包含ptC的Au(l)配合物催化烯丙基醋酸盐重排反应的理论研究
2016-09-09贾晓芳张聪杰
贾晓芳 张聪杰
(陕西师范大学化学化工学院,陕西省大分子科学重点实验室,西安710062)
包含ptC的Au(l)配合物催化烯丙基醋酸盐重排反应的理论研究
贾晓芳张聪杰*
(陕西师范大学化学化工学院,陕西省大分子科学重点实验室,西安710062)
理论上,采用密度泛函理论研究两类包含平面四配位碳的Au(I)配合物([Au(C3H2BR)]+,R=Me,t-Bu)催化烯丙基醋酸盐的重排反应。计算结果表明,配合物[Au(C3H2BR)]+催化烯丙基醋酸盐重排反应相比较文献中报道的催化剂[Au(NHC)]+而言,能垒降低了9.2-11.7 kJ∙mol-1。因此,除了配体CO和NHC之外,配合物[Au(C3H2BR)]+中的新型配体将会在配位化学以及有机化学中具有应用潜力。
密度泛函理论;金属;平面四配位碳;催化;重排反应
1 lntroduction
The gold complexes as carbophilic π-acids in homogeneous catalysis have a standing interest1.They are also efficient tools for coordination to the alkynes and allenes,as well as attacking interand intramolecular nucleophilic atoms2,3.Generally,the ligands in the gold complexes were CO,N-heterocyclic carbine(NHC),CNR (R=Me),CH2PR3(R=Me,t-Bu),cyclic alkyl(amino)carbene (cAAC)and so on4.In particular,[Au(NHC)]+complexes have attracted great attention of the experimental researchers and been used to catalyze organic transformations reactions5.For example,[Au(NHC)]+was used as catalysts in the rearrangement of the allylic acetates6,2-propargyl 2H-azirines7,and propargyl ester8. Because the allylic rearrangement reaction can efficiently obtain the primary oxo derivatives,the[3,3]sigmatropic rearrangement of allylic esters catalyzed by[Au(NHC)]+and the mechanism of the reactions were obtained using density functional theory (DFT)9,10.The mechanism indicates that the rearrangement process goes through a six-membered ring intermediate where gold moiety coordinates with one of the carbon atoms and the free energy barrier is 59.5 kJ∙mol-1in gas phase.Recently,we found that the derivatives of 2-borabicyclo[1.1.0]but-1(3)-ene(2BB)can also bind to Au(I)to form gold complexes11,12.The chemical bonding of the horizontal C―C bond in 2BB was illustrated in Scheme 1 in order to more clearly understand that it can be a ligand.The twocarbon atoms of horizontal C―C bond adopt sp2hybrid,then the two pzorbitals perpendicular to the molecular plane form a conjugate π bond,while the two outward stretch hybrid orbitals form a charge shift(CS)bond similar with that of[1.1.1]propellanes C5H613-15and C216,17.Thus,the ionic restructure of 2BB can coordinate with Au(I)to form[Au(2BB)]+complex12.Moreover,our previous theoretical calculations indicated that AuCl(2BB)complexes are stable and contain a planar tetracoordinate carbon (ptC)12,which are obviously different from the[Au(NHC)]+and [Au(CO)]+18-21.In this contribution,we applied[Au(2BB)]+to catalyze the allylic rearrangement reaction in order to check the catalyzed ability of[Au(2BB)]+.
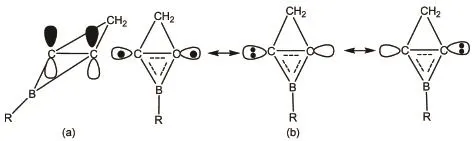
Scheme 1Horizontal C―C bonding of the derivatives of 2-borabicyclo[1.1.0]but-1(3)-ene(a)π bond and(b)charge shift bond arising from the mixture of covalent and ionic resonance structures
2 Computational methods
The geometries of the reactants,intermediates,transition states,and products were optimized using B3LYP functional,combination with the effective core potentials(SDD)for gold,and the 6-31+G(d,p)basis sets for other atoms.Frequency calculations were also carried out to verify all stationary points to be the transition states or minima at the same level of theory.We employed a continuum medium to calculate single point solvation energies for all the compounds,using UFF radii on the polarizable continuum model(PCM)22,23.In the PCM calculations,dichloroethane was used as the solvent,corresponding to the conditions in reference9.Intrinsic reaction coordinate(IRC)calculations were used to confirm the transition structures24.Wiberg bond indices (WBIs)of the complexes were obtained by natural bond orbital (NBO)analysis25.All calculations were performed with Gaussian 09 package26.
