Bioactive com pounds of red grapes from Dão region(Po rtuga l):Evalua tion of pheno lic and organic pro file
2016-09-07LuRodriguesSilvaMafaldaQueirozHealthSciencesResearchCentreFacultyofHealthSciencesUniversityofBeiraInteriorAvenueInfanteHenriqueCovilh601506PortugalDepartmentofChemicalEngineeringFacultyofEngineeringUniversityofPortoStreetDrR
Luís Rodrigues Silva,Mafalda QueirozHealth Sciences Research Centre Faculty of Health Sciences,University of Beira Interior,Avenue Infante D.Henrique,Covilhã601-506,PortugalDepartmentofChemical Engineering,Faculty of Engineering,University of Porto,StreetDr.Roberto Frias,Porto 00-65, PortugalPolytechnic Institute of Castelo Branco,School of Health Dr.Lopes Dias,Avenue Empres´ario,Campus da Talagueira,Castelo Branco 6000-767,PortugalLaboratory of Pharmacognosy,Department of Chem istry,Faculty of Pharmacy,University of Porto,Street Dr.Jorge Viterbo Ferreira 8,Porto 050-1,Portugal
Bioactive com pounds of red grapes from Dão region(Po rtuga l):Evalua tion of pheno lic and organic pro file
Luís Rodrigues Silva1,2,3*,Mafalda Queiroz41Health Sciences Research Centre Faculty of Health Sciences,University of Beira Interior,Avenue Infante D.Henrique,
Covilhã6201-506,Portugal
2DepartmentofChemical Engineering,Faculty of Engineering,University of Porto,StreetDr.Roberto Frias,Porto 4200-465, Portugal
3Polytechnic Institute of Castelo Branco,School of Health Dr.Lopes Dias,Avenue Empres´ario,Campus da Talagueira,
Castelo Branco 6000-767,Portugal
4Laboratory of Pharmacognosy,Department of Chem istry,Faculty of Pharmacy,University of Porto,Street Dr.Jorge Viterbo Ferreira 228,Porto 4050-313,Portugal
Original article http://dx.doi.org/10.1016/j.apjtb.2015.12.015
ARTICLE INFO
Article history:
Received in revised form 20Oct,2nd revised form 5Nov,3rd revised form 11 Nov 2015
Accepted 5Dec 2015
Availableonline 12 Jan 2016
ABSTRACT
Objective:To improve the know ledge on themetabolite pro fi le of fi ve red grapes from Dão region(Portugal),concerning to the phenolic characteristics(coloured and non-coloured phenolics)and organic acid composition.
M ethods:Five red grapes collected from Dão region were studied.The pro fi les of phenolic compounds and organic acids were estimated by high-performance liquid chromatography w ith diode-array detection and high-performance liquid chromatography w ith UV detector,respectively.
Resu lts:Totally 24 phenolic compounds were identi fi ed,and distributed by several classes:8 anthocyanins,1 hydroxybenzoic acid,4 hydroxycinnam ic acids,1 stilbene,4 fl avan-3-ols,6 fl avonols.Additionally,10 organic acidsweredetected inallsamples.Total contents of each phenolic classand organic acids amounts varied signi fi cantly among the differentgrape cultivars investigated.The principal componentsanalysisdifferentiates the Touriga Nacional from the other varieties due to their high contents in anthocyanins, non-coloured phenolics and organic acids.Touriga Nacional is an important red grape cultivar,highly esteemed in Dão region for itsability to produce high qualityw ines.
Conclusions:The results suggest that the red grapes from Dão region present a good composition in bioactive compounds,being important for the production of w inesw ith superior quality.
1.Introduction
The tradition of w ine production in Portugal dates back to centuries of history,w ith enviable potential for producing high quality wines.This country is responsible for producing of the best w ines in the world,being the fi rst to have a demarcated region(region of Douro,where it is produced the Portw ine), which ensures the production of genuine w ines originated in a particular region.Dão is a unique region w ith important viticulture traditions,located in north central Portugal,from which the excellent edapho-climatic conditions are turned to advantage for vineyard culture,w hich corresponds to 20000 ha.The Dão region presents a temperate climate,although cold and rainy in w inter and frequently is very hot in summer.Dão w ines w ith Denom ination d’Origine Contrˆol´ee arise from vineyards established in granite land,between 400 and 500m altitude.
Grapes from Vitis vinifera L.belong to the world's largest fruit crops,being a variety known for the bestquality w ines[1]. Grapes and products derived from them constitute an importantfactor worldwide. Viticulture is one of the activities with the highest impact in the Portuguese economy,representing about 15% of all agricultural production, and placing Portugal among the largest producers of wine worldwide.
Vitis vinifera Grapes
Dão region
Phenolics
2221-1691/Copyright©2016 Hainan Medical University.Production and hosting by Elsevier B.V.This is an open accessarticle under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
Grape ripening is a physiological period that in fl uences the composition of the grapes and determ ines varietal characteristics,which have in fl uence on the future ofw ine quality.Grapes undergo many changes during the ripening process which involves a number of physical and biochemicalmodi fi cations, like weight,volume,rigidity,sugar,acidity,colour and aroma [2].The optimum level of harvesting can be determ ined by the level of soluble solids,berry weight,titratable acidity,as well as full fl avour characteristics[2].To harvest the grapes at ideal maturity,it is necessary to investigate their pro fi le and composition in phenolics and organic acids in the fi eld throughoutmaturation.
