet.2015.05.23
(10)Li,W.Y.;He,Y.Q.;Li,Y.M.Acta Phys.-Chim.Sin.2015,31, 458.[李文有,贺蕴秋,李一鸣.物理化学学报,2015,31, 458.]doi:10.3866/PKU.WHXB201501093
(11)Dikin,D.A.;Stankovich,S.;Zimney,E.J.;Piner,R.D.; Dommett,G.H.B.;Evmenenko,G.;Nguyen,S.T.;Ruoff,R. S.Nature 2007,448,458.doi:10.1038/nature06016
(12)Rajasekar,R.;Kim,N.H.;Jung,D.;Kuila,T.;Lim,J.K.; Park,M.J.;Lee,J.H.Compos.Sci.Technol.2013,89,171. doi:10.1016/j.compscitech.2013.10.004
(13)Hu,M.;Mi,B.X.J.Membr.Sci.2014,469,80.doi:10.1016/j. memsci.2014.06.036
(14)Hummers,W.S.;Offeman,R.E.J.Am.Chem.Soc.1958,80, 1339.doi:10.1021/ja01539a017
(15)Wang,J.D.;Peng,T.J.;Xian,H.Y.;Sun,H.J.Acta Phys.-Chim.Sin.2015,31,91.[汪建德,彭同江,鲜海洋,孙红娟.物理化学学报,2015,31,91.]doi:10.3866/PKU. WHXB201411202
(16)Goh,K.L.;Setiawan,L.;Wei,L.;Si,R.M.;Fane,A.G.; Wang,R.;Chen,Y.J.Membr.Sci.2015,474,245.doi: 10.1016/j.memsci.2014.09.057
(17)Zhou,T.N.;Chen,F.;Tang,C.Y.;Bai,H.W.;Zhang,Q.; Deng,H.;Fu,Q.Compos.Sci.Technol.2011,71,1267.doi: 10.1016/j.compscitech.2011.04.016
(18)Suresh,D.;Udayabhanu;Kumar,M.A.P.;Nagabhushana,H.; Sharma,S.C.Mater.Lett.2015,151,93.doi:10.1016/j. matlet.2015.03.035
(19)Zhang,G.J.;Yan,H.H.;Ji,S.L.;Liu,Z.Z.J.Membr.Sci. 2007,292,2.doi:10.1016/j.memsci.2006.11.023
(20)Wang,N.X.;Ji,S.L.;Zhang,G.J.;Li,J.;Wang,L.Chem. Eng.Technol.2012,213,321.doi:10.1016/j.cej.2012.09.080
(21)Wang,X.;Guo,X.Y.;Shao,H.Q.;Zhou,Q.X.;Hu,W.L.; Song,X.J.Prog.Chem.2015,27,1475.[王茜,郭晓燕,邵怀启,周启星,胡万里,宋晓静.化学进展,2015,27,1475.] doi:10.7536/PC150321
(22)Schniepp,H.C.;Li,J.L.;McAllister,M.J.;Sai,H.;Herrera-A lonso,M.;Adamson,D.H.;Prud'homme,R.K.;Car,R.; Saville,D.A.;Aksay,I.A.J.Phys.Chem.2006,110,8537. doi:10.1021/jp060936f
(23)Zhang,J.G.;Xu,Z.W.;Shan,M.J.;Zhou,B.M.;Li,Y.L.;Li, B.D.;Niu,J.R.;Qian,X.M.J.Membr.Sci.2013,448,91. doi:10.1016/j.memsci.2013.07.064
(24)Ben Ameur,S.;Barhoum i,A.;Mimouni,R.;Am louk,M.; Guermazi,H.Superlattice.Microst.2015,84,110.doi: 10.1016/j.spmi.2015.04.028
(25)Marmur,A.Langmuir2004,20,3519.doi:10.1021/la036369u
(26)Malaisamy,R.;Talla-Nwafo,A.;Jones,K.L.Sep.Purif. Technol.2011,77,370.doi:10.1016/j.seppur.2011.01.005
Layer-by-Layer Assembly of Graphene Oxide and Polyelec trolyte Composite Membranes for Monovalent Cation Separation
ZHAO Sheng-Jun1ZHANGWei2DENG Hui-Ning2,*LIUWei2
(1SchoolofChemical Engineering,HebeiUniversity ofTechnology,Tianjin 300130,P.R.China;
2School ofMarine Science and Engineering,HebeiUniversity ofTechnology,Tianjin 300130,P.R.China)
Graphene oxide(GO)com positememb ranes w ere fabricated via layer-by-layer(LBL)assem bling poly(ethylenim ine)(PEI)and am ixture ofGO and poly(acrylic acid)(PAA)on a poly(acrylonitrile)(PAN)support membrane.The com positemembranes and theirapp lication performance were characterized and eva luated. The X-ray pow der diffrac tion(XRD)spectrum show s that GO w as successfully synthesized by them od ified Hummers method,and itwas homogenously dispersed in the com posite membranes.Scanning electron m icroscopy(SEM)show s the successfulassembly ofmultip le polyelectrolyte PEIand am ixture ofGO and PAA bilayers on the PAN supportmembrane.The ultraviolet-visible(UV-Vis)spectrum indicates that the uniform ity and continuity of the com positemembrane were enhanced with the increasing numbero fassembled layers. The hydrophilic and selectivity tests reveals that the add ition o f GO decreased the wa ter contact angle and enhanced the selectivity formonovalentcations of themultilayer polyelectrolyte compositemembranes.Allthese advantages combine to fabricate a high-flux,high selectivity,and anti-fouling com positemembrane for separation applications and water softening.
Multilayer structure;Layer-by-layerassembly;Deposition;Graphene oxide;Selectivity
1 Introduction
Graphene oxide(GO)has excellent properties ofmechanics, electricity,and heat,such propertiesmake itahot topic in the field ofmaterials science and physics1-6.Moreover,thanks to the specialhoneycomb lattice two dimensional(2D)structure,variousoxygen functionalgroupsand the frictionless surface,GO also can play a block role to create polyelectrolyte compositemembranes for separation7-10.Dikin et al.11reported that GO sheets could be stacked in anear-parallelmannerundera directional flow induced by vacuum filtration and uniform ly dispersed in polyelectrolyte, resulting in the higher tensile yield strength of compositematerials than those without GO.Rajasekar et al.12prepared poly(diallyldimethylammonium)chloride(PDDA)and sulfonated polyvinylidene fluoride-graphene oxide(SPVDF-GO)composites to enhance both themechanical and gas barrier properties of the membrane.Hu and Mi13fabricated a compositemembrane by assembling GO and poly(allylam ine hydrochloride)(PAH)and found that the addition of GO greatly im proved thew ater flux in a forward osmosissystem.
September 28,2015;Revised:December 8,2015;Published onWeb:December 14,2015.
O647
10.3866/PKU.WHXB201512141
*Corresponding author.Email:huiningd@163.com;Tel:+86-22-6020241.
The projectwas supported by theNationalNatural Science Foundation of China(20906017),NaturalScience Foundation ofHebeiProvince,China (B2013202087,D2014202074),and Tianjin Research Program of Application Foundation and Advanced Technology,China(14JCZDJC38900).
国家自然科学基金(20906017),河北省自然科学基金(B2013202087,D2014202074),天津市应用基础与前沿技术研究重点项目(14JCZDJC38900)资助©Editorialofficeof Acta Physico-Chim ica Sinica
