重金属检测荧光传感技术的研究进展
2016-09-10熊晓辉
冷 玲,李 壹,熊晓辉
(南京工业大学食品与轻工学院,江苏南京 211816)
重金属检测荧光传感技术的研究进展
冷玲,李壹,熊晓辉*
(南京工业大学食品与轻工学院,江苏南京 211816)
重金属离子是一类极具生理毒性的化学物质,其检测方法在化学传感领域引起了人们广泛的关注,而食品中重金属离子的检测也愈发重要。荧光探针因具有组织穿透性、低背景荧光干扰、高效灵敏和检测实时便捷等特点,成为重金属检测的重要手段之一。文中综述了近年来主要重金属离子检测荧光探针(香豆素类,罗丹明类、喹啉类和比率型)的研究进展,重点分析荧光探针的设计原理,检测机制及其结构与化学传感的关系,为荧光探针识别重金属的应用提供指导。最后展望了荧光探针在食品检测、环境监测和生物成像等领域的发展趋势和应用前景。
荧光探针,重金属,传感器,食品检测
随着人们生活水平的日益提高,食品安全问题也成为了百姓关心的头等大事。近年来,“毒大米”、“小龙虾中毒”等事件的频发,严重危害身体健康,让人们对重金属离子的检测愈发重视。
重金属广泛地存在于自然界中,主要指汞(Hg)、铜(Cu)和铅(Pb)等元素。即使在很低的浓度下,汞也具有很高的毒性[1],其主要通过消化道,呼吸道和皮肤吸收到体内,对神经和内分泌造成实质性损害[2],汞离子对蛋白质中的巯基有高亲和力[3],造成大脑,肾和中枢神经系统的诸多健康问题[4],如甲状腺肿大和水俣病等。铜离子是一个显著的金属污染物,美国环境保护署(EPA)规定饮用水中的铜离子含量上限是1.3 ppm[5]。研究表明铜离子的细胞毒性可导致严重疾病,如印第安人儿童肝硬化(ICC)[6],朊病毒病[7],肌萎缩性侧硬化症[8]和威尔逊疾病[9]。铅离子是一种对人体有害的神经毒素,通过食物链进入机体,在体内富集蓄积造成急慢性的中毒,对机体新陈代谢产生较大的干扰[10]。
由于重金属污染的扩散以及对环境造成污染的严重性,开发高灵敏度、高精密度的仪器检测方法是必要的。本文概述了传统重金属检测技术的研究与发展,重点阐述了重金属荧光传感快速检测技术,旨在推动重金属荧光传感技术的发展,进一步完善食品安全检测技术体系。
1 重金属检测技术的研究与发展

图1 基于香豆素的探针[20-22]Fig.1 Chemosensors based on coumarin[20-22]
当前,常见传统检测重金属的方法有:电化学分析[11],原子吸收光谱(AAS)[12],电感耦合等离子体质谱(ICP-MS)[13],电感耦合等离子体原子发射光谱法(ICP-AES)[14],伏安法[15],比色法[16],紫外光谱[17]。上述方法大都为检测重金属的传统方法,方法灵敏度高,但是也存在操作繁琐、样品前处理时间长、检测时间长和需要大型仪器等诸多局限,因此不能实现样品快速检测。为实现快速、有效准确的现场检测,出现了酶分析法、免疫分析法和目视比色法等重金属快速检测方法,其具有检测快速、操作简便、成本低廉的优点。重金属的快速检测方法正好与传统的仪器检测方法相互补,在重金属检测的常规化、现场检测、即时筛选等方面具有独特的优势。虽然当前多数快速检测方法对环境污染物的检测还只能达到定性(或半定量)检测的程度,而且检测的灵敏度和准确性也不如传统的仪器检测方法。
分子荧光探针技术也是一种重金属快速检测技术,已成为传感和成像的有力工具,广泛应用于环境和生物样品中,而在食品领域鲜有研究[18]。其中,有机染料香豆素、罗丹明、喹啉及其比率型为代表的荧光分子探针,在饮用水、茶叶和鱼类等各种实际食品样品重金属快速检测和现场分析方面有着重要应用,为我国食品安全做出贡献。