Chem ical composition is one of themost quality criteria for fruit products.The grapes content in phenolic compounds and organic acids is of great importance for the organoleptic characteristics of grapes and w ines,being related w ith the degree of grape ripening[2,3].
Grapes are a rich source of phenolic compounds(mainly in skin and seed),which play an important role in oenology due to their in fl uence on some important sensory properties of grapes and w ines,such as colour,stability,bitterness and astringency [1,4].Due to their antioxidant and anti-in fl ammatory properties, phenolic compounds are associated w ith several bene fi cial physiological effects that are derived from moderate w ine consumption[5].The study of phenolic composition of grapesmay allow theestablishmentof oneormore biomarkers speci fi c for a particularly type of grapes,allow ing to assess their chem ical evolution during grow th andmaturation[1,5–7].
The most abundant non-coloured phenolics in skin are fl avonols,while fl avan-3-ols monomer such as(+)-catechin and (−)-epicatechin,as well as dimers,trimmers and polymeric forms,also called procyanidins(2–10 units),are presentmainly in grape seeds.These compoundsmay contain subunits of gallic acid,epigallocatechin of epicatechin gallate linked by an interfl avan bond[4,5,8–10].
Anthocyanins are themain pigments of red grapes located in skins and appearedmanly during the ripening,which aremainly responsible for the colour of red w ine[1,10].The major anthocyanins found in grapes are derived from cyanidin, peonidin,delphinidin,petunidin and malvidin,and they generally occuras glycosidesand acylglycosides;malvidin-3-O-glucoside is themostabundant[5,6,10,11].
The organic acids composition of grapes and w ines is important,because they have in fl uence on the organoleptic properties(fl avor,colour and aroma)and on the stability and m icrobiological control of the products.Tartaric and malic acids are the predom inantorganic acids in grapes juices,on the other hand,succinic and citric acids are present in m inor proportion[6,12].
Therefore,in this work we aimed to contribute to the know ledge of themetabolite pro fi le of themain red grapes from Dão region(Portugal),and determ ine simultaneously their non-coloured and coloured phenolicsand organic acids contents. The phenolic compoundswere determ ined by high-performance liquid chromatography w ith diode-array detection(HPLC/DAD) and organic acids by high-performance liquid chromatography w ith ultraviolet detection(HPLC/UV).Then,the principal components analysis(PCA)was used to analyze the results previously obtained.
2.Materials and methods
2.1.Standards and reagents
A ll chem icals used were of analytical grade.The standard compounds were purchased from various suppliers:oxalic, aconitic,citric,ketoglutaric,tartaric,malic,quinic,succinic, shikim ic,fumaric,cafeic,p-coumaric and ferulic acids were from Sigma–A ldrich(St.Louis,MO,USA).Delphinidin-3-O-glucoside,cyanidin-3-O-glucoside,petunidin-3-O-glucoside, peonidin-3-O-glucoside,malvidin-3-O-glucoside were from Extrasynth`ese(Genay,France).trans-Caffeoyltartaric acid (t-CAFTA)and trans-p-coumaroyltartaric acid(t-COUTA)were kindly supplied by Dr.C.Garcia-Viguera(CEBAS-CSIC, M urcia,Spain).Epigallocatechin,catechin,epicatechin, epigallocatechin gallate,epicatechin gallate,resveratrol-3-O-glucoside,quercetin-3-O-galactoside,quercetin-3-O-rutinoside, quercetin-3-O-glucoside,laricitrin-3-O-glucoside,isorhamnetin-3-O-glucoside and syringetin-3-O-glucoside were from Extrasynth`ese(Genay,France);gallic acid was from Fluka(Buchs, Sw itzerland).Water was deionized using a M illi-Q water purifi cation system(M illipore,Bedford,MA,USA).
2.2.Grape samples
Five samples of fi ve red grapes varieties from Dão region (Portugal)were harvested during September of 2012,in Quinta das Cam´elias,located in Sabugosa:Tondela(Portugal).The varieties under study were:“Jaen”,“Touriga Nacional”,“A lfrocheiro”,“Tinta Roriz”and“Syrah”.A fter harvested,the grapes were preserved at−20°C and dried in a lyophilizer apparatus(Labconco 4.5,Kansas City,MO,USA).
2.3.Phenolic compounds
2.3.1.Extraction
The non-coloured and coloured phenolic compounds were extracted according to the procedure described by Oszm ianski and Lee[13].Aliquotsof5 g of powder samplewereweighed and extracted w ith 100m L ofM eOH(80%)along 2 h under stirring after fl ushing w ith nitrogen to avoid oxidations.Then,the extract was centrifuged for 10 m in at 4000 r/m in.Continuing thematerialwasagain extracted during 15min w ith 100m L of M eOH(80%).The both supernatants were evaporated to dryness under reduced pressure at 30°C.The resultant extract was dissolved w ith 50 m L of deionised water and placed into the column.The solid-phase extraction cartridge was preconditionedw ith20m Lofethylacetate,20m L ofmethanoland 20m L of 0.01mol/L HCl.A fter passage of the sample,the column was washed w ith 3 m L of 0.01 mol/L HCl.Then,the fraction I, designed by non-coloured phenolicswas eluted w ith 20m L of ethyl acetate.The fraction II,designed by anthocyanins was eluted w ith 40m L ofmethanol containing 0.1%HCl.The fractions Iand IIwere evaporated under reduced pressure,and the dried extractsobtainedwere re-dissolved w ith 1m L ofmethanol (non-coloured phenolics)and in 20m L ofacidi fi edwater,pH 3.0 (anthocyanins),using amembrane-fi ltered(0.45μm).
