In vitro antibacterialactivity and major bioactive com ponents of Cinnamom um verum essentialoils against cariogenic bacteria,Streptococcus m utans and Streptococcus sobrinus
2016-09-07OkheeChoiSuKyungChoJunheonKimChungGyooParkJinwooKimDivisionofAppliedLifeScienceBKPlusGyeongsangNationalUniversityJinju588SouthKoreaInstituteofAgricultureLifeScienceGyeongsangNationalUniversityJinju588SouthKorea
Okhee Choi,Su Kyung Cho,Junheon Kim,Chung Gyoo Park,Jinwoo Kim,*Division of Applied Life Science(BK Plus),Gyeongsang National University,Jinju 588,South KoreaInstitute of Agriculture&Life Science,Gyeongsang National University,Jinju 588,South Korea
In vitro antibacterialactivity and major bioactive com ponents of Cinnamom um verum essentialoils against cariogenic bacteria,Streptococcus m utans and Streptococcus sobrinus
Okhee Choi1,#,Su Kyung Cho1,#,Junheon Kim2,#,Chung Gyoo Park1,Jinwoo Kim1,2*1Division of Applied Life Science(BK21 Plus),Gyeongsang National University,Jinju 52828,South Korea
2Institute of Agriculture&Life Science,Gyeongsang National University,Jinju 52828,South Korea
Original article http://dx.doi.org/10.1016/j.apjtb.2016.01.007
ARTICLE INFO
Article history:
Received in revised form 28Oct,2nd revised form 2 Nov 2015
Accepted 4Nov 2015
Availableonline 16 Feb 2016
Streptococcusmutans Streptococcus sobrinus Cariogenic bacteria
Essential oil
Antibacterial activity
Cinnamaldehyde
ABSTRACT
Ob jective:To evaluate theantibacterialactivity of Cinnamomum verum(C.verum)from 32 different essential oils against cariogenic bacteria,Streptococcusmutans(S.mutans) and Streptococcus sobrinus(S.sobrinus).
M ethods:The antibacterialactivities of each essential oilwere individually investigated against S.mutans and S.sobrinus.The essential oil of C.verum was selected for further evaluation against S.mutans and S.sobrinus.Gas chromatography mass spectrometry was used to determ ine themajor constituents of C.verum essential oil.In addition,the m inimum inhibitory concentration(M IC)and m inimum bactericidal concentration of the most effective constituentwas investigated.
Resu lts:The essentialoil from C.verum exhibited thegreatestantibacterialactivity.Gas chromatography mass spectrometry analysis revealed that the major components of C.verum essential oil were cinnamaldehyde(56.3%),cinnamyl acetate(7.1%)andβphellandrene(6.3%).The M IC of cinnamaldehyde was measured using broth dilution assays.TheM IC of cinnamaldehydewas0.02%(v/v)againstboth bacterialstrains tested. The m inimum bactericidal concentration of cinnamaldehyde against S.mutans and S.sobrinus were 0.2%and 0.1%(v/v),respectively.
Conclusions:The essential oil of C.verum and itsmajor component cinnamaldehyde possessed considerable in vitro antibacterial activities against cariogenic bacteria, S.mutans and S.sobrinus strains.These results showed that the essential oil of C.verum and its bioactive component,cinnamaldehyde,have potential for application as natural agents for the prevention and treatment of dental caries.
1.Introduction
Dental caries is a major oral infectious disease of bacterial origin.The tooth surface can be damaged due to the production of acid by bacterial fermentation of sugars such as sucrose, fructose and glucose in foods or drinks[1,2].The bacteria responsible for dental caries are the mutans streptococci including Streptococcus mutans(S.mutans),Streptococcus sobrinus(S.sobrinus),Streptococcus downei,Streptococcus rattus,and Streptococcus cricetus,but most prom inently S.mutans and S.sobrinus[3].S.mutans and S.sobrinus are the major bacteria associated w ith dental plaque bio fi lms,a deposit of proteins and cell-free enzymes.In addition,these bacteria are embedded in exopolysaccharides thatadhere fi rm ly to the tooth surface[4–7].
Treatment w ith antim icrobial agents for the prevention of dental caries has been investigated for over fi ve decades[8–10]. Fluoride has been used as a major agent for prevention of dental caries.Previous reports have shown that fl uoride has antibacterial activity againstmutans streptococci.In addition, fl uoride works by slow ing dem ineralization,which causes calcium and phosphate loss from the tooth enamel[11]. However,the clinical antibacterial effects of fl uoride againstmutans streptococcihave been questioned as the decrease in this disease has not always been correlated w ith a decline in the numbers of mutans streptococci[12,13].Moreover,large concentrations and frequent applications of fl uoride are required to reduce both the numbers of mutans streptococci and enamel dem ineralization[14].Chlorhexidine has broadspectrum antifungal and antibacterial activity[15].A lthough chlorhexidine has been used clinically formutans streptococci reduction in both saliva and dental plaque,its unpleasant taste and tooth staining have led to the search for appropriate alternatives[15].Among the alternative therapeutic agents, plant essential oils have become interesting candidates against mutans streptococci.A lthough over 2000 publications have addressed the antim icrobial activity of essential oils,few studies have evaluated the effectiveness of alternative or complementary treatments w ith essential oils against mutans streptococci.