2 荧光探针对重金属离子的检测
荧光探针一般由荧光信号报告单元(reportor)和底物接收单元(receptor)两个主要基本要素构成。底物与探针受体单元通过各种作用力(如分子间氢键,静电作用,共价键等)相互作用,引起信号报告单元荧光信号的改变,进而实现对目标物的传感检测。其信号传递机制包括:光致电子转移(PET)、荧光共振能量转移(FRET)和分子内电荷转移(ICT)等。常见的荧光探针大致可分为:香豆素类,罗丹明类,喹啉类和比率型等。
2.1香豆素类
香豆素具有相对光稳定性,低毒性,易修饰和较长的激发波长。香豆素及其衍生物具有很大的斯托克斯位移,一定可调性的光物理参数和优良的量子产率,进而有效地防止了激发和发射光谱之间的重叠[19]。Xu等[20]设计合成了一种基于香豆素的新型汞离子荧光探针1,即一种氨基硫脲衍生物,该探针可检测哺乳动物细胞或大肠杆菌中的Hg2+。Hg2+诱导氨基硫脲衍生物的脱硫反应,在495 nm处有明显的绿色荧光增强,最大发射波长有10 nm的红移表明发生了荧光分子内电荷转移(ICT)。该新型荧光探针在乙醇-水溶液体系中表现出良好的选择性和实用性,具有更广的应用和生物学意义。Yeh等[21]报道了一种含苯酚腙基团的香豆素类荧光探针2,当加入Cu2+时,体系出现从淡黄色到红色的颜色变化,向传感系统中加入CN-,发射峰强度显著增大;再次加入Cu2+,荧光探针2的荧光再次猝灭,证实了探针2和Cu2+之间的结合是可逆的。即使存在其他金属离子的干扰,Cu2+也是造成显著荧光猝灭的唯一金属;Cu2+处理Hela活细胞后,细胞内部的强荧光淬灭,实现了活细胞中Cu2+的识别。Yu等[22]设计的荧光探针3是含亚胺和羟基的香豆素类衍生物,因Hg2+抑制了亚胺的异构化,导致探针3蓝色荧光大大增强。荧光显微镜实验证实该探针能用于活细胞内检测Hg2+,其他重金属离子对检测无显著干扰。图1是基于香豆素的探针。
2.2罗丹明类

图2 基于罗丹明衍生物的探针[26-28]Fig.2 Chemosensors based on rhodamine analogue[26-28]

2.3喹啉类
Sarkar等[29]将8-氨基喹啉和3,5-二氯-2-羟基苯甲醛以1∶1摩尔比的席夫碱缩合,合成了一种基于喹啉的可逆探针10,可高效率检测生理和环境样品中的Zn2+,其检出限为5.0×10-9mol/L。由于激发态分子内质子转移(ESIPT),传感器具有弱荧光强度;存在Zn2+时,激发态分子内质子转移过程受到配位作用的抑制,导致该探针在553 nm处的发射强度提高53倍;良好螯合剂EDTA使探针脱离分析物,造成荧光猝灭;再次加入Zn2+,探针可再次使用。EDTA的加入反映了探针的一种强选择性的“关-开-关”荧光信号变化。密度泛函理论(DET)和含时密度泛函理论(TDDFT)计算出了探针的电子结构和传感机制。晶体结构显示Zn2+中心的周围以N,N,O-三齿单阴离子形式结合了两个有伪八面体几何的受体分子(探针)。Basa等[30]探讨了一种少见的多离子反应和单分子系统的化学传感器和化学计量器11,即蒽酮-喹啉亚胺衍生物的选择性还原,在95%乙醇中使用1.0当量的NaBH4得到相应的蒽-9-醇衍生物。Zn2+的配位作用诱导1,5-质子化转移,使喹啉取代蒽酮-亚胺分子,导致延伸的π共轭蒽荧光团通过亚胺-烯胺互变异构途径形成。