血清异常凝血酶原水平在胰腺癌诊治中的应用价值
2016-09-08雷旦生喻晶
雷旦生 喻晶
·论著·
血清异常凝血酶原水平在胰腺癌诊治中的应用价值
雷旦生喻晶
目的检测胰腺癌患者血清异常凝血酶原(PIVKA-Ⅱ)水平,探讨其在胰腺癌诊断、疗效评估中的临床应用价值。 方法收集湖北省肿瘤医院收治的112例胰腺癌、28例胰腺炎及79例健康者血清,应用化学发光法检测血清PIVKA-Ⅱ水平,同时应用电化学发光法检测血清CEA、CA19-9水平。绘制受试者工作特征(ROC)曲线,确定诊断临界值,计算诊断敏感性、特异性,准确性。 结果112例胰腺癌患者的血清PIVKA-Ⅱ浓度为39.0(22.61,137.67)U/L,显著高于30例胰腺炎患者的23.0(17.32,29.67)U/L及79例健康者的21.21(17.90,25.12)U/L,差异均有统计学意义(P值均<0.01),而胰腺炎与健康者的差异无统计学意义。血清PIVKA-Ⅱ、CEA、CA19-9的ROC曲线下面积分别为0.793、0.707、0.849,诊断胰腺癌的临界值分别为32.4、4.7、27.0 U/L;敏感性为61.6%、38.0%、66.1%;特异性为86.3%、96.3%、89.9%;准确性为71.7%、62.3%、75.0%。PIVKA-Ⅱ联合CEA、CA19-9检测的敏感性、特异性、准确性分别为90.2%、76.0%、84.3%。联合检测的敏感性显著提高,特异性略有下降。胰腺癌患者血清PIVKA-Ⅱ水平与肿瘤分期有关。20例追踪监测的患者中15例经手术或介入治疗后血清PIVKA-Ⅱ水平较治疗前显著下降(50.6U/L比110.5 U/L,P<0.001),5例姑息治疗者中4例有不同程度升高。 结论检测血清PIVKA-Ⅱ水平对胰腺癌的诊断及疗效评估具有一定的临床应用价值。
胰腺肿瘤;异常凝血酶原;诊断;监测
PIVKA-Ⅱ是出现在维生素K缺乏、用华法林或苯丙香豆素治疗的患者的一种异常脱羧基凝血酶原[1-3]。正常个体中无高浓度的PIVKA-Ⅱ,但恶性肿瘤患者中即便未出现维生素K缺乏,也可能存在高浓度的PIVKA-Ⅱ[3]。最早在1984年就发现部分肝癌患者的血中PIVKA-Ⅱ水平升高,且具有较高的诊断特异性。此外,PIVKA-Ⅱ对其他消化道肿瘤也有一定的临床诊断价值[4-6]。胰腺癌因生理结构特征很容易发生肝转移,且一般不易早期发现,死亡率高,素有“癌中之王”之称。传统的胰腺癌血清标志物有CEA、CA19-9[7-8]。CEA对胰腺癌的诊断敏感性不高,CA19-9诊断胰腺癌的敏感性虽高,但存在一定的假阳性,易受胆汁淤积的影响,因此临床上一直在寻找胰腺癌新的诊断标志物。本研究检测胰腺癌患者血PIVKA-Ⅱ水平,探讨其在胰腺癌诊治中的应用价值。
资料与方法
一、一般资料
选择2015年2月20日至2015年10月20日湖北省肿瘤医院肝胆胰科收治的经病理证实的胰腺癌患者112例,其中男性83例,女性29例,年龄25~75岁,平均58岁。112例胰腺癌患者中局限期(Ⅰ~Ⅱ)14例,进展期(Ⅲ~Ⅳ)98例,其中12例晚期胰腺癌患者肝功能异常,被证实为肝转移,其余患者观察期内肝功能正常。急、慢性胰腺炎患者28例,其中男性19例,女性9例,平均46岁,均无其他疾病。体检健康者79例,其中男性45例,女性34例,平均41岁。排除标准:肝癌转移性胰腺癌患者、伴有肝硬化的胰腺癌患者、存在凝血功能障碍的胰腺癌患者均不纳入肿瘤组;伴有肝硬化、凝血功能障碍的胰腺炎患者也不纳入良性病组。