The aim of the present study was to identify essential oils show ing antibacterial activity against cariogenic bacteria, S.mutans and S.sobrinus.Disk diffusion assaysshowed that the essential oil of Cinnamomum verum(C.verum)exhibited the greatest activity against both bacterial strains.Using gas chromatography mass spectrometry(GC-MS),we determ ined the major constituents of C.verum essential oil.In addition,the m inimum inhibitory concentration(M IC)and m inimum bactericidal concentration(MBC)of the most effective constituent was investigated.
2.M aterials and m ethods
2.1.Essential oils
Thirty-two essential oilswere purchased from HerbM all Co. Ltd.,(Seoul,Korea).Table 1 lists names of the essential oils used in this study,extractionmethods and plant parts extracted.
2.2.Bacterial strains and culture conditions
S.mutans KCOM 1054 and S.sobrinus KCOM 1061 were used in this study.The strains were obtained from the Korean Collection forOralM icrobiology(KCOM)and cultured in Todd-Hew itt(TH,Difco,Lab.,USA)broth or agar plates at 37°C.
2.3.Disk diffusion assay
A bacterial suspension was prepared from an overnightgrown culture,and adjusted to an optical density of 0.5 (600 nm)containing approximately 108CFU/m L.A sterile swab immersed in the bacterial suspension was used to spread the entire surface of a Todd-Hew itt agar plate.A total of 10μL of each essential oil or essential oil component was applied to a sterile paper disc aseptically placed on the center of the inoculated plates.After 36 h of incubation at 37°C,the diameter of the zone of grow th inhibition was measured in centimeters. Ampicillin was used asa positive control.A ll experimentswere carried out in triplicate.
The averageof inhibition diameterswas calculated to classify theessentialoilsas follows:the strainswere termed notsensitive (0)for a diameter smaller than 0.8 cm,moderately sensitive(+) fora 0.8–2.0 cm diameter,sensitive(++)fora 2–3 cm,and very sensitive(+++)for a diameter greater than 3 cm.
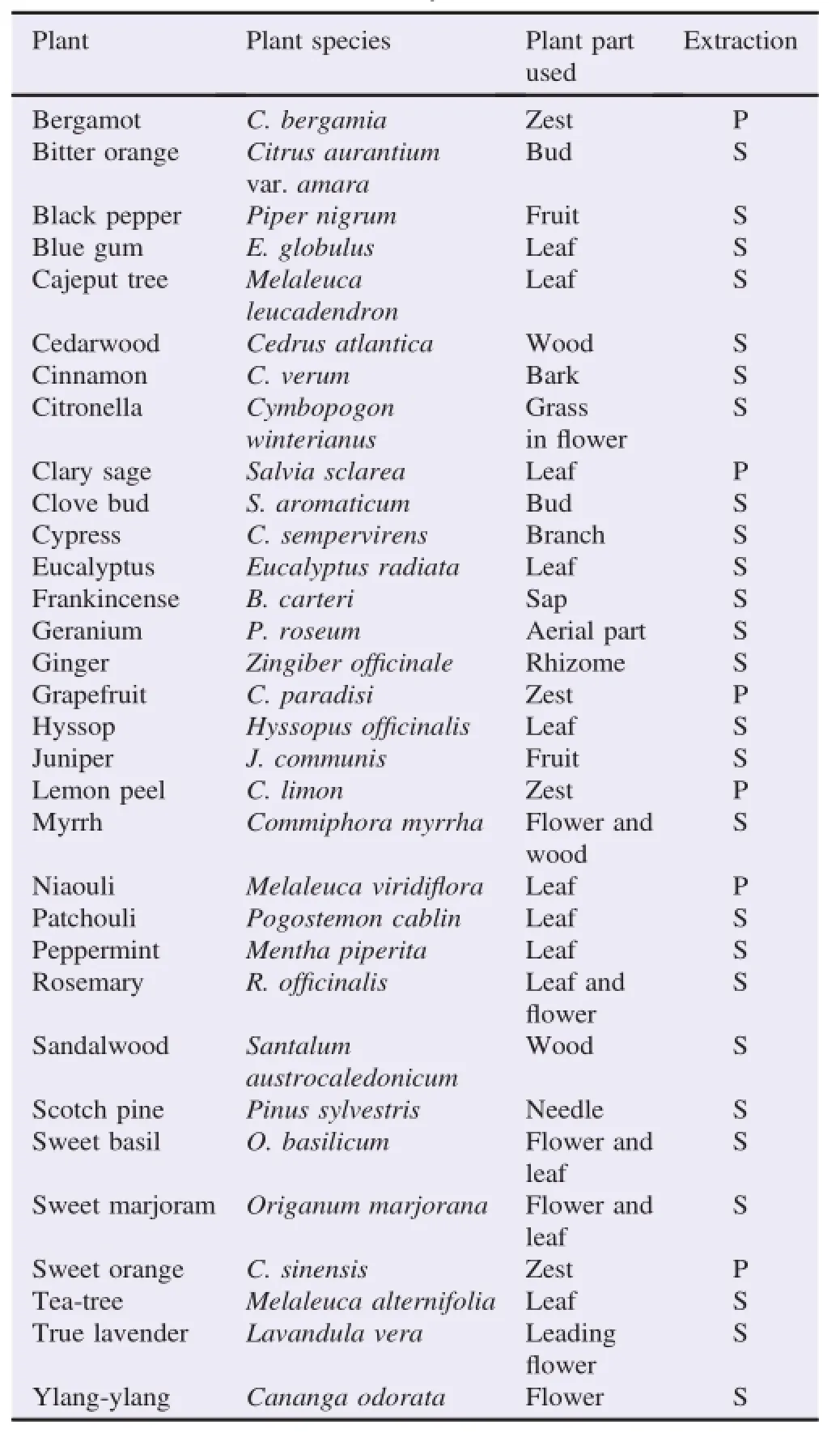
Table1 Listof essential oils used in this study.
