外源过氧化氢提高燕麦耐盐性的生理机制
2016-09-05刘建新王金成王瑞娟贾海燕陇东学院生命科学与技术学院甘肃省高校陇东生物资源保护与利用省级重点实验室甘肃庆阳745000
刘建新,王金成,王瑞娟,贾海燕(陇东学院生命科学与技术学院,甘肃省高校陇东生物资源保护与利用省级重点实验室,甘肃庆阳745000)
外源过氧化氢提高燕麦耐盐性的生理机制
刘建新*,王金成,王瑞娟,贾海燕
(陇东学院生命科学与技术学院,甘肃省高校陇东生物资源保护与利用省级重点实验室,甘肃庆阳745000)
以燕麦品种‘定莜6号'为材料,采用水培法,研究喷施过氧化氢(H2O2)对盐胁迫下燕麦幼苗生长、渗透调节物质积累和活性氧代谢的影响。结果表明:1)150 mmol/L NaCl胁迫显著抑制燕麦幼苗生长,提高叶片游离氨基酸和脯氨酸水平,降低谷胱甘肽(GSH)和可溶性糖含量;喷施0.01 mmol/L H2O2对NaCl胁迫引起的生长抑制有明显的缓解作用,并提高了幼苗叶片可溶性蛋白质、可溶性糖和脯氨酸含量,降低了游离氨基酸含量。2)NaCl胁迫下,虽然燕麦叶片超氧化物歧化酶、过氧化氢酶、过氧化物酶和抗坏血酸过氧化物酶活性提高,但O2·-、H2O2和丙二醛(MDA)积累;喷施H2O2进一步提高了NaCl胁迫下燕麦的上述抗氧化酶活性和GSH含量,却降低了O2·-产生速率及H2O2和MDA含量,说明外施H2O2能够增强盐胁迫燕麦的抗氧化能力,减轻氧化伤害。以上结果表明,外源H2O2可通过调控渗透调节物质积累和活性氧代谢提高燕麦耐盐性。
盐胁迫;过氧化氢;燕麦;活性氧代谢;渗透调节物质
http://cyxb.lzu.edu.cn
刘建新,王金成,王瑞娟,贾海燕.外源过氧化氢提高燕麦耐盐性的生理机制.草业学报,2016,25(2):216-222.
LIU Jian-Xin,WANG Jin-Cheng,WANG Rui-Juan,JIA Hai-Yan.The physiological mechanisms through which exogenous H2O2increases the resistance of Avena nuda to salt stress.Acta Prataculturae Sinica,2016,25(2):216-222.
盐害是农业生产的主要障害之一,通过对作物渗透调节和离子平衡的破坏,造成活性氧积累和生长抑制,甚至死亡[1]。作物能通过感受刺激和信号转导启动各种生理生化反应适应盐胁迫[2]。过氧化氢(hydrogen peroxide,H2O2)是细胞代谢过程中产生的一种具有毒害作用的活性氧[3]。但近来的研究表明,H2O2也是植物体内一种重要的信号分子[4],参与调控植物的生长发育[5]及对各种非生物逆境胁迫的应答过程[6-8]。H2O2作为第二信使参与了ABA诱导的拟南芥(Arabidopsis thaliana)气孔关闭过程[9];外源H2O2预处理通过提高抗氧化系统活性缓解了干旱对黄瓜(Cucumis sativus)叶绿体膜的伤害[7],提高了玉米(Zea mays)[10]和小麦(Triticum aestivum)[6]的耐盐性。张波和张怀刚[11]研究表明,外源H2O2通过提高叶绿素、可溶性糖和谷胱甘肽含量有效增强了小麦幼苗的耐盐性。Uchida等[2]研究证明,H2O2能够诱导细胞抗氧化机制提高水稻(Oryza sativa)对盐胁迫的耐受性。谷文英等[12]研究发现,H2O2处理对菊苣(Cichorium intybus)幼苗盐胁迫的缓解效应与其上调抗氧化酶活性和逆境蛋白表达有关。此外,H2O2还在寄主-病原物互作过程中的过敏反应[13]、细胞程序性死亡[14]和诱导植物抗病性[15]等过程中发挥着重要作用。