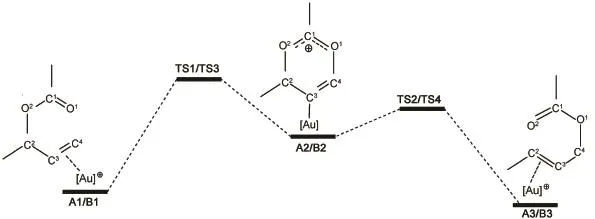
Scheme 2Scheme of rearrangement reaction of allylic acetates catalyzed by[Au(2BB)]+
3 Results and discussion
Previous studies showed that the uncatalyzed rearrangement reaction of allylic acetates took place in one step with high energy barrier of 132.7 kJ∙mol-1.9Furthermore,the mechanism of the rearrangement of allylic acetates catalyzed by[Au(2BB)]+has been obtained and found that is has low energy barrier.9The structure of[Au(2BB)]+is similar with that of[Au(NHC)]+because[Au(2BB)]+and[Au(NHC)]+are formed by Au(I)bonded to the ligands 2BB and NHC toward carbon atoms.Thus,the mechanism of the rearrangement of allylic acetates catalyzed by [Au(2BB)]+should be the same as that of by[Au(NHC)]+,and the potential energy profile was illustrated in Scheme 2.Because [Au(2BB)]+originates from Au(C3H2BR)Cl(R=Me,t-Bu),the stability of the complexes of Au(C3H2BR)Cl(R=Me,t-Bu)is considered,Calculated vibrational frequencies of the two complexes are positive,in addition,their dissociation energies ofAu (C3H2BR)Cl(R=Me,t-Bu)are 652.3 and 274.7 kJ∙mol-1,respectively,indicating that the two complexes are stable and consistent with the results in reference12.
Optimized geometries and the smallest frequencies of the complexes of[Au(C3H2BCH3)]+(C1)and[Au(C3H2BCMe3)]+(C2)are displayed in Fig.1.The smallest frequencies of C1 and C2 are positive,indicating that they are true minima on the potential energy surfaces.The bond distances of Au―C bonds in C1 and C2 are 0.200 and 0.200 nm,respectively,which are in well agreement with that ofAu―C(0.200 nm)bonds in(C3H2BCH3)AuCl12and Au―C bond in[Au(NHC)]+20,21.The distances of horizontal C―C bonds in C1 and C2 are 0.143 and 0.145 nm,respectively,showing the horizontal C―C bonds in both C1 and C2 are between single and double bonds.The lengths of C(Au)―C(H2)and C(Au)―B bonds in C1 and C2 are close to 0.156 and 0.158 nm,respectively,which are single bonds.The sum of the bond angles of Au―C―B,Au―C―C(H2),C(H2)―C(Au)―C,and C―C(Au)―B in C1 and C2 are 360.1°and 363.0°,respectively,as a result,the C(Au)in C1 and C2 are planar tetracoordinate carbons(ptCs)because the sum of the bond angles associated with the C(Au)atom are close to 360.0°.The WBIs of the C―C(horizontal),Au―C,C(Au)―C(H2),and C(Au)―B bonds of C1 and C2 were also displayed in Fig.1.The WBIs of theAu―C bonds in C1 and C2 are 0.64 and 0.63 and the WBIs of C(Au)―C,C(Au)―C(H2),and C(Au)―B in C1 and C2 are close to 1.20,0.87 and 0.83,respectively,which confirm that the C(Au)are ptCs.In addition,the total WBI of ptCs in C1 and C2 are 3.68 and 3.70,respectively,thus the ptC obeys the octal rule.According to the rearrangement mechanism of allylic acetates as shown in Scheme 2,the geometries of the three intermediates(A1,A2,and A3)and two transition structures(TS1 and TS2)of allylic acetates catalyzed by C1 were optimized and illustrated in Fig.2.The reaction from A1 to A3 goes through the cleavage of the C2―O2bond and formation of the C4―O1bond.The distances of C3…Au and C4…Au in A1 are 0.232 and 0.223 nm,respectively,showing thatAu binds to the C=C bond.Subsequently,A1 leads to a sixmembered ring structure A2 via the transition structure TS1.The Au bonds to the six-membered ring towards C3atom in A3.The distances of Au―C3,O1―C4,and O2―C2in A2 are 0.212,0.152,and 0.156 nm,respectively.A2 further arrives at the A3 through transition state TS2,the length of O1―C4bond inA3 is 0.144 nm andAu bonds to C2=C3double bond.