2.4.Gas chromatography–fl ame ionization detector and GC-MS analyses and chemical synthesis
Theactive chem ical constituents of the C.verum essentialoil were determ ined using gas chromatography–fl ame ionization detector,a GC-17A(Shimadzu,Japan)fi tted w ith a DB-5MS [30mm×0.25 mm(inner diameter)×0.25μm,J&W Scienti fi c Co.,USA]non-polar column and GC-MS,a GC puls-2010 coupled w ith GCMS-QP2010(Shimadzu,Japan),which was fi tted w ith a HP-Innowax polar column[30 mm×0.25 mm (inner diameter)×0.25μm,J&W Scienti fi c Co.,USA].The temperature program started at40°C for 1m in and increased to 250°C at6°C/m in,and then held for 4m in.Split injection(1:5 ratio)was performed w ith 5 mg sample diluted w ith 1 m L of hexane.Themass detectorwas fi tted w ith an electron ionization source operated at 70 eV w ith a source temperature of 230°C.Helium was the carrier gas at a fl ow rate of 1 mL/m in.Identifi cation of essential oil compositions was based on the mass spectral information in amass spectra library(W iley Registry of Mass Spectra Data,2000),and sample peakswere con fi rmed by comparison w ith the retention indices(RI)and mass spectra of authentic standards.
For the identi fi cation of the essential oil components,αpinene,camphene,3-carene,α-terpinene,(+)-limonene, (−)-limonene,p-cymene,linalool,α-terpineol,andβ-caryophyllene oxide were purchased from A ldrich.(+)-β-Pinene, (−)-β-pinene,α-phellandrene,α-terpinolene,andα-humulene were purchased from Fluka.β-Caryophyllene and benzyl benzoate were purchased from TCI(Tokyo Chem ical Industry Co., Ltd.).Eugenol and cinnamyl alcoholwere purchased from A lfa Aesar.β-Phellandrenewas prepared as described previously[16].
Benzaldehyde,hydrocinnam ic aldehyde and cinnamaldehyde were synthesized from the corresponding alcohol by pyridinium chlorochromate oxidation[17].Hydrocinnamyl acetate and cinnamyl acetate were obtained by acetylation of the corresponding alcohol.Hydrocinnamyl alcoholwas synthesized by hydrogenation of cinnamyl alcoholw ith Pd on the carbon.

Figure1.Antibacterialeffectofessentialoilsagainstcariogenic bacteria S.mutans and S.sobrinus.Average inhibition diameterswere calculated to classify the essential oils as follow s:the tested strains were not sensitive for a diameter smaller than 0.8 cm(0),moderately sensitive for a 0.8–2 cm diameter(+), sensitive for a 2–3 cm(++),and very sensitive for a diameter larger than 3 cm(+++).BC:B.carteri;CO:Cananga odorata;CA:Cedrus atlantica;CV:C.verum;CAA:Citrus aurantium var.amara;CB:C.bergamia;CL:C.limon;CP: C.paradisi;CS:C.sinensis;CM:Commiphoramyrrha;CSE:C.sempervirens;CW:Cymbopogonwinterianus;EG:E.globulus;ER:Eucalyptus radiata; HO:Hyssopusof fi cinalis;JC:J.communis;LV:Lavandula vera;MA:Melaleuca alternifolia;ML:Melaleuca leucadendron;MV:Melaleuca viridi fl ora; MP:Mentha piperita;OB:O.basilicum;OM:Origanum marjorana;PR:P.roseum;PS:Pinus sylvestris;PN:Piper nigrum;PC:Pogostemon cablin;RO: R.of fi cinalis;SS:Salvia sclarea;SA:Santalum austrocaledonicum;SAR:S.aromaticum;ZO:Zingiber of fi cinale;AM:Ampicillin.