2.3.2.HPLC-DAD analysis
The extracts were analyzed on an analytical HPLC unit (Gilson),using a Spherisorb ODS2 column(25.0 cm×0.46 cm; 5μm particle size waters,M ilford,MA,USA).
2.3.2.1.Anthocyanins
The conditionsdescribed by Kammerer etal.were employed [14].Themobile phase consisted ofwater/form ic acid/acetonitrile (87:10:3,v/v/v;eluent A)and water/form ic acid/acetonitrile (40:10:50,v/v/v;eluentB)using a gradientprogram as follows: from 10%to 25%B(10 m in),from 25%to 31%B(5 m in), from 31%to 40%(5m in),from 40%to 50%B(10m in),from 50%to 100%B(10m in),from 100%to 10%B(5m in).Total run time was 50m in.Flow ratewas 0.8m L/m in.The injection volumewas 20μL.Detection wasachieved w ith a Gilson diode array(DAD)detector.The compounds in each sample were identi fi ed by comparing their retention times and UV–Vis spectra in the 200–600 nm range w ith the library of spectra previously compiled by the authors.Peak purity was checked by means of the Gilson 160 SpectraViewer Software Contrast Facilities.Anthocyanin quanti fi cation was achieved by the absorbance recorded in the chromatograms relative to external standards.Anthocyanins quanti fi cation was achieved using a calibration plot of external standard at 500 nm.Malvidin-3-O-p-coumaroylglucoside and petunidin-3-O-p-coumaroylglucoside were quanti fi ed as malvidin-3-O-glucoside.Additionally,the peonidin-O-p-coumaroylglucoside was quanti fi ed as peonidin-3-O-glucoside.
2.3.2.2.Non-coloured phenolics
Themethod for quanti fi cation of the non-coloured phenolic was previously described by Dopico-García et al.[6].The mobile phase used is composed by 2%(v/v)acetic acid in water(eluent A)and 0.5%(v/v)acetic acid in water and acetonitrile(50:50,v/v,eluent B).The solvent system starting w ith 10%of B,and installing a gradient to obtain(24%B at 20 m in,30%B at 40 m in,55%B at 60 m in,70%B 65 m in, 80%B at 70 m in),100%B at 75 m in,and maintain 100%B isocratic during 5 m in(80 m in).A solvent fl ow rate was 1.0 m L/m in.The injection volume was 20μL.Detection was achieved w ith a Gilson diode array detector(DAD).Spectral data from all peaks were accumulated in the range of 200–400 nm and chromatograms were recorded at 280,320 and 350 nm.The data were processed on Unipoint System Software(Gilson Medical Electronics,Villiers le Bel,France). Peak purity was checked by the software contrast facilities. Phenolic compounds quanti fi cation was achieved by the absorbance recorded in the chromatograms relative to external standards.The quanti fi cation of phenolic compounds was archived by the absorbance recorded in the chromatogram s relative to external standard at 350 nm for quercetin-3-O-galactoside,quercetin-3-O-rutinoside,quercetin-3-O-glucoside,myricetin-3-O-rhamnoside and isorhamnetin-3-O-glucoside,at 320 nm for p-coumaric acid,caffeic acid,ferulic acid and resveratrol-3-O-glucoside.280 nm was used for gallic acid,epigallocatechin,catechin,epicatechin, epigallocatechin gallate and epicatechin gallate.As the available amounts of t-CAFTA and t-COUTA standards were not enough,these compounds were quanti fi ed as caffeic and p-coumaric acids,respectively.Syringetin-3-O-glucoside and laricitrin-3-O-glucoside were quanti fi ed as myricetin-3-O-rhamnoside.
2.4.Organic acids
2.4.1.Extraction
Organic acids extraction was performed according to a described procedure[3].A total of 0.25 g of dried grapeswere m ixed w ith 50 m L of H2SO40.005 mol/L under stirring (300 r/m in)for 30 m in.The extracts were fi ltered and then passed through a C18 solid-phase extraction column(70mL/ 10000 mg;Macherey–Nagel),previously conditioned w ith 30m L ofmethanol and 70m L of acid water(pH 2 w ith HCl). The aqueous extract,containing the organic acids,was evaporated until dryness under reduced pressure(30°C)and redissolved in 0.005 mol/L H2SO4(1 m L)for HPLC/UV analysis (20μL).
2.4.2.HPLC/UV analysis
The separation and quanti fi cation of organic acids were carried outaccording to a procedure described by Silva etal.[15] in analytical HPLC unit(Gilson),using an ion exclusion Nucleogel Ion 300 OA(300 mm×7.7 mm)(Germany) column,in conjunction w ith a column heating device set at 30°C.Detection was performed w ith a Gilson UV–vis detector at 214 nm.Organic acids quanti fi cation was achieved by measuring the absorbance recorded in the chromatograms relative to external standards.This procedure was performed in triplicate.