燕麦(Avena nuda)是中国北方广泛种植的一种粮饲作物,但种植区较高的土壤含盐量往往是其生长发育和产量提高的重要限制因子之一,尤其在春季返盐季节对幼苗期生长的影响更大。不同品种燕麦对盐胁迫的生理响应及耐盐性存在很大差异[16]。‘定莜6号'是甘肃省定西市旱作农业科研推广中心选育的燕麦新品种,具有抗旱性强、丰产性好、品质优和抗坚黑穗病强等特点。然而,‘定莜6号'对盐胁迫的响应机制及H2O2的调节作用目前尚不了解,也未见有关H2O2对燕麦盐胁迫生理影响的报道。本研究通过渗透调节物质含量和活性氧代谢的变化探讨外源H2O2对‘定莜6号'响应盐胁迫生理机制的调节作用,以期为应用化学调控提高燕麦耐盐性提供依据。
1 材料与方法
1.1供试材料和处理
试验于2013年3-8月在甘肃省高校陇东生物资源保护与利用省级重点实验室生物科技园进行。供试燕麦品种‘定莜6号'种子(由甘肃省定西市旱作农业科研推广中心提供)经3%NaClO表面消毒10 min后催芽,选露白一致的种子播种在装有珍珠岩的底部带孔塑料钵(口径20 cm,高14 cm)中,浇水后置温室培养,昼/夜温度(26±5)℃/(20±6)℃,相对湿度70%~80%,光照强度520~710μmol/(m2·s),常规管理。幼苗2叶1心期进行疏苗,每钵保留一致壮苗约100株,3叶1心期进行处理:1)CK(对照),根部浇灌Hoagland营养液,叶面喷雾蒸馏水;2)NaCl,根部浇灌含150 mmol/L NaCl的Hoagland溶液,叶面喷雾蒸馏水;3)NaCl+H2O2,根部浇灌含150 mmol/L NaCl的Hoagland溶液,叶面喷雾0.01 mmol/L H2O2;4)H2O2,根部浇灌Hoagland营养液,叶面喷雾0.01 mmol/L H2O2(根据预试验0.01 mmol/L H2O2对150 mmol/L NaCl胁迫下燕麦生长抑制的缓解作用最明显)。叶面喷雾于每天7:00和19:00进行,为降低表面张力,喷雾溶液配制时加入2滴吐温-80,喷雾量以叶面滴液为限,约8 m L/盆。根部浇灌每天19:00进行,浇施量为珍珠岩持水量的2倍(约1000 m L)以保持处理浓度的恒定。每个处理3盆,重复3次,随机排列。处理5 d后取全钵所有幼苗的倒数第2~3片展开叶[11]用液氮速冻后-70℃保存,及时测定相关生理指标。
1.2测定指标与方法
1.2.1植株干重的测定处理10 d后,每个处理取30株幼苗,洗净后在105℃杀青30 min,70℃烘干至恒重,称干重。
1.2.2可溶性蛋白质、可溶性糖、游离氨基酸和脯氨酸含量的测定分别按李合生[17]的考马斯亮蓝法、蒽酮比色法、茚三酮染色法和磺基水杨酸法测定可溶性蛋白质、可溶性糖、游离氨基酸和脯氨酸含量。
1.2.3O2·-产生速率、H2O2和MDA含量的测定O2·-产生速率按陈建勋和王晓峰[18]的方法测定。H2O2含量参照Sergiev等[19]的方法测定;丙二醛(MDA)含量采用硫代巴比妥酸法测定[18]。
1.2.4抗坏血酸(ASA)和谷胱甘肽(GSH)含量的测定称取0.20 g叶片,分别用2.0 m L 15%偏磷酸和5%三氯乙酸溶液研磨,将匀浆液14470 r/min离心20 min,上清液定容至2.0 m L。按Arakawa等[20]的方法测定ASA含量;采用Ellman[21]的方法测定GSH含量。
1.2.5SOD、CAT、POD和APX活性的测定采用陈建勋和王晓峰[18]的方法测定超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、过氧化物酶(POD)、抗坏血酸过氧化物酶(APX)活性。