2.5.Determination of M IC and MBC
M IC of cinnamaldehyde was determ ined using the broth dilutionmethod in Todd-Hew ittbroth asdescribed by Sfeir etal. [18].Brie fl y,each compound was fi rst diluted to 40%(v/v)in dimethyl sulfoxide.Serial dilutions were carried out in sterile distilled water at concentrations of 0.01%–0.50%(v/v).One m illiliter of bacterial suspension(106CFU/m L)and 0.1 m L of each compound show ing antibacterial activity were added to 2.9 m L of Todd-Hew itt broth.Controls w ithout test compoundswere prepared.A fter 24 h of incubation at 37°C under agitation in culture tubes,the M IC was determ ined as the lowest concentration that visibly inhibited bacterial grow th.
To determ ine the MBC,10μL of bacterial inoculum were removed from tubes thathad not presented visible turbidity and spread onto Todd-Hew itt agar.These plates were incubated at 37°C for 48 h.The MBC was considered as the lower concentration thatshowed no bacterialgrow th on Todd-Hew ittagar plates.Each M IC and MBC value was obtained from three independent experiments.
3.Resu lts
3.1.Screening of antibacterial activity
Antibacterial activities of plant essential oils against S.mutans and S.sobrinus strains were presented in Figure 1.
Results obtained from disk diffusion assay showed that essential oil of C.verum was the most active against the tested bacterial strains,w ith zones of inhibition ranging from 4.0 to 4.6 cm(+++).S.sobrinus was sensitive(++)to essential oils from O.basilicum and P.roseum.M ost essential oils tested showed moderate inhibitory activities(+)against both tested strains.Seven essential oils(C.bergamia,C.limon, C.paradisi,C.sinensis,C.sempervirens,E.globulus and R.of fi cinalis)showed no activity(0)against both test strains. Additionally,two essential oils(B.carterii and J.communis) showed no activity(0)against S.mutans.The zone of inhibition of essential oils were smaller than that of the positive control, ampicillin.
3.2.Chemical composition of the essential oil of C.verum
The results of the chemical analysis of C.verum essential oil were presented in Table2.The compoundsare listed according to their elution order,which was in agreementwith their RIon HP-Innowax columns[19].Of the27 componentsof C.verum essential oil,24were identi fi ed(Table2 and Figure2).Three peaksshowed nomatch w ith the MS library.Cinnamaldehyde(56.3%)was the main compound in C.verum essentialoil,followed by cinnamyl acetate andβ-phellandrene(Table 2 and Figure2).

Table2 Chemical composition of the essentialoil of C.verum.
3.3.Antibacterial activity of the C.verum essential oil components
The antibacterial activities of the chem ical constituents of C.verum essential oil against S.mutans and S.sobrinus strains were presented in Figure 2.Results obtained from the disk diffusion assay showed thatcinnamaldehydewas themostactive against the tested bacterial strains,w ith zones of inhibition ranging from 4.2 to 5.7 cm(+++).S.sobrinus wassensitive(++) to eugenol and cinnamyl alcohol,whereas S.mutans was moderately sensitive(+).Both tested strains were moderately sensitive(+)to 3-carene,α-terpinene,benzaldehyde,linalool,βcaryophyllene,α-humulene,α-terpineol,hydrocinnam ic aldehyde,hydrocinnamyl acetate and cinnamyl acetate.No activity (0)was detected against either strain in 11 compounds:αpinene,camphene,(+)-β-pinene,(−)-β-pinene,α-phellandrene, α-terpinene,(+)-limonene,(−)-limonene,p-cymene,β-caryophyllene oxide and benzyl benzoate(Figure 3).The zone of inhibition of all the essential oils were smaller than that of the positive control,ampicillin.
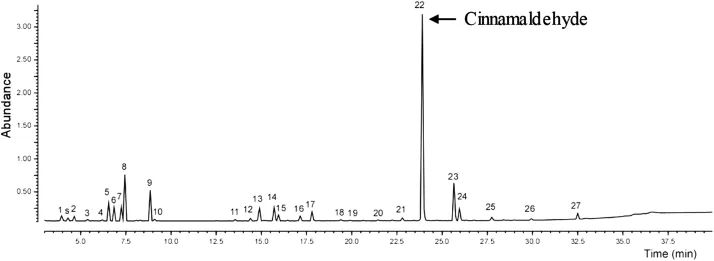
Figure 2.GC-MS chromatograms show ing chem ical compositions of cinnamon essential oil.Total ion chromatogram of cinnamon oil on HP-Innowax column;Numbersof peaks are the same as those in Table 2;s:Toluene from solvent(hexane).