当加入5.0当量的Zn2+,该传感器的蓝色发射荧光增强了42倍,吸收光谱有一个红移。Cu2+诱导传感器出现了从浅黄色到橙红色的比色变化,传感器发生不可逆的亚胺水解,荧光完全猝灭。Li等[31]以喹啉基团作为荧光单元,吡啶-2-甲胺作为金属离子的结合单元,合成了一个简单的荧光探针12。X射线晶体结构分析表明该传感器与Zn2+以1∶1的比例进行配位,而和Cd2+以2∶1的比例进行结合,这导致这些络合物的荧光单元有不同的空间排布。加入Zn2+,该传感器在316 nm处的吸收峰逐渐下降,而在363 nm处出现一个新的吸收峰,表明络合物中的Cd2+能被Zn2+取代。乙腈溶液中,用Zn2+滴定传感器,会使498 nm处出现荧光发射并有75倍的荧光增强。HOMO和传感器的LUMO上的π-电子主要集中在喹啉上,当加入Cd2+时,LUMO和HOMO上的能级高于传感器;存在Zn2+时,HOMO和LUMO上的π-电子分布有一个明显的变化。通过与Zn2+结合,电子转移过程大大抑制LUMO的稳定,相比Cd2+,导致更大荧光增强。当用Zn2+处理MCF-7细胞时,明场成像中有绿色荧光,表明传感器的生物相容性适用于快速检测活细胞内的Zn2+。图3表示基于喹啉的探针。

图3 基于喹啉的探针[29-31]Fig.3 Chemosensors based on quinoline[29-31]
2.4比率型
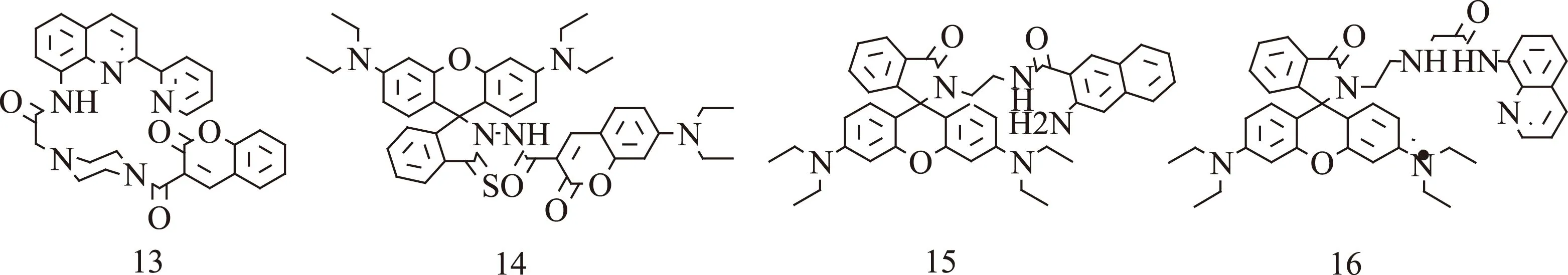
图4 比率型探针[40-43]Fig.4 Radiometric chemosensors.[40-43]
通常荧光传感器分为基于强度的和比率传感器。在实际分析中,荧光探针的荧光强度通常受很多因素影响[32-33],如光照强度,光路长度,探针浓度,激发强度和检测环境(pH,极性,温度等)[34-35]。因此,基于强度的传感器是在荧光强度的单变量测量易受定量检测的干扰[36-37]。原则上,比率传感器通过测量在两个不同波长下的荧光强度比率[38],提供一个环境因素的内置校正[39]。图4是四种比率型探针。Zhang等[40]合成了一种新型香豆素和喹啉的双荧光基团比率Cu2+探针13。空白传感器在322 nm处有最大吸收,当加入Cu2+时,由于喹啉环和吡啶基之间的共面效应,增加了共轭面积和减少了HOMO-LUMO之间的能隙,Cu2+络合物导致最大吸收出现从322 nm到415 nm的红移。当设定290 nm处激发时,传感器溶液在355 nm处荧光强度没有变化,而470 nm处有明显荧光猝灭,这是因为光激发喹啉环到低能空d-轨道的能量转移。355 nm处的发射光可作为稳定的内标,且在比率检测中,荧光信号的双变到单变量的转化。470、355 nm处的发射率(I470 nm/I355 nm)线性下降,说明Cu2+浓度不断增加。Gong等[41]设计合成了一种香豆素-罗丹明TBET系统的比率探针14。通过采用Hg2+脱硫反应作为识别机理,呈现对Hg2+的强选择性和高灵敏性。空白探针([Hg2+]=0 mol/L在420 nm处有明显的吸收峰,伴随供体(香豆素)发出的微黄色;且无受体的吸收峰特性(罗丹明B,近560 nm),证明了螺内结构的存在。但是,当Hg2+加入缓冲溶液后,供体的吸收条带出现红移。这种结果可能是因为Hg2+-触发氨基硫脲向1,3,4-恶二唑转化,增加了共轭的程度,表明对香豆素有较强的吸电子作用。