1.3统计分析
所有数据以单位材料干重计算,平均值±标准误表示,SPSS 19.0方差分析和Duncan法多重比较(P<0.05)。
2 结果与分析
2.1外源H2O2对NaCl胁迫下燕麦幼苗生长的影响
图1表明,150 mmol/L NaCl胁迫10 d导致燕麦植株干重下降20.5%,喷施0.01 mmol/L H2O2显著提高了NaCl胁迫下燕麦的植株干重,比单独NaCl处理提高了12.6%。而单独H2O2处理的植株干重与CK无显著差异。
2.2外源H2O2对NaCl胁迫下燕麦幼苗叶片渗透调节物质含量的影响
从表1可见,与CK相比,单独NaCl胁迫显著降低了燕麦叶片中可溶性糖的含量,下降幅度达55.9%,却明显提高了游离氨基酸和脯氨酸含量,分别提高了31.4%和145.4%,而可溶性蛋白质含量无明显改变;NaCl+H2O2处理的可溶性蛋白质、可溶性糖和脯氨酸含量比单独NaCl处理分别提高了39.5%、135.2%和39.3%,游离氨基酸含量下降了43.1%,差异显著。单独H2O2处理与CK相比,可溶性蛋白质和脯氨酸含量分别提高了17.8%和39.7%,而可溶性糖和游离氨基酸含量变化不大。

图1 外源H2O2对NaCl胁迫下燕麦幼苗干重的影响Fig.1 Effect of exogenous H2O2on dry weight of oat seedlings under NaCl stress
2.3外源H2O2对NaCl胁迫下燕麦幼苗叶片O2·-产生速率、H2O2和MDA含量的影响O2·-和H2O2是2种主要的细胞质膜过氧化活性氧,MDA是膜脂过氧化的产物之一。图2结果表明,单独NaCl处理显著提高了燕麦叶片的O2·-产生速率及H2O2和MDA含量,分别比CK提高了87.2%、64.5%和53.2%;NaCl+H2O2处理与单独NaCl处理相比,O2·-产生速率、H2O2和MDA含量分别下降了40.4%、24.7%和16.4%,差异显著,说明外源H2O2能够降低盐胁迫诱导的活性氧积累对膜脂的氧化伤害。与CK相比,单独H2O2处理显著提高了燕麦叶片内源H2O2的含量,但O2·-产生速率和MDA含量无明显差异。
2.4外源H2O2对NaCl胁迫下燕麦幼苗叶片抗氧化物质含量的影响
ASA和GSH是2种重要的活性氧清除抗氧化物质。图3显示,不同处理并没有引起燕麦叶片ASA含量的显著改变,但GSH含量却发生了明显变化。与CK相比,单独NaCl处理下GSH含量降低了39.6%,单独H2O2处理GSH含量无显著变化;而NaCl+H2O2处理的GSH含量比单独NaCl处理提高了132.8%。
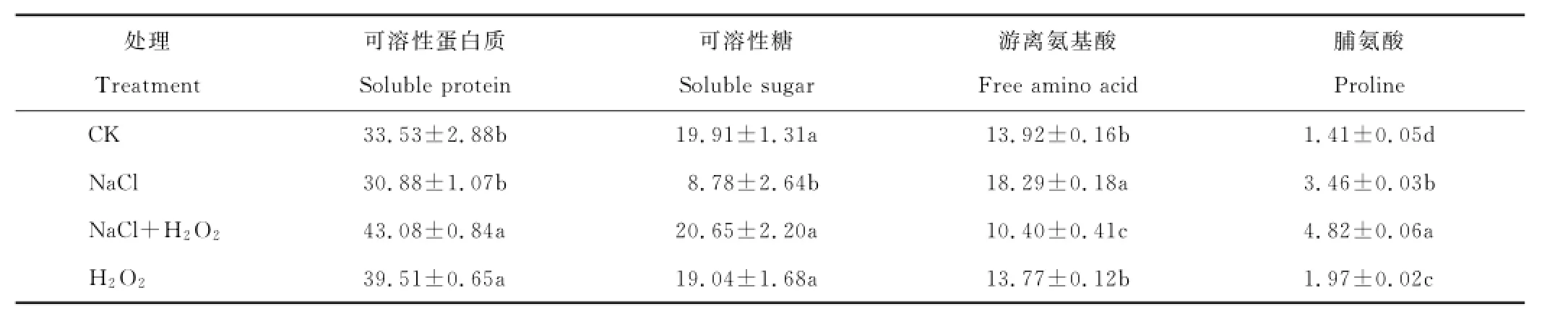
表1 外源H2O2对NaCl胁迫下燕麦幼苗叶片渗透调节物质含量的影响Table1 Effects of exogenous H2O2on content of osmotica in leaves of oat seedlings under NaCl stress mg/g

图2 外源H2O2对NaCl胁迫下燕麦幼苗叶片O2·—产生速率及H2O2和MDA含量的影响Fig.2 Effects of exogenous H2O2on O2·—production rate,contents of H2O2and MDA in leaves of oat seedlings under NaCl stress

图3 外源H2O2对NaCl胁迫下燕麦幼苗叶片ASA和GSH含量的影响Fig.3 Effects of exogenous H2O2on contents of ASA and GSH in leaves of oat seedlings under NaCl stress