The relative energy of A1 with respect to the individual reactants H2C=CHC(CH3)OCOCH3and C1 is significantly reduced,which is 138.6 kJ∙mol-1.Then,we used the energy ofA1 as origin for relative energies of A2,A3,TS1 and TS2 in order to compare with the results in reference9and listed the relative energies in Table 1.The free energy barrier of forming intermediate A2 from A1 is 63.2 kJ∙mol-1in solvent,which is 9.2 kJ∙mol-1lower than that in reference9.The formation of A3 fromA2 is both kinetically and thermodynamically favorable,for which the free energy barrier and the reaction free energy are 52.3 and 3.3 kJ∙mol-1,respectively.Moreover,the free energy barrier and the reaction free energy fromA2 toA3 are also lower than those in reference9. Therefore,the[Au(C3H2BCH3)]+complexes with ptC is slightly better than that of[Au(NHC)]+.
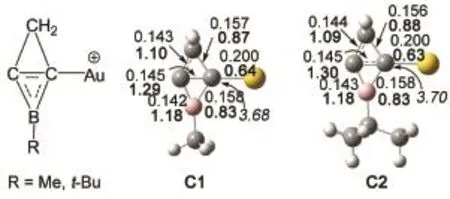
Fig.1 Optimized geometries(in plain,bond length in nm),WBIs(in bold)and the total bond indices of ptCs(in italics)of catalysts C1 and C2
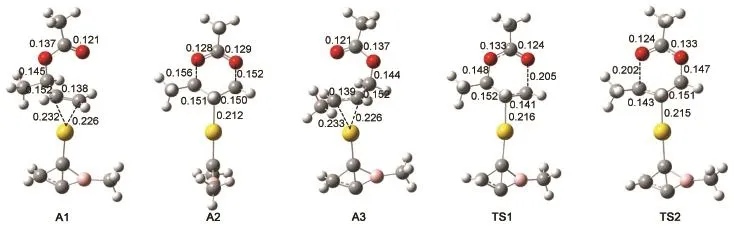
Fig.2Optimized geometries of the intermediates(A1,A2,andA3)and transition states(TS1 and TS2)bond length in nm
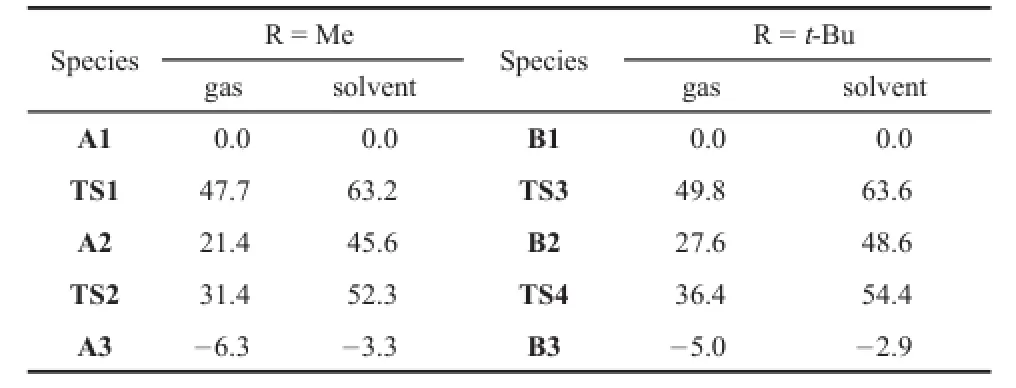
Table 1Relative Gibbs energies(kJ∙mol-1)of the intermediates and transtition structures of the rearrangement reactions of allylic acetates in gas and solvent
We further constructed[Au(C3H2BCMe3)]+complex(C2)whose group bonded to B atom in C1 was replaced with bulky t-Bu group to catalyze the rearrangement of allylic acetates.Optimized geometries of three intermediates(B1,B2,and B3)and transition state structures(TS3 and TS4)are illustrated in Fig.3.The bond distances in B1,B2,and B3 have no significant variation compared toA1,A2,andA3.For example,the lengths of C2―O2bond in B1,C4―O1bond in B3,as well as C2―O2and C4―O1bonds in B2 are 0.145,0.144,0.156,and 0.153 nm,respectively,which are very close to those of inA1,A3,andA2.Thus,the groups Me and t-Bu bonded to B in C1 and C2 do not influence the geometries of the structures involved in the reactions.