除了香豆素吸收峰的红移,在567 nm处出现了一个新的对应罗丹明B开环结构的特征吸收峰,具有很高的摩尔消光系数,随着Hg2+浓度增加,吸收强度增加。同时,肉眼很容易观察到,传感器颜色从微黄色到红色的显著变化,荧光从青色到粉红色的变化(365 nm处激发)。Sun等[42]报道了一种萘胺-罗丹明比率型和比色型探针15。探针能通过荧光成像用于监测活鼠中Pd2+。在金属离子中,探针对Pd2+有很强地选择性。无Pd2+时,乙醇/水(1∶1)中的探针(10 μmol/L)在490 nm处有最大发射峰。无论是固态还是溶液中,探针发射出蓝绿色的荧光(萘胺部分的特性),红色荧光消失表明无金属离子的溶液中,罗丹明部分的螺内环关闭。加入Pd2+离子后,荧光强度比率(I590nm/I490nm)前5 min内迅速增加,10 min内达到最大值。不断加入Pd2+,490 nm处的荧光强度逐渐降低,而590 nm处出现一个新的最大发射峰,且逐渐增强;同时,萘胺的蓝绿色荧光猝灭,罗丹明的螺内环打开,伴随红色荧光。向上述溶液中滴加EDTA-2Na,590 nm处的荧光强度逐渐降低,而490 nm处的最大峰强度增加,过量的EDTA-2Na使590 nm处的荧光完全猝灭。这归因于探针上Pd2+被除去,罗丹明部分的螺内环重构。Zhou等[43]设计了一个含罗丹明和氨基喹啉基团的简单荧光探针16。根据Job plot 实验,探针和Hg2+以1∶1化学计量连接。Hg2+逐渐加入探针溶液中,探针(5×10-5mol/L)在497 nm处的发射峰逐渐下降,伴随581 nm处一个新的峰形成,且581 nm处的荧光强度随着Hg2+浓度增加而逐渐增加,之后达到平衡,伴随溶液由无色变为红色,发出亮绿色荧光。在420 nm处,激发络合物喹啉-锌部分,探针可能发生荧光能量共振转移(FRET)现象。此外,荧光光谱的比率变化在547 nm处分离发射点。
3 总结和展望
综上所述,近年来,荧光探针用于检测重金属的研究快速发展,新型材料[44-47]的设计合成使得重金属检测在食品检测、环境监测和生物毒理学等领域不断拓宽。实际样品的基质复杂,荧光探针易受多种检测环境干扰,其检测准确性和利用率较低,因此突破传统的设计理念,构建多功能和高性能检测体系,开发新的检测机理是一项重要的研究内容。同时保持传感器优良的检测性能,开发可重复利用,环境友好和实时监测的传感器,也是目前和未来的一个研究热点。总之,多种技术之间交叉融合,取长补短,新型化学传感器的设计合成将指日可待。
[1]Samuel K,Montserrat S,Cristal F G,et al. New insights into mercury bioaccumulation in deep-sea organisms from the NW Mediterranean and their human health implications[J]. Science of the Total Environment,2013,442(1):329-335.
[2]Dommergue A,Ferrari C P. Influence of anthropogenic sources on total gaseous mercury variability in Grenoble suburban air(France).[J]. Science of the Total Environment,2002,297(1-3):203-213.