2.5外源H2O2对NaCl胁迫下燕麦幼苗叶片抗氧化酶活性的影响
SOD、CAT、POD和APX是植物体内清除活性氧的主要抗氧化酶。由表2可见,与CK相比,单独NaCl处理使燕麦幼苗叶片SOD、CAT、POD和APX活性均显著提高,NaCl+H2O2处理进一步提高了上述4种抗氧化酶的活性,与单独NaCl处理比较,SOD、CAT、POD和APX活性分别提高了30.3%、71.1%、32.5%和22.4%,差异显著。单独H2O2处理的CAT和APX活性分别比CK提高了64.3%和40.0%,而SOD和POD活性则无明显差异。

表2 外源H2O2对NaCl胁迫下燕麦幼苗叶片抗氧化酶活性的影响Table2 Effects of exogenous H2O2on anti-oxidative enzyme activities in oat seedling leaves under NaCl stress U/g
3 讨论
植物在盐胁迫下首先遭受渗透胁迫和离子毒害,进而引起活性氧代谢失调,造成细胞代谢紊乱和生长受抑[22]。本研究结果显示,燕麦幼苗在150 mmol/L NaCl胁迫10 d后植株干重明显降低,叶面喷施0.01 mmol/L H2O2能够有效减轻NaCl胁迫对燕麦幼苗生长的抑制作用(图1),这与外源H2O2能够缓解受旱小麦[23]和Cd胁迫水稻[8]生长受抑的结果一致。说明外源H2O2可以缓解逆境胁迫对植物生长的抑制作用。其原因可能与H2O2能够促进植物次生壁的发育有关[24]。植物次生壁随细胞分化在初生壁内侧逐渐形成,次生壁组成中除半纤维素和纤维素外,还含有木质素。木质素是一种高度交联的酚类聚合物,由此增强了植物向上生长所需要的机械支持力,有利于植物生长[25]。另外,H2O2对盐胁迫燕麦生长的促进作用可能还与渗透调节和活性氧代谢有关。
正常细胞内离子保持平衡稳态。盐胁迫下会导致胞质Na+积累,过多的Na+则通过跨质膜转运或跨液泡膜区域化到液泡中以减轻盐离子毒害[26]。液泡中Na+的积累,不仅可以减轻对细胞质中酶和膜系统的伤害,而且可作为渗透调节剂降低渗透势,以利于植物吸收水分。胞质中则积累可溶性糖、脯氨酸等来维持细胞的渗透平衡[1]。魏小红等[27]研究表明,H2O2能够调节烟草(Nicotiana tabacum)脯氨酸、游离氨基酸和可溶性蛋白质的含量;张波和张怀刚[11]报道,外源H2O2可提高盐胁迫下小麦幼苗的可溶性糖和谷胱甘肽含量。本研究结果显示,150 mmol/L NaCl胁迫下,燕麦叶片可溶性蛋白质含量并没有明显改变,但可溶性糖含量明显下降,游离氨基酸和脯氨酸含量显著提高(表1)。说明燕麦通过积累游离氨基酸和脯氨酸增强盐胁迫下的渗透适应能力。喷施0.01 mmol/L H2O2提高了150 mmol/L NaCl胁迫下燕麦叶片中的可溶性蛋白质、可溶性糖和脯氨酸含量,而游离氨基酸含量明显降低。可溶性蛋白质有较强的持水力,其中大多数是参与代谢反应的酶类;可溶性糖和脯氨酸等是细胞重要的有机渗透调节物质,它们含量的变化与植物抗逆性密切相关[11]。说明外源H2O2能够调节可溶性蛋白质和有机渗透调节物质的含量,提高燕麦的耐盐性。这与H2O2预处理能够提高铝胁迫下黑豆(Ribes nigrum)可溶性蛋白质含量[28]及Cd胁迫下水稻谷胱甘肽转硫酶活性[8]的结果类似。但H2O2提高盐胁迫下燕麦可溶性蛋白质、可溶性糖和脯氨酸含量的分子机制还需进一步探讨。