Similarly,the rearrangement reactions of allylic acetates catalyzed by C2 is also the cleavage of the C2―O2bond and for-mation of the C4―O1bond,that is,B1→B3 via a 6-membered ring intermediate B2 as shown in Fig.3.The reaction of B1→B3 is thermodynamically favorable in solvent because the reaction free energy is 2.9 kJ∙mol-1.The free energy barrier of B1→B2 is 63.6 kJ∙mol-1in solvent.Subsequently,B2→B3 goes through TS4 with the free energy barrier of 54.4 kJ∙mol-1.It is clear that free energy barriers and reaction free energies of the rearrangement allylic acetates catalyzed by C2 are almost the same as with C1.Thus the substituent of the groups bonded to B atom in the derivatives of 2-borabicyclo[1.1.0]but-1(3)-ene has little influence on their catalyzed ability.The same results were also found in the rearrangement allylic acetates catalyzed by[Au(NHC)]+9.
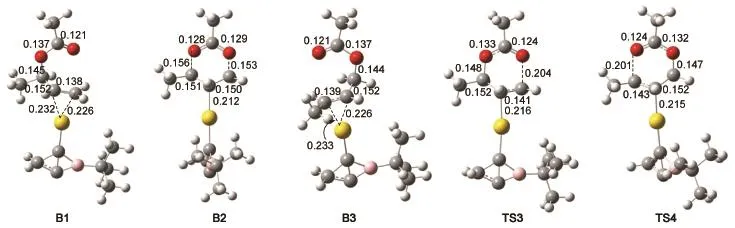
Fig.3Optimized geometries of intermediates(B1,B2,and B3)and transition states(TS3 and TS4)bond length in nm
4 Conclusions
In summary,we theoretically designed the novel complexes([Au(C3H2BR)]+,R=Me,t-Bu).Moreover,we applied the complexes to catalyze the rearrangement reactions of allylic acetates. The main conclusions are envisioned:(i)[Au(C3H2BR)]+(R=Me,t-Bu)complexes contain planar tetracoordinate carbon(ptC);(ii)the catalyzed ability of[Au(C3H2BR)]+are little influenced by the groups R bonded to B in the derivatives of 2-borabicyclo[1.1.0]but-1(3)-ene;(iii)the free energy barrier and the reaction free energy of rearrangement reactions of allylic acetates catalyzed by [Au(C3H2BR)]+are lower than those of by[Au(NHC)]+;(iv)the third type ligand toward carbon atom besides CO and NHC can coordinate with Au to form complexes with catalyzed activation. Therefore,the gold complexes with ptC were suggested to have potential application in the organic reactions.
References
(1)Li,Z.;Brouwer,C.;He,C.Chem.Rev.2008,108,3239. doi:10.1021/cr068434l
(2)Toups,K.L.;Liu,G.T.,Widenhoefer,R.A.J.Organomet. Chem.2009,694,571.doi:10.1016/j.jorganchem.2008.11.058
(3)Marion,N.;Nolan,S.P.Angew.Chem.Int.Edit.2007,46,2750. doi:10.1002/anie.200604773
(4)(a)Cotton,F.A.;Wilkinson,G.;Murillo,C.A.;Bochmann,M. Advanced Inorganic Chemistry,6th ed.;Wiley:New York,1999.(b)Dumas,J.B.Ann.Chim.Phys.1835,58,28. (c)Romanov,A.S.;Bochmann,M.Organometallics 2015,34,2439.doi:10.1021/om501211p
(5)(a)de Fremont,P.;Marion,N.;Nolan,S.P.J.Organomet. Chem.2009,694,551.doi:10.1016/j.jorganchem.2008.10.047. (b)Kappe,C.O.Angew.Chem.Int.Edit.2004,43,6250. doi:10.1002/anie.200400655 (c)de la Hoz,A.;Dıʹaz-Ortiz,A.;Moreno,A.Chem.Soc.Rev. 2005,34,164.doi:10.1039/B411438H
(6)Marion,N.;Gealageas,R.;Nolan,S.P.Org.Lett.2007,9,2653. doi:10.1021/ol070843w
(7)Jin,L.;Wu,Y.;Zhao,X.J.Org.Chem.2015,80,3547. doi:10.1021/acs.joc.5b00180
(8)Rettenmeier,E.;Hansmann,M.M.;Alexander,A.;Rubenacker,K.;Saboo,T.;Massholder,J.;Meier,C.;Rudolph,M.;Rominger,F.;Hashmi,A.S.K.Chem.Eur.J.2015,21,14401. doi:10.1002/chem.201501725