[3]Jalal I,Ahmida E A. A water soluble fluorescent BODIPY dye with azathia-crown ether functionality for mercury chemosensing in environmental media[J]. Analyst,2013,138(13):3809-3819.
[4]Elizabeth M,Nolan,Stephen J,Lippard. Tools and tactics for the optical detection of mercuric ion[J]. Chemical Reviews,2008,108(9):3443-3480.
[5]Meng L,Haobo G,Arrowsmith R L,et al. Ditopic boronic acid and imine-based naphthalimide fluorescence sensor for copper(II)[J]. Chemical Communications,2014,50(80):11806-11809.
[6]Hahn S H,Tanner M S,Danke D M,et al. Normal Metallothionein Synthesis in Fibroblasts Obtained from Children with Indian Childhood Cirrhosis or Copper-Associated Childhood Cirrhosis[J]. Biochemical & Molecular Medicine,1995,54(54):142-145.
[7]Brown D R. Copper and prion disease[J]. Brain Research Bulletin,2001,55(2):165-173.
[8]Valentine J S,Hart P J. Misfolded CuZnSOD and amyotrophic lateral sclerosis[J]. Proceedings of the National Academy of Sciences of the United States of America,2003,100(7):3617-3622.
[9]D J Waggoner,T B Bartnikas,J D Gitlin. The role of copper in neurodegenerative disease[J]. Neurobiology of Disease,1999,6(4):221-230.
[10]Zhang Z,Lu S,Sha C,et al. A single thiourea-appended 1,8-naphthalimide chemosensor for three heavy metal ions:Fe3+,Pb2+,and Hg2+[J]. Sensors & Actuators B Chemical,2015,208:258-266.
[11]Meng Z,Lei G,Ge S,et al. Three-dimensional paper-based electrochemiluminescence device for simultaneous detection of Pb2+and Hg2+based on potential-control technique[J]. Biosensors & Bioelectronics,2012,41(6):544-550.
[12]Zhang Y,Adeloju S B. Speciation of mercury in fish samples by flow injection catalytic cold vapour atomic absorption spectrometry[J]. Analytica Chimica Acta,2012,721(7):22-27.
[13]Moreno F,García-Barrera T,Gómez-Ariza J L. Simultaneous analysis of mercury and selenium species including chiral forms of selenomethionine in human urine and serum by HPLC column-switching coupled to ICP-MS[J]. Analyst,2010,135(10):2700-2705.
[14]Moreda A. Evaluation of commercial C18 cartridges for trace elements solid phase extraction from seawater followed by inductively coupled plasma-optical emission spectrometry determination[J]. Analytica Chimica Acta,2005,536(1):213-218.
[15]Andria S E,Seliskar C J,Heineman W R. Simultaneous detection of two analytes using a spectroelectrochemical sensor.[J]. Analytical Chemistry,2010,82(5):1720-1726.
[16]Liang Z Q,Wang C X,Yang J X,et al. A highly selective colorimetric chemosensor for detecting the respective amounts of iron(II)and iron(III)ions in water[J]. New Journal of Chemistry,2007,31(6):906-910.
[17]Bin-Cheng Y,Bang-Ce Y,Weihong T,et al. An Allosteric Dual-DNAzyme Unimolecular Probe for Colorimetric Detection of Copper(II)[J]. Journal of the American Chemical Society,2009,131(41):14624-14625.
[18]Wrobel A T,Johnstone T C,Deliz L A,et al. A Fast and Selective Near-Infrared Fluorescent Sensor for Multicolor Imaging of Biological Nitroxyl(HNO)[J]. Journal of the American Chemical Society,2014,136(12):4697-4705.
[19]Formica M,Fusi V,Giorgi L,et al. New fluorescent chemosensors for metal ions in solution[J]. Coordination Chemistry Reviews,2012,256(s 1-2):170-192.
[20]Xu Y,Jiang Z,Xiao Y,et al. A new fluorescent turn-on chemodosimeter for mercury ions in solution and its application in cells and organisms[J]. Analytica Chimica Acta,2014,807:126-134.