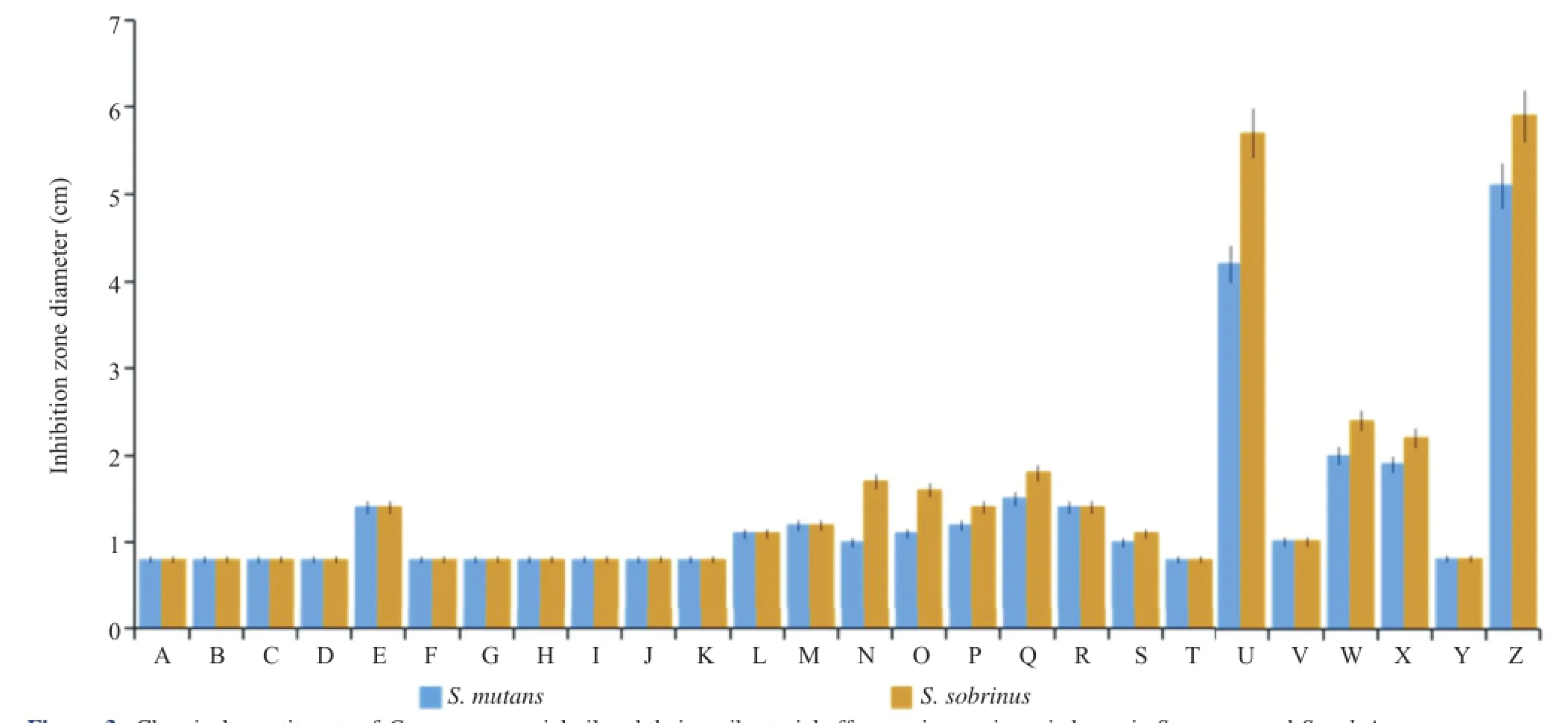
Figure 3.Chem ical constituents of C.verum essentialoiland their antibacterialeffectagainstcariogenic bacteria S.mutans and S.sobrinus.Averageof inhibition diameterswere calculated to classify theessentialoilsas follows:the tested strainswerenotsensitive foradiametersmaller than 0.8 cm(0), moderately sensitive for a 0.8–2.0 cm diameter(+),sensitive fora 2–3 cm(++),and very sensitive fora diametergreater than 3 cm(+++);A:α-Pinene;B: Camphene;C:(+)-β-Pinene;D:(−)-β-Pinene;E:3-Carene;F:α-Phellandrene;G:β-Phellandrene;H:α-Terpinene;I:(+)-Limonene;J:(−)-Limonene; K:p-Cymene;L:α-Terpinolene;M:Benzaldehyde;N:Linalool;O:β-Caryophyllene;P:α-Humulene;Q:α-Terpineol;R:Hydrocinnam ic aldehyde;S:Hydrocinnamylacetate;T:β-Caryophylleneoxide;U:Cinnamaldehyde;V:Cinnamylacetate;W:Eugenol;S:Cinnamylalcohol;Y:Benzylbenzoate;Z:Ampicillin.
3.4.MIC and MBC values
Disk diffusion assays for cinnamaldehyde were used to determ ine the most effective compound,and the M IC values were determ ined bymeans of broth dilution assays.The M IC of cinnamaldehyde was 0.02%(v/v)against both bacterial strains tested(Figure 4).The MBC values of cinnamaldehyde against S.mutans and S.sobrinus were 0.2%and 0.1%(v/v),respectively(Figure 4).