活性氧(reactive oxygen species,ROS)的产生在植物代谢过程中不可避免,本底或自稳态水平的ROS在植物的生长发育以及对环境胁迫的适应中具有重要作用[3-4],但植物遭受逆境胁迫时O2·-和H2O2等ROS水平明显提高[1]。植物体内ROS代谢能否保持平衡与抗氧化酶的活性和抗氧化剂的含量密切相关[29]。SOD催化O2·-歧化反应生成H2O2,CAT可直接催化H2O2生成H2O和O2[30]。POD催化H2O2与酚类反应清除ROS;APX通过ASA-GSH循环利用ASA、GSH等抗氧化剂将H2O2还原为H2O[31]。本研究结果显示,150 mmol/L NaCl胁迫5 d显著提高了燕麦叶片O2·-的产生速率和H2O2含量(图2),此时尽管SOD、CAT、POD 和APX活性均提高(表2),但GSH含量的下降(图3)使抗氧化系统未能及时清除过量的ROS导致其积累。积累的ROS使膜脂不饱和脂肪酸过氧化产生MDA,MDA能与酶蛋白发生链式聚合反应,使膜系统变性[29]。喷施0.01 mmol/L H2O2显著提高了150 mmol/L NaCl胁迫下燕麦幼苗叶片的SOD,CAT、POD和APX等抗氧化酶活性(表2)及GSH含量(图3)。说明盐胁迫破坏了燕麦ROS清除的ASA-GSH循环系统,外施H2O2有效促进了ASA-GSH循环系统的有效运转,从而加强了植株的ROS清除能力,降低了O2·-和H2O2的积累,减轻了盐胁迫诱导的氧化损伤(图2)。这与前人[10,32]的研究结果一致,可能与H2O2能够诱导抗氧化酶基因的表达有关[33],但具体机制尚待进一步研究。
4 结论
盐胁迫下,喷施H2O2能够增加燕麦幼苗可溶性蛋白质、可溶性糖和脯氨酸等渗透调节物质含量,提高SOD、CAT、POD和APX等抗氧化酶活性及抗氧化剂GSH含量,降低O2·-和H2O2积累,减轻膜脂氧化伤害和幼苗生长受抑程度,从而增强燕麦耐盐性。
References:
[1]Munns R,Tester M.Mechanisms of salinity tolerance.Annual Review of Plant Biology,2008,59:651-681.
[2]Uchida A,Jagendorf A T,Hibino T,et al.Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice.Plant Science,2002,163(3):515-523.
[3]Dat J F,Vandendede F,Vranova E,et al.Dual action of the active oxygen species during plant stress response.Cellular and Molecular Life Sciences,2000,57(5):779-795.
[4]Hung S H,Yu C W,Lin C H.Hydrogen peroxide functions as a stress signal in plants.Botanical Bulletin of Academia Sinica,2005,46(1):1-10.
[5]Liao W B,Huang G B,Yu J H,et al.Nitric oxide and hydrogen peroxide are involved in indole-3-butyricacid-induced adventitious roots development in marigold.Journal of Horticultural Science&Biotechnology,2011,86(2):159-165.