二、标本采集及检测
采用黄盖含分离胶的进口真空采血管抽取待检者空腹静脉血2~4 ml,待血液自然凝固后以2 300 g(离心半径5 cm)离心15 min分离血清,-80℃保存待测。采用ARCHITECT-IU2000化学发光分析仪及PIVKA-Ⅱ检测试剂盒(美国Abbott公司)测定血清PIVKA-Ⅱ浓度,标准品及质控品均为原装配套试剂。采用Cobas e601电化学发光免疫分析仪及CEA、CA19-9检测试剂盒(瑞士Roche公司)测定血CEA、CA19-9浓度,应用BIORAD的肿瘤标志物质控品,室内质控、室间质评均合格。
三、统计学处理
采用SPSS17.0软件进行统计学分析。因样本浓度均呈偏态分布,故以M(P25,P75)表示,组间比较采用非参数Kruskal-Wallis检验,两两比较采用MannWhitneyU检验。P<0.05为差异有统计学意义。敏感性=真阳性人数/(真阳性人数+假阴性人数)×100%,特异性=真阴性人数/(真阴性人数+假阳性人数)×100%,准确性=(真阳性人数+真阴性人数)/总人数。绘制受试者工作特征(ROC)曲线,计算曲线下面积(AUC),确定临界值。
结 果
一、胰腺癌、胰腺炎及健康者血清PIVKA-Ⅱ浓度
胰腺癌、胰腺炎及健康者血清PIVKA-Ⅱ水平分别为39.0(22.61,137.67)、23.0(17.32,29.67)、21.21(17.90,25.12)U/L,胰腺癌患者显著高于胰腺炎患者及健康者,差异均有统计学意义(U=254.0,Z=-2.733;U=578.5,Z=-4.292;P值均<0.05),而胰腺炎患者与健康者之间的差异无统计学意义(U=98.4,Z=-1.116,P>0.05)。
二、胰腺癌患者血清PIVKA-Ⅱ、CEA、CA19-9浓度对胰腺癌的诊断价值
胰腺癌患者血清PIVKA-Ⅱ、CEA、CA19-9的ROC曲线见图1,各指标的AUC分别为0.793±0.058、0.707±0.069、0.849±0.053,诊断临界值分别为32.4、4.7、27.0 U/L。
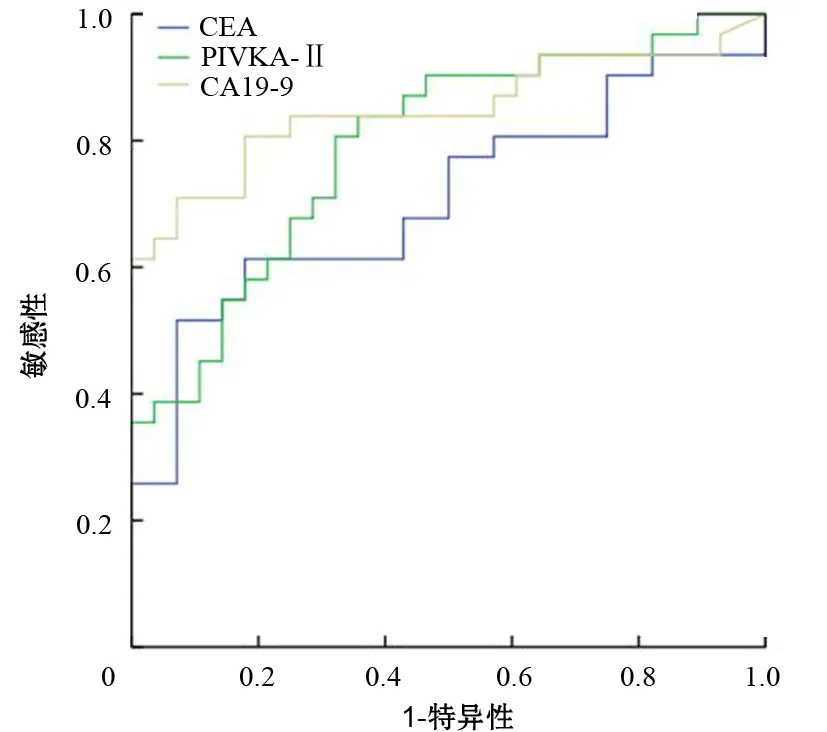
图1 不同标志物诊断胰腺癌的ROC曲线
PIVKA-Ⅱ、CEA、CA19-9单指标及CEA+CA19-9、PIVKA-Ⅱ+CEA+CA19-9联合检测对胰腺癌诊断的敏感性、特异性、准确性见表1。2项或3项联合检测诊断胰腺癌的敏感性可从单项检测的60%左右提高至80%或90%,特异性保持在88%或略降至76%。而2项、3项联合检测的差异无统计学意义(χ2=2.296,P=0.13)。
表1血清PIVKA-Ⅱ及CEA、CA19-9对胰腺癌的诊断价值

检测指标阳性例数敏感性(%)特异性(%)准确性(%)PIVKA-Ⅱ6961.686.371.7CEA4338.096.362.3CA19-97466.189.975.0CEA+CA19-99080.488.683.8PIVKA-Ⅱ+CEA+CA19-910190.276.084.3
三、胰腺癌患者PIVKA-Ⅱ水平与肿瘤分期的关系
14例Ⅰ~Ⅱ期患者、98例Ⅲ~Ⅳ期患者血清PIVKA-Ⅱ水平分别为25.2(20.54,40.33)、39.7(23.45,152.23)U/L。Ⅲ~Ⅳ期患者显著高于Ⅰ~Ⅱ期患者,Ⅰ~Ⅱ期患者又显著高于健康者,差异均有统计学意义(U=251.6,Z=-2.971,P=0.014;U=226.5,Z=-2.052,P=0.021)。Ⅲ、Ⅳ期胰腺癌患者PIVKA-Ⅱ阳性率为63.3%,显著高于Ⅰ~Ⅱ期患者的35.7%,差异有统计学意义(χ2=3.869,P=0.048)。