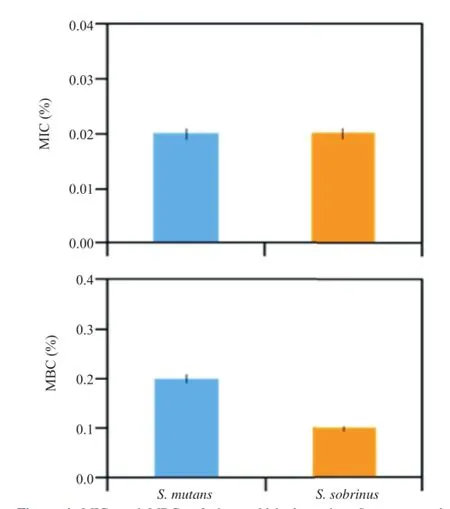
Figure 4.MICs and MBCs of cinnamaldehyde against S.mutans and S.sobrinus(means±SD).
4.Discussion
Plant-derived essential oils are ideal for use in oral care formulations,including toothpastes,mouthwashes,sprays and gels because they are non-toxic and have antiseptic properties [20].Scienti fi c investigations to evaluate the antim icrobial activity of essential oils are needed.
The present work evaluated the antibacterial activities of essential oils speci fi cally against the S.mutans and S.sobrinus strains responsible for dental caries.W e analyzed the chem ical composition of C.verum essential oil show ing the greatest antibacterial activity against the tested bacteria.To our know ledge,no previous publications have reported the antibacterial activity of cinnamaldehyde,the major bioactive component of C.verum essential oil,against cariogenic bacteria.
In this study,we used a disk diffusion assay to identify essential oils w ith the highest inhibitory activity against S.mutans and S.sobrinus strains among 32 essential oils tested. The essential oil of C.verum exhibited the highest inhibitory activity against both bacterial strains compared.These results were consistent w ith previous work.In the literature,the essential oilof C.verum inhibited the grow th of fungi including Aspergillus spp.,Penicillium spp.,Alternaria spp.,Fusarium spp.and Mucor spp.It also inhibited the grow th of various bacterial taxa,including Lactobacillus sp.,Bacillus thermoacidurans,Salmonella sp.,Corynebacterium michiganense,Pseudomonas striarfacines and Streptococcus pyogenes[21–24]. Cinnamaldehyde has been proposed to inhibit the grow th of food-spoilage bacteria,including Escherichia coli O157:H7 and Salmonella typhimurium[25].
Using GC-MS analysis,we showed that cinnamaldehyde (56.3%)was themajor componentof C.verum essential oil;and other components identi fi ed were cinnamyl acetate(7.1%)and β-phellandrene(6.3%).The chem ical components of C.verum essential oil used this study was very sim ilar to that used in previous reports[26,27].The three principal components, eugenol,cinnamaldehyde,and linalool,were detected in both bark and leaf essential oils of Cinnamomum sp.[26].According to Wang et al.[27],the essential oil of C.osmophloeum was classi fi ed into 9 types:cassia,cinnamaldehyde,coumarin, linalool,eugenol,camphor,4-terpineol,linalool-terpineol,and am ixed-type oil.Our present GC-MS results indicated that the essential oils of C.verum were of the cinnamaldehyde type.
Among themajor components,cinnamaldehyde exhibited the greatest antibacterial activity against S.mutans and S.sobrinus strains(Figure 2).Therefore,the essential oil of C.verum containing cinnamaldehydeshowed thehighestactivity.Essentialoils containingmainly aromatic phenols or aldehydes have been reported to exhibit considerable antim icrobial activity,whereas essential oils containing terpene ether,ketone or oxide have weakeractivity[22,28].Eugenolexhibited sensitiveandmoderately sensitiveactivitiesagainst S.sobrinus and S.mutans,respectively (Figure 2).Therefore,essential oils from O.basilicum and S.aromaticum,which are known to contain 70%–85%eugenol, showed antibacterialactivities against S.sobrinus and S.mutans. Xu etal.[29]have shown that the inhibitory effectsof eugenolon dental caries development caused by S.mutans.
Essential oils have a complex mode of antibacterial action [30].However,the antibacterial actions are intimately attached to their major characteristic,hydrophobicity,which produces an increase in the bacterial membrane permeability and the consequent loss of their cellular elements[31–34].In Staphylococcus aureus,cells treated w ith the essential oil of C.verum showed a considerable decrease in the metabolic activity and replication capacity,leading to a viable but noncultivable state[35].The essential oil of C.verum damages the cellular membrane of Pseudomonas aeruginosa,which leads to the collapse of membrane potential and loss of membraneselective permeability[35].Cinnamaldehyde exposure causes morphological changes in foodborne pathogenic bacteria, including Staphylococcus aureus,Staphylococcus anatum,and Bacillus cereus[36].
The essential oil of C.verum was not harm ful when consumed in food additives and may be used as an antibacterial agent.However,there are occasional case reports of allergic contact dermatitis and stomatitis among the consumers[37,38].
Con fl ict of interest statement
We declare thatwe have no con fl ictof interest.
Acknow ledgm ents
This research was supported by Basic Science Research Program through the NationalResearch Foundation of Korea funded by theM inistry of Education(2015R1A6A1A03031413).
References
[1]Soames JV,Southam JC.Oral pathology.4th ed.Oxford:Oxford University Press;2005.
[2]Nishikawara F,Nomura Y,ImaiS,Senda A,Hanada N.Evaluation of cariogenic bacteria.Eur JDent2007;1(1):31-9.