[21]Yeh J T,Chen W C,Liu S R,et al. A coumarin-based sensitive and selective fluorescent sensor for copper(II)ions[J]. New Journal of Chemistry,2014,38(9):4434-4439.
[21]Yu S Y,Wu S P. A highly selective turn-on fluorescence chemosensor for Hg(II)and its application in living cell imaging[J]. Sensors & Actuators B Chemical,2014,201(4):25-30.
[23]Tomá P,Peter S,JiIna M,et al. Near-Infrared Fluorescent 9-Phenylethynylpyronin Analogues for Bioimaging[J]. Journal of Organic Chemistry,2014,79(8):3374-3382.
[24]Yang Y K,Cho H J,Lee J,et al. A Rhodamine-Hydroxamic Acid-Based Fluorescent Probe for Hypochlorous Acid and Its Applications to Biological Imagings[J]. Organic Letters,2009,11(4):859-861.
[25]Zhang L,Wang J,Fan J,et al. A highly selective,fluorescent chemosensor for bioimaging of Fe3+[J]. Bioorganic & Medicinal Chemistry Letters,2011,21(18):5413-5416.
[26]Wang F H,Cheng C W,Duan L C,et al. Highly selective fluorescent sensor for Hg2+ion based on a novel rhodamine B derivative[J]. Sensors & Actuators B Chemical,2015,206:679-683.
[27]Jong Woo Jeong,Boddu Ananda Rao,Young-A Son. Rhodamine-chloronicotinaldehyde-based “OFF-ON” chemosensor for the colorimetric and fluorescent determination of Al3+ions[J]. Sensors & Actuators B Chemical,2015,208(208):75-84.
[28]Zhang Y,Zeng X,Mu L,et al. Rhodamine-triazine based chemosensors for Cu2+in aqueous media and living cells[J]. Sensors & Actuators B Chemical,2014,204:24-30.
[29]Sarkar D,Pramanik A,Jana S,et al. Quinoline based reversible fluorescent ‘turn-on’ chemosensor for the selective detection of Zn2+:Application in living cell imaging and as INHIBIT logic gate[J]. Sensors & Actuators B Chemical,2015,209:138-146.
[30]Basa P N,Sykes A G. Differential sensing of Zn(II)and Cu(II)via two independent mechanisms[J]. Journal of Organic Chemistry,2012,77(19):8428-8434.
[31]Li P,Zhou X,Huang R,et al. A highly fluorescent chemosensor for Zn2+and the recognition research on distinguishing Zn2+from Cd2+[J]. Dalton Trans,2013,43(2):706-713.
[32]Kawanishi Y,Kikuchi K,Takakusa H,et al. Design and Synthesis of Intramolecular Resonance-Energy Transfer Chemosensors for Use in Ratiometric Measurements in Aqueous Solution[J]. Angewandte Chemie International Edition,2000,39(19):3438-3440.
[33]Carolyn C,Woodroofe,Stephen J,Lippard. A novel two-fluorophore approach to ratiometric sensing of Zn2+[J]. Journal of the American Chemical Society,2003,125(38):11458-11459.
[34]Liu K,Zhou Y,Yao C. A highly sensitive and selective ratiometric and colorimetric sensor for Hg2+based on a rhodamine-nitrobenzoxadiazole conjugate[J]. Inorganic Chemistry Communications,2011,14(11):1798-1801.
[35]Zhaochao X,Kyung-Hwa B,Ha Na K,et al. Zn2+-triggered amide tautomerization produces a highly Zn2+-selective,cell-permeable,and ratiometric fluorescent sensor[J]. Journal of the American Chemical Society,2010,132(132):601-610.
[36]Zhu B,Gao C,Zhao Y,et al. A 4-hydroxynaphthalimide-derived ratiometric fluorescent chemodosimeter for imaging palladium in living cells[J]. Chemical Communications,2011,47(30):8656-8658.