2.5.Statistical analysis
A ll data were recorded as mean±SD of triplicate determ inations.Mean values were compared using Two-way ANOVA and Bonferroni test,as post-hoc test,was used to determ ine differences w ith statistical signi fi cance.Differences were considered signi fi cant for P<0.05.Statisticalanalysiswas carried out using Graphpad Prism 5 Software(San Diego,CA, USA).PCA was carried out using XLSTAT 2013.5 software. The PCA method shows sim ilarities between samples projected on a plane and makes it possible to identify which variables determine these similarities and in whatway.
3.Resu lts
3.1.Anthocyanins
The analysis of red grapes from Dão region(Portugal)by HPLC/DAD allowed the identi fi cation of eight anthocyanins: delphinidin-3-O-glucoside,cyanidin-3-O-glucoside,petunidin-3-O-glucoside,peonidin-3-O-glucoside,malvidin-3-O-glucoside, petunidin-3-O-p-coumaroyl-glucoside,peonidin-3-O-p-coumaroyl-glucoside,malvidin-3-O-p-coumaroyl-glucoside(Table 1 and Figure 1).A ll compoundswere previously described in the red grapes of Touriga Nacional and Syrah[10,11,16–18]. Delphinidin-3-O-glucoside,cyanidin-3-O-glucosideandmalvidin-3-O-glucosidewere previously described in Jaen and A lfrocheiro varieties from Dão region[18],being the other anthocyanins described herein for the fi rst time in Jaen,A lfrocheiro and Tinta Roriz grape varieties.
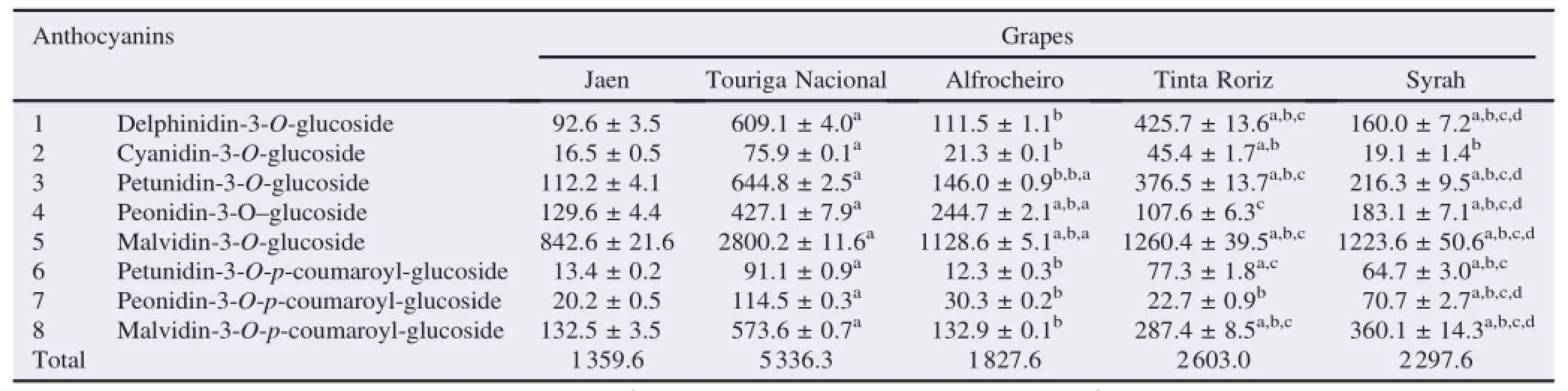
Table1 Anthocyanin contents of red“Dão”grape sam ples(mg/kg of lyophilized sample).
Figure1.HPLC/DAD anthocyaninspro fi leof TourigaNacional“Dão”red grapes from Quinta das Cam´elias,harvested in 2012(detected at 500 nm).
Despite the difference observed in the contents of each anthocyanin,all theanalysed samplesexhibited a sim ilar pro fi le, w ith amounts ranging between 1359.6 and 5336.3 mg/kg of lyophilized grapes(Table 1).Jaen was the poorest variety and Touriga Nacional showed remarkable higher amounts,which was at least more than tw ice higher than the other analised varieties(Table 1).This fact can be visualized by the colour of the skins and themust produced by maceration of these grapes.
3.2.Non-coloured phenolics
The analysis by HPLC/DAD allowed the identi fi cation and quanti fi cation of 16 non-coloured phenolics:one hydroxybenzoic acid(1),four hydroxycinnam ic acids(2,3,6,8),one stilbene(10),four fl avan-3-ols(4,5,7,9)and six fl avonols (11–16)(Table 2 and Figure 2).
A ll of these compounds were previously reported in black grapes[6,9].Nevertheless,the fi ve grape varieties showed different qualitative and quantitative composition(Table 2). From the identi fi ed compounds,epigallocatechin gallatewas not detected in Jaen;whereas epicatechin was not found in Touriga Nacional.The ferulic acidwasabsent in Tinta Roriz,A lfrocheiro and Syrah.Finally,isorhamnetin-3-O-glucoside was not found in A lfrocheiro and Syrah(Table 2).
The phenolic contents of the fi ve analysed grape varieties ranged between 343.8 and 1328.3 mg/kg of lyophilized material,being Touriga Nacional the richest one.Jaen presented the lowestamounts in non-coloured phenolic compounds(Table 2).