[3]Whiley RA,Bieghton D.Current classi fi cation of the oral streptococci.Oral Microbiol Immunol 1998;13(4):195-216.
[4]Fejerskov O,Nyvad B,Kidd E,editors.Dental caries:the disease and its clinical management.3rd ed.Oxford:W iley Blackwell; 2015.
[5]Lamont RJ,Hajishengallis GN,Jenkinson HF,editors.Oral microbiology and immunology.2nd ed.Washington DC:ASM Press;2014.
[6]Hiremath SS.Textbook ofpreventive and community dentistry.2nd ed.India:Elsevier;2011.
[7]Inui T,Walker LC,Dodds MW,Hanley AB.Extracellular glycoside hydrolase activities in the human oral cavity.Appl Environ Microbiol 2015;81(16):5471-6.
[8]Li Y,Tanner A.Effect of antimicrobial interventions on the oral m icrobiota associated w ith early childhood caries.Pediatr Dent 2015;37(3):226-44.
[9]Tut OK,M ilgrom PM.Topical iodine and fl uoride varnish combined ismore effective than fl uoride varnish alone for protecting erupting fi rst permanent molars:a retrospective cohort study. J Public Health Dent2010;70(3):249-52.
[10]Erdem AE,Sepet E,KulekciG,Trosola SC,Guven Y.Effects of two fl uoride varnishes and one fl uoride/chlorhexidine varnish on Streptococcus mutans and Streptococcus sobrinus bio fi lm formation in vitro.Int JMed Sci2012;9(2):129-36.
[11]Lee BS,Chou PH,Chen SY,Liao HY,Chang CC.Prevention of enameldemineralization w ith a novel fl uoride strip:enamelsurface com position and depth pro fi le.Sci Rep 2015;http://dx.doi.org/ 10.1038/srep13352.
[12]Kishi M,Abe A,Kishi K,Ohara-Nem oto Y,K imura S, Yonem itsu M.Relationship of quantitative salivary levels of Streptococcus mutans and S.sobrinus in mothers to caries status and colonization ofmutans streptococciin plaque in their2.5-yearold children.Community DentOral Epidemiol 2009;37(3):241-9.
[13]Weyant RJ,Tracy SL,Anselmo TT,Beltr´an-Aguilar ED, Donly KJ,FreseWA,etal.Topical fl uoride for caries prevention: executive summary of the updated clinical recommendations and supporting system atic review.J Am Dent Assoc 2013;144(11): 1279-91.
[14]Gluzman R,Katz RV,Frey BJ,M cGowan R.Prevention of root caries:a literature review of primary and secondary preventive agents.Spec Care Dentist2013;33(3):133-40.
[15]Youse fi manesh H,Amin M,Robati M,Goodarzi H,Otou fi M. Comparison of the antibacterial properties of threemouthwashes containing chlorhexidine againstoralm icrobialplaques:an in vitro study.Jundishapur J Microbiol 2015;http://dx.doi.org/10.5812/ jjm.17341.
[16]Kang JS,Kim E,Lee SH,Park IK.Inhibition of acetylcholinesterasesof the pinewood nematode,Bursaphelenchus xylophilus,by phytochemicals from plant essential oils.Pestic Biochem Physiol 2013;105(1):50-6.
[17]AL-Ham dany AJ,Jihad TW.Oxidation of some primary and secondary alcohols using pyridinium chlorochromate.Tikrit JPure Sci 2012;17(4):72-6.
[18]Sfeir J,Lefrançois C,Baudoux D,Derbr´e S,Licznar P.In vitro antibacterial activity of essential oils against Streptococcus pyogenes.Evid Based ComplementAltern Med 2013;http://dx.doi.org/ 10.1155/2013/269161.
[19]Peng CT.Prediction of retention indices.VI:isothermal and tem perature-programmed retention indices,methylene value, functionality constant,electronic and steric effects.JChromatogr A 2010;1217(23):3683-94.
[20]Ocheng F,Bwanga F,Joloba M,Softrata A,Azeem M,P¨utsep K, et al.Essential oils from Ugandan aromatic medicinal plants: chemical composition and grow th inhibitory effects on oral pathogens.Evid Based ComplementAltern Med 2015;http://dx.doi.org/ 10.1155/2015/230832.
[21]Naveed R,Hussain I,Tawab A,Tariq M,Rahman M,Hameed S, et al.Antimicrobial activity of the bioactive com ponents of essential oils from Pakistani spices against Salmonella and othermulti-drug resistantbacteria.BMC Complement Altern Med 2013; http://dx.doi.org/10.1186/1472-6882-13-265.
[22]Nabavi SF,Di Lorenzo A,IzadiM,Sobarzo-S´anchez E,Daglia M, Nabavi SM.Antibacterial effects of cinnamon:from farm to food, cosmeticand pharmaceuticalindustries.Nutrients2015;7(9):7729-48.
[23]Bassol´e IH,Juliani HR.Essential oils in combination and their antimicrobial properties.Molecules 2012;17(4):3989-4006.