四、PIVKA-Ⅱ水平监测对胰腺癌患者疗效及预后评估的价值
对112例胰腺癌患者中20例进行追踪监测,其中15例在手术或介入治疗后1个月的血PIVKA-Ⅱ、CEA、CA19-9水平分别为50.6、11.3、88.2 U/L,较治疗前的110.5、36.4、234.5 U/L显著下降,差异均有统计学意义(U值分别为327.4、209.3、368.1,Z值分别为-2.734、-1.875、-2.865,P值分别为<0.001、0.004、<0.001)。5例行姑息治疗的晚期患者每2周检测1次,至2015年10月20日截止,其中4例患者3项标志物均有不同程度升高(升高40%~4倍),1例患者在观察期内病情稳定,各项指标均未见显著升高。
讨 论
胰腺癌是死亡率最高的肿瘤之一,不易早期发现,患者生存期短[9-10]。目前临床常用的胰腺癌标志物有CEA、CA19-9[7-8]。PIVKA-Ⅱ即维生素K缺乏或拮抗剂Ⅱ诱导的蛋白,Liebman等最早于1984年发现其在部分肝癌患者中升高,且具有较高的诊断特异性[1-3]。检测PIVKA-Ⅱ水平可用于辅助诊断肝细胞癌(HCC)、监测HCC高危患者(丙型肝炎病毒感染、肝炎/肝硬化、乙型肝炎病毒感染)和评估HCC疗效[2]。此后有报道PIVKA-Ⅱ在纵膈卵巢囊肿[3]、消化道的结肠癌[4]、胃癌[5-6]、胰腺的肝样癌[11]中也有高表达。
本研究结果显示,胰腺癌患者血清PIVKA-Ⅱ浓度显著高于急、慢性胰腺炎和健康人群。PIVKA-Ⅱ与CEA、CA19-9[12-13]单项检测诊断胰腺癌时,以CA19-9敏感性最高,CEA特异性最好。PIVKA-Ⅱ诊断胰腺腺癌的敏感性为61.6%,特异性为86.3%,PIVKA-Ⅱ联合CEA、CA19-9检测诊断胰腺癌的敏感性可提高到90.2%,特异性保持在76%,大大提高胰腺癌诊断的灵敏度。
本结果还显示,胰腺癌患者血清PIVKA-Ⅱ水平与肿瘤分期显著相关,分期晚的患者血清PIVKA-Ⅱ水平高,甚至可高达1 000 U/L以上。血清PIVKA-Ⅱ水平还可监测病情的变化及预后。治疗前PIVKA-Ⅱ水平越高的胰腺癌患者预后差,手术或介入治疗有效患者血PIVKA-Ⅱ水平下降,部分晚期胰腺癌肝转移患者经介入治疗后PIVKA-Ⅱ水平也有一定程度下降,因此,检测血清PIVKA-Ⅱ水平对胰腺癌的诊断及疗效评估具有重要的临床应用价值。
[1]Seo SI, Kim HS, Kim WJ, et al. Diagnostic value of PIVKA-Ⅱ and alpha-fetoprotein in hepatitis B virus-associated hepatocellular carcinoma[J]. World J Gastroenterol, 2015, 21(13):3928-3935. DOI: 10.3748/wjg.v21.i13.3928.
[2]Poté N, Cauchy F, Albuquerque M, et al. Performance of PIVKA-Ⅱ for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion[J]. J Hepatol, 2015,62(4):848-854. DOI: 10.1016/j.jhep.2014.11.005.