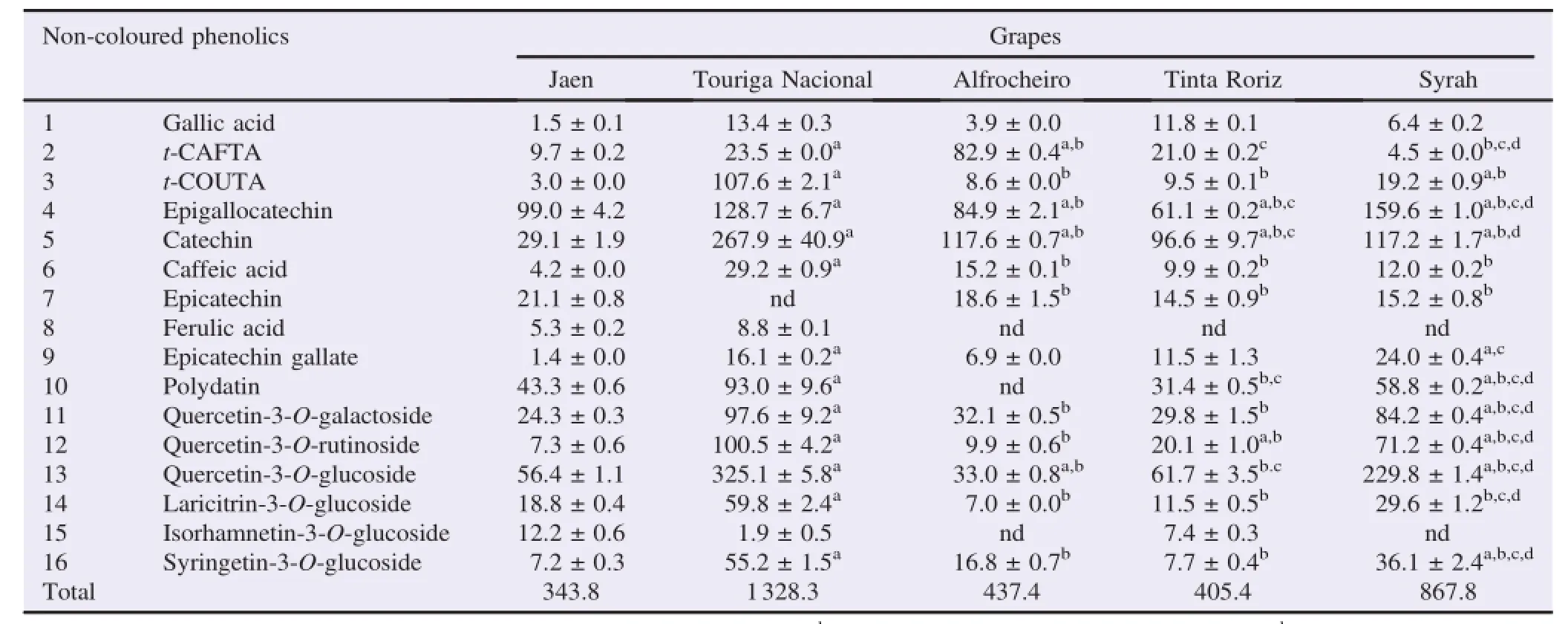
Table2 Non-coloured phenolic composition of red“Dão”grape sam ples(mg/kg of lyophilized sample).

Figure 2.HPLC/DAD non-coloured phenolics pro fi le of TourigaNacional“Dão”red grapes from Quinta das Cam´elias,harvested in 2012.A:Detected at 280 nm;B:Detected at 350 nm.
3.3.Organic acids
The HPLC/UV analysis of red grapes extracts revealed a pro fi le composed by 10 identi fi ed organic acids:oxalic,aconitic, ketoglutaric,citric,tartaric,malic,quinic,succinic,shikim ic and fumaric acids(Table 3).The total amounts of organic acids in the analysed samples ranged from ca.20.5 to 69.0 g/kg of lyophilized grape samples,Touriga Nacional being the variety that showed high levels(Table 3).
3.4.PCA analysis
A ll data of anthocyanins,non-coloured phenolic compounds and organic acids were analysed by PCA,being expressed as mass by weight of lyophilized grapes.Figure 3 shows the projection of variables,grouped by chemical classes obtained for the fi ve grape varieties in the plane composed by principal axes F1 and F2,containing 85.1%of the total variance(Figure 3).

Figure 3.PCA of all phenolic compounds and organic acids in red“Dão”grapes.
4.Discussion
Grapes including anumberof polyphenolic constituentshave shown interesting biological properties,related to their antioxidant capacities[1,5,11,16].Among them,anthocyanins,which are extracted from the skins of black grapes,are natural colorants which have aroused grow ing interest of researchers due their extensive range colour,innocuous and bene fi cial health.
The results obtained for anthocyanins revealed that the malvidin-3-O-glucoside was the major compound representing ca.48%–62%of total compounds.These results are in accordance w ith previous works,that reported malvidin-3-O-glucoside as themain compound in black grapes[6,9,16,19]and in Dão red wines[10].Anthocyanins are characterized by antioxidant potential,anti-in fl ammatory activity and their cellular signaling activity[5].