[37]Chun-Yan L,Xiao-Bing Z,Li Q,et al. Naphthalimide-
porphyrin hybrid based ratiometric bioimaging probe for Hg2+:well-resolved emission spectra and unique specificity[J]. Analytical Chemistry,2009,81(24):9993-10001.
[38]Carolyn C,Woodroofe,Stephen J,Lippard. A novel two-fluorophore approach to ratiometric sensing of Zn2+[J]. Journal of the American Chemical Society,2003,125(38):11458-11459.
[39]Yuan L,Lin W,Zheng K,et al. FRET-based small-molecule fluorescent chemosensors:rational design and bioimaging applications[J]. Accounts of Chemical Research,2013,46(7):1462-1473.
[40]Zhang Y,Guo X,Tian X,et al. Carboxamidoquinoline-coumarin derivative:A ratiometric fluorescent sensor for Cu(II)in a dual fluorophore hybrid[J]. Sensors & Actuators B Chemical,2015,218:37-41.
[41]Gong Y J,Zhang X B,Zhang C C,et al. Through bond energy transfer:a convenient and universal strategy toward efficient ratiometric fluorescent probe for bioimaging applications[J]. Analytical Chemistry,2012,84(24):10777-10784.
[42]Shiguo Sun,Bo Qiao,Na Jiang,et al. Naphthylamine-rhodamine-based ratiometric fluorescent probe for the determination of Pd2+ions[J]. Organic Letters,2014,16(4):1132-1135.
[43]Zhou X,Yan W,Zhao T,et al. Rhodamine based derivative and its zinc complex:synthesis and recognition behavior toward Hg(II)[J]. Tetrahedron,2013,69(46):9535-9539.
[44]Bradley M,Alexander L,Duncan K,et al. pH sensing in living cells using fluorescent microspheres[J]. Bioorganic & Medicinal Chemistry Letters,2008,18(1):313-317.
[45]Hashemi P,Zarjani R A. A wide range pH optical sensor with mixture of Neutral Red and Thionin immobilized on an agarose film coated glass slide[J]. Sensors & Actuators B Chemical,2008,135(1):112-115.
[46]Wang J Q,Huang L,Xue M,et al. Architecture of a Hybrid Mesoporous Chemosensor for Fe3+by Covalent Coupling Bis-Schiff Base PMBA onto the CPTES-Functionalized SBA-15[J]. Journal of Physical Chemistry C,2008,112(13):5014-5022.
[47]Uttamlal Mahesh,Sloan William D,Millar David. Covalent immobilization of fluorescent indicators in photo and electropolymers for the preparation of fibreoptic chemical sensors[J]. Polymer International,2002,51(11):1198-1206.
Study on fluorescent chemosensors for heavy metal ions
LENG Ling,LI Yi,XIONG Xiao-hui*
(College of Food Science and Light Industry,Nanjing Tech University,Nanjing 211816,China)
As a great toxic chemical substance in physiology,heavy metal ions in food causes severe healthy problems and the detection methods of them have aroused widespread concern,especially in sensing area. Owing to their unique advantages,such as tissue penetration,minimum interference from background auto-fluorescence and efficient detection,fluorescent chemosensors have been one of the most important sensing methods for heavy metals. The advances in the progress of fluorescent chemosensors including coumarin,rhodamine analogue,quinoline and radiometric chemosensors were reviewed in this paper. The design principles of chemosensors,as well as sensing mechanisms and the relationship between structure and chemical sensing were analyzed,which provided guidance for fluorescent chemosensors to identify the heavy metals. Finally,in the aspect of food testing,environmental monitoring and biological imaging,the development and prospect of fluorescent chemosensors were addressed to our understanding.
fluorescent probes;heavy metal ions;chemosensors;food detection
2015-12-16
冷玲(1992-),女,硕士研究生,研究方向:食品安全,E-mail:learning92@163.com。
熊晓辉(1964-),男,博士,教授,研究方向:食品安全,E-mail:xxh@njtech.edu.cn。
“十二五”农村领域国家科技计划(2013BAD19B09);江苏省科技基础设施建设计划( BM2012026)。
TS201.6
A
1002-0306(2016)15-0380-06
10.13386/j.issn1002-0306.2016.15.066