[3]Akutsu N, Adachi Y, Isosaka M, et al. Mediastinal yolk sac tumor producing protein induced by vitamin K absence or antagonist-Ⅱ[J]. Intern Med, 2015,54(12):1531-1536. DOI: 10.2169/internalmedicine.54.4025.
[4]Kato K, Iwasaki Y, Taniguchi M, et al. Primary colon cancer with a high serum PIVKA-Ⅱ level[J]. Int J Surg Case Rep, 2015,6(3):95-99. DOI: 10.1016/j.ijscr.2014.11.072.
[5]Ogasawara N, Takahashi E, Matsumoto T, et al. Prolonged survival in a case of chemotherapy-sensitive gastric cancer that produced alpha-fetoprotein and protein induced by vitamin K antagonist-II[J]. Case Rep Gastroenterol, 2015, 9(1):113-119. DOI: 10.1159/000382072.
[6]Takahashi Y, Inoue T, Fukusato T. Protein induced by vitamin K absence or antagonist Ⅱ-producing gastric cancer[J]. World J Gastrointest Pathophysiol, 2010, 1(4):129-136. DOI: 10.4291/wjgp.v1.i4.129.
[7]Dranka-Bojarowska D, Lekstan A, Olakowski M, et al. The assessment of serum concentration of adiponectin, leptin and serum carbohydrate antigen-19.9 in patients with pancreatic cancer and chronic pancreatitis. J Physiol Pharmacol, 2015, 66(5):653-663.
[8]Reitz D, Gerger A, Seidel J, et al. Combination of tumour markers CEA and CA19-9 improves the prognostic prediction in patients withpancreatic cancer[J]. J Clin Pathol, 2015, 68(6):427-433. DOI: 10.1136/jclinpath-2014-202451.
[9]杨尹默.胰腺癌外科治疗的热点与难点[J].中华消化外科杂志,2015,14(8):612-614.DOI:10.3760/cma.j.issn.1673-9752.2015.08.004.