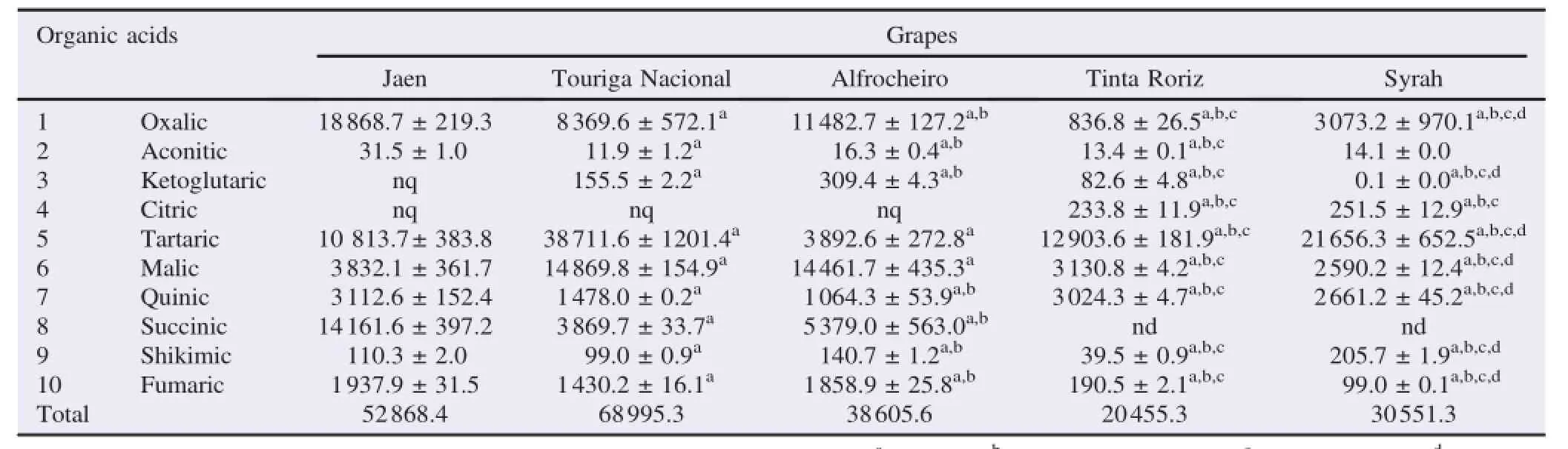
Table3 Organic acids composition of red“Dão”grape samples(mg/kg of lyophilized sample).
In respect to non-coloured phenolic,gallic acid was the only hydroxybenzoic acid found in allanalysed grapes(Table2).It is the only benzoic acid that has been formally identi fi ed native state in grapes,found in the solid parts of theberry,either in free form of fl avanol ester(i.e.epicatechin-3-O-gallate)[5].
Regarding the hydroxycinnam ic acids,they corresponded to ca.4.1%–24.4%of the total of non-coloured phenolic compounds in the analysed grape varieties,t-CAFTA being the major one in Jaen,A lfrocheiro and Tinta Roriz.Additionally,t-COUTA was the biggest one in Touriga Nacional and Syrah (Table 2).The hydroxycinnam ic acids are located in the vacuoles of the skin and pulp cells,essentially in the form of tartaric esters[5].
One stilbene compound,polydatin(resveratrol-3-O-glucoside)was identi fi ed in all samples,except in A lfrocheiro (Table 2).The highest level in polydatin was found in Touriga Nacional(93.0 mg/kg of determ ined phenolics),followed by Syrah,Jaen and Tinta Roriz(58.8,43.3 and 31.4 mg/kg of determ ined phenolics,respectively).Stilbenes are phytoalexins synthesized by plant,especially in skins,leaves and roots in response to fungal infectionsand UV light,being the grapesand their derived products considered as themost important dietary sources[5,20].Resveratrol and their derivatives have been reported once that could prevent some kinds of cancer, cardioprotective and neuroprotective effects,protection against obesity,among others[20].
In respect to fl avonoids(fl avan-3-ols and fl avonols),they showed percentages that ranged from 74.4%to 88.4%.Epigallocatechin was themostabundant in Jaen and Syrah(28.8% and 18.4%of determ ined phenolics,respectively).Quercetin-3-O-glucoside was themajor one in Touriga Nacional(24.5%of determ ined phenolics),and catechin showed higher amounts in Alfrocheiro and Tinta Roriz(26.9%and 23.8%of the determ ined phenolics,respectively)(Table 2).Flavonols are secondarymetabolites present in almostall higher plants.They are considered to act as UV-and photo-protectors because they absorb strongly at both UV-A and UV-B wavelengths.A lso, fl avonols playing an important role in w ine copigmentation together w ith anthocyanins,are usefulmarkers in grape taxonomy,and are considered bioactive grape/w ine compounds of possible importance for human health and nutrition[5].
The nature and concentration of organic acids are important factors in fl uencing the organoleptic characteristics of fruit and vegetables.Acids are known to have a lower susceptibility to change during processing and storage than other compounds, such as pigments and fl avour components[21].They are primary metabolites,which can be found in great amounts in all plant materials.As phenolics,this class of compounds may also have a protective role against various diseases due to their antioxidant properties[22].
Oxalic acid was themost abundant in Jaen(35.7%).Oxalic acid is an organic acid present in various organisms,has shown some antioxidantactivities and thus could play an important role in system ic resistance,programmed cell death,redox homeostasis in plants,and an anti-senescence effect in harvested fruits [23].