[24]Seow YX,Yeo CR,Chung HL,Yuk HG.Plant essential oils as active antim icrobial agents.Crit Rev Food Sci Nutr 2014;54(5): 625-44.
[25]Hyldgaard M,Mygind T,Meyer RL.Essential oils in food preservation:mode of action,synergies,and interactions w ith food matrix components.Front Microbiol 2012;http://dx.doi.org/ 10.3389/fmicb.2012.00012.
[26]Jeong EJ,Lee NK,Oh J,Jang SE,Lee JS,Bae IH,etal.Inhibitory effect of cinnamon essential oils on selected cheese-contaminating fungi(Penicillium spp.)during the cheese-ripening process.Food Sci Biotechnol 2014;23(4):1193-8.
[27]Wang SY,Chen PF,Chang ST.Antifungal activities of essential oils and their constituents from indigenous cinnamon(Cinnamomum osmophloeum)leaves against wood decay fungi.Bioresour Technol 2005;96(7):813-8.
[28]Fabio A,CermelliC,Fabio G,Nicoletti P,Quaglio P.Screening of the antibacterial effects of a variety of essential oils on microorganisms responsible for respiratory infections.Phytother Res2007; 21(4):374-7.
[29]Xu JS,LiY,Cao X,CuiY.The effectof eugenolon the cariogenic properties of Streptococcusmutans and dental caries development in rats.Exp Ther Med 2013;5(6):1667-70.
[30]Stoeken JE,ParaskevasS,van derWeijden GA.The long-term effect of a mouthrinse containing essential oils on dental p laque and gingivitis:asystematic review.JPeriodontol2007;78(7):1218-28.
[31]Dorman HJ,Deans SG.Antimicrobial agents from plants:antibacterial activity of plant volatile oils.J Appl Microbiol 2000; 88(2):308-16.
[32]Andrade-Ochoa S,Nev´arez-Moorill´on GV,S´anchez-Torres LE, Villanueva-García M,S´anchez-Ramírez BE,Rodríguez-Valdez LM,et al.Quantitative structure-activity relationship of molecules constituent of different essential oils w ith antimycobacterial activity against Mycobacterium tuberculosis and Mycobacterium bovis.BMC Complement Altern Med 2015;http:// dx.doi.org/10.1186/s12906-015-0858-2.
[33]TurgisM,Han J,M illetteM,SalmieriS,Borsa J,Lacroix M.Effect of selected antimicrobial com pounds on the radiosensitization of Salmonella Typhi in ground beef.Lett Appl Microbiol 2009; 48(6):657-62.
[34]Kothiwale SV,Patwardhan V,Gandhi M,Sohoni R,Kumar A. A comparative study of antiplaque and antigingivitis effects of herbal mouthrinse containing tea tree oil,clove,and basil w ith commercially available essential oil mouthrinse.J Indian Soc Periodontol 2014;18(3):316-20.
[35]Bouhdid S,AbriniJ,AmensourM,ZhiriA,Espuny MJ,Manresa A. Functionaland ultrastructural changes in Pseudomonasaeruginosa and Staphylococcus aureus cells induced by Cinnamomum verum essentialoil.JApplM icrobiol2010;109(4):1139-49.
[36]Shan B,Chai YZ,Brooks JD,Corke H.Antibacterial properties and major bioactive componentsof cinnamon stick(Cinnamomum burmannii):activity against of foodborne pathogenic bacteria. JAgric Food Chem 2007;55(14):5484-90.
[37]Lauriola MM,De Bitonto A,Sena P.A llergic contact dermatitis due to cinnamon oil in galenic vaginal suppositories.Acta Derm Venereol 2010;90(2):187-8.
[38]Biron JF,Iovino JP,Bailey JR,B rown RS.Cinnam on-induced oral contact stomatitis.Dent Today 2013;32(2):82.
16 Oct 2015
*Corresponding author:Jinwoo Kim,Division of Applied Life Science and Instituteof Agriculture&Life Science,Gyeongsang NationalUniversity,Jinju 52828, South Korea.
Tel:+82 55 552 1927
Fax:+82 55 772 1929
E-mail:jinwoo@gnu.ac.kr
Foundation Project:Supported by Basic Science Research Program through the National Research Foundation o f Korea funded by the M inistry of Education (2015R1A6A 1A 03031413).
Peer review under responsibility of Hainan M edical University.The journal implements double-blind peer review practiced by specially invited international editorial board members.
#These authors contributed equally to thiswork.
2221-1691/Copyright©2016 Hainan Medical University.Production and hosting by Elsevier B.V.This is an open accessarticle under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- Sudden death in a captive mee rkat(Suricata surica tta)w ith arterial m edial and m yocardial calcification
- Characteristics o f obese or overweight dogs visiting private Japanese vete rinary c linics
- Risk factors from HBV infection among blood donors:A system atic review
- Com putational in telligence in tropicalm edicine
- The African Moringa is to change the lives ofm illions in Ethiopia and far beyond
- Pediculosis capitis among p rimary and m idd le school children in Asadabad,Iran:An epidem iological study