[10]张太平,曹喆,赵玉沛.胰腺癌的化疗与放疗[J].中华消化外科杂志,2015,14(8):619-622.DOI:10.3760/cma.j.issn.1673-9752.2015.08.006.
[11]Matsueda K, Yamamoto H, Yoshida Y, et al. Hepatoid carcinoma of the pancreas producing protein induced by vitamin K absence or antagonist Ⅱ(PIVKA-Ⅱ) and alpha-fetoprotein (AFP)[J]. J Gastroenterol, 2006, 41(10):1011-1019. DOI:10.1007/s00535-006-1889-8.
[12]曾彦博,王凯旋,李兆申.胰腺癌标志物的新进展[J].中华胰腺病杂志,2011,11(6):448-450. DOI: 10.3969/j.issn.1673-8640.2014.03.017.
[13]张泉东,金政锡,郝迪斯.肿瘤标志物与胰腺癌相关性的研究进展[J].国际免疫学杂志,2014,37(1):57-60. DOI: 10.3760/cma.j.issn.1674-1935.2011.06.025.
(本文编辑:屠振兴)
Clinical application of serum PIVKA-Ⅱ level in the diagnosis and treatment of pancreatic cancer
LeiDansheng,YuJing.
DepartmentofClinicalLaboratory,CancerHospitalofHubeiProvince,Wuhan430079,China
Correspondingauthor:YuJing,Email:14043237@qq.com
ObjectiveSerum abnormal prothrombin (PIVKA-Ⅱ) level was detected in pancreatic cancer patients and its clinical value in diagnosing and treating pancreatic cancer was explored. MethodsA total of 112 pancreatic cancer patients, 28 benign pancreatic diseases patients and 79 healthy controls were collected from Cancer Hospital of Hubei Province. Serum PIVKA-Ⅱ level was determined by chemiluminescent immunoassay (CLIA). CEA and CA19-9 level was detected by electrochemiluminescence immunoassay (ECLIA). Receiver operation characteristic (ROC) curve was drawn and the cut-off value was set. The sensitivity, specificity and accuracy were calculated. ResultsPIVKA-Ⅱ level in 112 pancreatic cancer patients was 39.0(22.61, 137.67)U/L[median(quartile range)],which was significantly higher than that in 30 patients with benign pancreatic disease [23.0(17.32, 29.67)U/L] and 79 healthy controls[21.21(17.9, 25.12)U/L].The differences were statistically significant (P<0.01). However, there was no significant difference between benign pancreatic disease patients and healthy controls. ROC analysis showed that area under curve(AUC) for PIVKA-Ⅱ, CEA and CA19-9 was 0.793, 0.707 and 0.849.The cut-off value for discriminating pancreatic cancer was established at 32.4, 4.7 and 27.0 U/L for PIVKA-Ⅱ, CEA and CA19-9. The sensitivity was 61.6%, 38.0% and 66.1%, and the specificity was 86.3%, 96.3% and 89.9%,and the accuracy was 71.7%, 62.3% and 75.0%, respectively. The sensitivity, specificity and accuracy of PIVKA-Ⅱ combined with CEA and CA19-9 was 90.2%, 76.0% and 84.3%. The sensitivity was obviously higher, while the specificity was lower. Serum PIVKA-Ⅱ level was associated with advanced tumor stage. There were 20 patients who were followed up. PIVKA-Ⅱ level in 15 cases were decreased after surgery or interventional therapy (50.6 U/Lvs110.5 U/L,P<0.001), which was increased in 4 of the five cases who received palliative care to a various degree. ConclusionsPIVKA-Ⅱ detection could benefit the diagnosis and prognosis monitoring of pancreatic cancer.
Pancreatic neoplasms;Abnormal prothrombin;Diagnosis;Monitoring
10.3760/cma.j.issn.1674-1935.2016.04.001
430079武汉,湖北省肿瘤医院检验科
喻晶,Email:14043237@qq.com
2015-12-18)