Additionally,tartaric acidwas themain compound in Touriga Nacional,Tinta Roriz and Syrah(56.1%,63.1%and 70.9%, respectively)and malic acid was themain one in A lfrocheiro (37.5%)(Table 3).Tartaric and malic acids are themost abundant organic acids in grapes and w ines.The levels in which these acidsare presentare related to the chem icaland biological stability ofw ines.Their levels in grapes are the data frequently used to determ ine the harvesting date,particularly since each acid presents a different behaviour during grape ripening process.Malic acid shows a continuous decrease during ripening whereas tartaric acid remains almost unchanged.Therefore, different ratios can beobtained during ripening and the optimum harvest date can be established from their ratio[3,24].
The PCA analysis showed that the Touriga Nacional is clearly separated from the other varieties,being projected into the plane formed by F1 positive and F2 neutral axis due to their high concentration in phenolic compounds(anthocyanins and non-coloured)and organic acids.
Tinta Roriz and Syrah are presented into the planes formed by F1 negative and F2 positive axis.On the other hand,A lfrocheiro and Jaenwere projected into the planes formed by F1 and F2 negative axis(Figure 3).This different position between the last both groups is due the high amounts in hydroxybenzoic acids,fl avonols and anthocyanins in Tinta Roriz and Syrah (Tables 1 and 2).Otherw ise,A lfrocheiro and Jaen are richer in organic acids(Table 3).These resultsagreew ith those observed in other studies,which indicate that it is possible to differentiate between grape varietiesw ith PCA of their chem ical constituents [6,25].
Despite the anthocyanin pro fi le revealed to bemore suited to characterize the red“Dão”grape varieties,our results revealed some relationships among the several varieties and their composition in anthocyanins and non-coloured phenolic compounds.This is very interesting thatonce thequality and typical characteristics of red w ine are greatly dependent on the composition of the grapes in these bioactive compounds,they have in fl uence on astringency,color,fl avour,among others.
In summary,the non-coloured phenolics,anthocyanins and organic acids pro fi leswere performed in fi ve red varieties from“Dão”region(Portugal).Malvidin-3-O-glucoside was themost abundant anthocyanin.Concerning non-coloured phenolics, catechin and epigallocatechin were the main fl avan-3-ols and quercetin-3-O-glucoside proved to be the fl avonol found in higher amounts.In respect to organic acids,in general way, tartaric and malic acidswere themain ones.
The PCA analysis revealed in more detail the relationship among the several red grapes and their chem ical composition to perform the characterization of the evaluated varieties taking into account to their contents in hydroxybenzoic and hydroxycinnam ic acids,stilbenes,fl avan-3-ols,fl avonols,anthocyanins and organic acids.Touriga Nacional proved to be distinctly different for the other varieties due their high contents in all chem ical classes.These results justify the reason why this variety is themost important red grape cultivar in Dão region for its ability to produce high quality w ines,being responsible for the prestige that Dão w ines have acquired over the years.
Con fl ict of interest statement
We declare thatwe have no con fl ict of interest.
Acknow ledgments
We are grateful to the fi nancial support from the European Union(FEDER funds through COMPETE)and National Funds (FCT,Fundação para a Ciˆencia e Tecnologia)through project Pest-C/EQB/LA0006/2013 and from the European Union (FEDER funds)under the framework of QREN through Project NORTE-07-0124-FEDER-000069.Luís R.Silva acknow ledges FCT the fi nancial support for the Post-doc grant(SFRH/BPD/ 105263/2014).
References
[1]Fraige K,Pereira-Filho ER,Carrilh E.Fingerprinting of anthocyanins from grapes produced in Brazil using HPLC–DAD–MS andexploratory analysis by principal com ponentanalysis.Food Chem 2014;145:395-403.
[2]Yang C,Wang Y,W u B,Fang J,Li S.Volatile compounds evolution of three table grapes w ith different fl avour during and after maturation.Food Chem 2011;128:823-30.
[3]Dopico-García MS,Valentão P,Guerra L,Andrade PB, Seabra RM.Experimental design for extraction and quanti fi cation of phenolic compounds and organic acids in white“Vinho Verde”grapes.Anal Chim Acta 2007;583:15-22.
[4]Di Lecce G,Arranz S,J´auregui O,Tresserra-Rimbau A,Quifer-Rada P,Lamuela-Ravent´os RM.Phenolic pro fi ling of the skin, pulp and seeds of Albariño grapes using hybrid quadrupole timeof-fl ight and triple-quadrupole mass spectrometry.Food Chem 2014;145:874-82.
[5]Flamini R,M attivi F,De Rosso M,A rapitsas P,Bavaresco L. Advanced know ledge of three importantclassesof grape phenolics: anthocyanins,stilbenes and fl avonols.Int J Mol Sci 2013;14: 19651-69.
[6]Dopico-García MS,Fique A,Guerra L,Afonso JM,Pereira O, Valentão P,etal.Principal componentsof phenolics to characterize red Vinho Verde grapes:anthocyanins or non-coloured compounds?Talanta 2008;75:1190-202.
[7]Garrido J,Borges F.W ine and grape polyphenols–a chem ical perspective.Food Res Int 2013;54:1844-58.
[8]Costa R,Silva LR.Health bene fi ts of nongallated and gallated fl avan-3-ols:a prospectus.In:Kinsey AL,editor.Recentadvances in gallate research.New York:Nova Science Publishers,Inc.; 2014,p.1-50.
[9]Silva LR,Andrade PB,Valentão P,Seabra RM,Trujillo ME, Vel´azquez E.Analysis of non-coloured phenolics in red w ine:effect of Dekkera bruxellensis yeast.Food Chem 2005;89:185-9.