(9)Gourlaouen,C.;Marion,N.;Nolan,S.P.;Maseras,F.Org.Lett. 2009,11,81.doi:10.1021/ol802430m
(10)Siebert,R.;Tantillo,D.J.J.Am.Chem.Soc.2007,129,8686. doi:10.1021/ja072159i
(11)Hoffmann,R.;Alder,R.W.;Wilcox,C.F.J.Am.Chem.Soc. 1970,92,4992.doi:10.1021/ja00719a044
(12)Zhang,C.;Li,F.J.Phys.Chem.A 2012,116,9123. doi:10.1021/jp305871ml
(13)Zhang,C.;Ning,L.;Li,J.Organometallics 2013,32,7083.doi: 10.1021/om400807w
(14)Wu,W.;Gu,J.;Song,J.;Shaik,S.;Hiberty,P.C.Angew.Chem. Int.Edit.2009,48,1407.doi:10.1002/anie.200804965
(15)Shaik,S.;Danovich,D.;Wu,W.;Hiberty,P.C.Nat.Chem. 2009,1,443.doi:10.1038/NCHEM.327
(16)Shaik,S.;Danovich,D.;Wu,W.;Su,P.F;Rzepa,H.S.;Hiberty,P.C.Nat.Chem.2012,3,195.doi:10.1038/NCHEM.1263
(17)Danovich,D.;Hiberty,P.C.;Wu,W.;Rzepa,H.S.;Shaik,S. Chem.Eur.J.2014,20,6220.doi:10.1002/chem.201400356
(18)Shao,L.;Zhang,L.;Zhou,M.;Qin,Q.Organometallics 2001,20,1137.doi:10.1021/om000911h
(19)Ray,L.;Shaikh,M.M.;Ghosh,P.Inorg.Chem.2008,47,230. doi:10.1021/ic701830m
(20)Hashmi,A.S.K.;Yu,Y.;Rominger,F.Organometallics 2012,31,895.doi:10.1021/om2008919
(21)Cure,J.;Poteau,R.;Gerber,I.C.;Gornitzka,H.;Hemmert,C. Organometallics 2012,31,619.doi:10.1021/om2009183
(22)Ho,J.M.;Klamt,A.;Coote,M.L.J.Phys.Chem.A 2010,114,13442.doi:10.1021/jp107136j
(23)Mazo,J.;El Badry,A.T.;Carreras,J.;Delgado,M.;Boer,D.; Zalba,B.Applied Thermal Engineering 2015,90,596. doi:10.1016/j.applthermaleng.2015.07.047
(24)Maeda,S.;Harabuchi,Y.;Ono,Y.;Taketsugu,T.;Morokuma,K. Int.J.Quantum Chem.2015,115,258.doi:10.1002/qua.24757
(25)Reed,A.E.;Curtiss,L.A.;Weinhold,F.Chem.Rev.1988,88,899.doi:10.1021/cr00088a005
(26)Frisch,M.J.;Trucks,G.W.;Schlegel,H.B.;et al.Gaussian 09,Revision C.01;Gaussian Inc.:Wallingford,CT,2009.
A Theoretical lnvestigation of the Rearrangement Reaction of Allylic Acetates Catalyzed by Au(l)Complexes with PtC
JIAXiao-FangZHANG Cong-Jie*
(Key Laboratory of Macromolecular Science of Shaanxi Province,School of Chemistry&Chemical Engineering,Shaanxi Normal University,Xi′an 710062,P.R.China)
The rearrangements of allylic acetates catalyzed by[Au(C3H2BR)]+(R=Me,t-Bu)with planar tetracoordinate carbon(ptC)have been theoretically investigated.Calculated results show that the free energy barriers and the reaction free energies of the[Au(C3H2BR)]+-catalyzed rearrangement of allylic acetates are lower than those using[Au(NHC)]+by 9.2-11.7 kJ∙mol-1,indicating that a third type of containing C-terminal ligand besides CO and NHC might have potential applications in coordination and organic chemistry as well.
Density functional theory;Metal;Planar tetracoordinate carbon;Catalysis;Rearrangement reaction
January 15,2016;Revised:March 9,2016;Published on Web:March 10,2016.
O641
[Article]10.3866/PKU.WHXB201603101www.whxb.pku.edu.cn
*Corresponding author.Email:zcjwh@snnu.edu.cn;Tel:+86-15291919063.
The project was supported by the National Natural Science Foundation of China(21373133).
国家自然科学基金(21373133)资助项目
©Editorial office ofActa Physico-Chimica Sinica