[10]Valentão P,Seabra RM,Lopes G,Silva LR,M artins V, Trujillo ME,et al.In fl uence of Dekkera bruxellensis on the contentsofanthocyanins,organic acidsand volatilephenolsof Dão red w ine.Food Chem 2007;100:64-70.
[11]Ferreira V,Fernandes F,Pinto-Carnide O,Valentão P,Falco V, Martín JP,etal.Identi fi cationof Vitisvinifera L.grapeberry skincolor mutantsand polyphenolic pro fi le.Food Chem 2016;194:117-27.
[12]M ato I,Su´arez-Luque S,Huidobro JF.Simple determ ination of main organic acids in grape juice and w ine by using capillary zone electrophoresis w ith direct UV detection.Food Chem 2007;102: 104-12.
[13]Oszm ianski J,Lee CY.Isolation and HPLC determ ination of phenolic com poundsin red grapes.Am JEnolVitic 1990;41:204-6.
[14]Kammerer D,Claus A,Carle R,Schieber A.Polyphenolscreening of pomace from red and white grape varieties(Vitis vinifera L.)by HPLC-DAD-MS/MS.JAgric Food Chem 2004;52:4360-7.
[15]Silva LR,Pereira MJ,Azevedo J,Gonçalves RF,Valentão P,de Pinho PG,et al.Glycine max(L.)Merr.,Vigna radiata L.and Medicago sativa L.sprouts:a natural source of bioactive compounds.Food Res Int2013;50:167-75.
[16]Mateus N,M achado JM,de Freitas V.Development changes of anthocyanins in Vitis vinifera grapes grown in the Douro Valley and concentration in respectivew ines.JSci Food Agric 2002;82: 1689-95.
[17]Vian MA,Tomao V,Coulomb PO,Lacombe JM,Dangles O. Com parison of the anthocyanin composition during ripening of Syrah grapes grown using organic or conventional agricultural practices.J Agric Food Chem 2006;54:5230-5.
[18]Jordão AM,Correia AC.Relationship between antioxidant capacity,proanthocyanidin and anthocyanin content during grape maturation of Touriga Nacional and Tinta Roriz grape varieties. SAfr JEnol Vitic 2012;33:214-24.
[19]Alcalde-Eon C,García-Est´evez I,M artín-Baz A,Rivas-Gonzalo JC,Escribano-Bail´on MT.Anthocyanin and fl avonol pro fi les of Vitis vinifera L.cv Rufete grapes.Biochem Syst Ecol 2014;53:76-80.
[20]Rotches-Ribalta M,Andres-Lacueva C,Estruch R,Escribano E, Urpi-Sarda M.Pharmacokinetics of resveratrolmetabolic pro fi le in healthy humansaftermoderate consum ption of red w ine and grape extract tablets.Pharmacol Res 2012;66:375-82.
[21]C´amara MM,Díez C,Torija ME,Cano MP.HPLC determination of organic acids in pineapple juices and nectars.Z Leb Unters Forsch 1994;198:52-6.
[22]Couto C,Silva LR,Valentão P,Vel´azquez E,Peix A,Andrade PB. Effects induced by the nodulation w ith Bradyrhizobium japonicum on Glycine max(soybean)metabolism and antioxidant potential. Food Chem 2011;127:1487-95.
[23]Huang H,Jing G,Guo L,Zhang D,Yang B,Duan X,et al.Effect of oxalic acid on ripening attributes of banana fruitduring storage. Postharvest Biol Technol 2013;84:22-7.
[24]Liang Z,Sang M,Fan P,Wu B,Wang L,Duan W,etal.Changes of polyphenols,sugars,and organic acid in 5 Vitis genotypes during berry ripening.JFood Sci2011;76(9):C1231-8.
[25]Burin VM,Ferreira-Lima NE,Panceri CP,Bordignon-Luiz MT. Bioactive compounds and antioxidantactivity of Vitis vinifera and Vitis labrusca grapes:evaluation of different extraction methods. Microchem J 2014;114:155-63.
30 Sep 2015
*Corresponding author:Luís Rodrigues Silva,Health Sciences Research Centre Faculty of Health Sciences,University of Beira Interior,Avenue InfanteD.Henrique, Covilhã6201-506,Portugal.
Tel:+351 272 340 560
Fax:+351 272 340 568
E-mail:lmsilva@ff.up.pt
Foundation Project:Supported by the European Union(FEDER funds through COMPETE)and National Funds(FCT,Fundação para a Ciˆencia e Tecno logia) through project Pest-C/EQB/LA0006/2013,the European Union(FEDER funds) under the framew ork o f QREN through Pro ject NORTE-07-0124-FEDER-000069, and FCT the fi nancial support for the Post-doc grant(SFRH/BPD/105263/2014).
Peer review under responsibility of Hainan M edical University.The journal implements double-blind peer review practiced by specially invited international editorial boardmembers.
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- Sudden death in a captive mee rkat(Suricata surica tta)w ith arterial m edial and m yocardial calcification
- Characteristics o f obese or overweight dogs visiting private Japanese vete rinary c linics
- Risk factors from HBV infection among blood donors:A system atic review
- Com putational in telligence in tropicalm edicine
- The African Moringa is to change the lives ofm illions in Ethiopia and far beyond
- Pediculosis capitis among p rimary and m idd le school children in Asadabad,Iran:An epidem iological study