[6]Wahid A,Perveen M,Gelani S,et al.Pretreatment of seed with H2O2improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress protein.Journal of Plant Physiology,2007,64(3):283-294.
[7]Liu Z J,Guo Y K,Lin S H,et al.Effects of exogenous hydrogen peroxide on ultra-structure of chloroplasts and activities of antioxidant enzymes in greenhouse-ecotype cucumber under drought stress.Acta Horticulturae Sinica,2009,36(8):1140-1146.
[8]Bai X J,Liu L J,Zhang C H,et al.Effect of H2O2pretreatment on Cd tolerance of different rice cultivars.Chinese Journal of Rice Science,2010,24(4):391-397.
[9]Bright J,Desikan R,Hancock J T,et al.ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2synthesis.The Plant Journal,2006,45(1):113-122.
[10]Azevedo N A D,Prisco J T,Eneas-Filho J,et al.Hydrogen peroxide pre-treatment induces salt stress acclimation in maize plants.Journal of Plant Physiology,2005,162(10):1114-1122.
[11]Zhang Bo,Zhang H G.Regulation of exogenous hydrogen peroxide on wheat seedling salinity tolerance.Acta Botanica Boreali-Occidentalia Sinica,2007,27(12):2491-2495.
[12]Gu W Y,Mo P H,Yang JS,et al.Exogenous nitric oxide and hydrogen peroxide regulate the acclimation of chicory(Cichorium intybus)to salt stress.Chinese Journal of Ecology,2014,33(1):89-97.
[13]Delledonne M,Xia Y,Dixon R A,et al.Nitric oxide functions as a signal in plant disease resistance.Nature,1998,394 (6693):585-588.
[14]Lara L,Nello C,Piero P,et al.Nitric oxide and hydrogen peroxide involvement during programmed cell death of Sechium edule nucellus.Physiologia Plantarum,2010,140(1):89-102.
[15]Fan B,Shen L,Liu K L,et al.Interaction between nitric oxide and hydrogen peroxide in postharvest tomato resistance response to Rhizopus nigricans.Journal of the Science of Food and Agriculture,2008,88(7):1238-1244.
[16]Liu F Q,Liu J L,Zhu R F,et al.Physiological responses and tolerance of four oat varieties to salt stress.Acta Prataculturae Sinica,2015,24(1):183-189.
[17]Li H S.Principles and Techniques of Plant Physiological Biochemical Experiment[M].Beijing:Higher Education Press,2000.
[18]Chen J X,Wang X F.Plant Physiology Experimental Guidance[M].Guangzhou:South China University of Technology Press,2002.
[19]Sergiev I,Alexieva V,Karanov E.Effect of spermine,atrazine and combination between them on some endogenous protective systems and stress markers in plants.Comptes Rendus de I'Academie Bulgare des Sciences,1997,51(2):121-124.
[20]Arakawa N,Tsutsumi K,Sanceda N G,et al.A rapid and sensitive method for the determination of ascorbic acid using 4,7-diphenyl-1,10-phenanthroline.Agricultural and Biological Chemistry,1981,45(5):1289-1290.
[21]Ellman G L.Tissue sulfhydryl groups.Archives of Biochemistry and Biophysics,1959,82(1):70-77.
[22]Zhu X J,Liang Y C,Yang J S,et al.Effect of exogenous calcium on antioxidant enzyme activity and lipid peroxidation of rice seedlings under salt stress.Acta Pedologica Sinica,2005,42(3):453-459.
[23]Qiu Z B,Sun L,Li J T,et al.Protecting effect of exogenous hydrogen peroxide on wheat seedlings damage by water stress. Bulletin of Botanical Research,2010,30(3):294-298.
[24]Pnueli L,Liang H,Rozenberg M,et al.Growth suppression,altered stomatal responses,and augmented induction of heat shock proteins in cytosolic ascorbate peroxidase(Apx1)-deficient Arabidopsis plants.The Plant Journal,2003,34(2):187-203.
[25]Song D L,Shen J H,Li L G.Cellulose synthesis in the cell walls of higher plants.Plant Physiology Journal,2008,44(4):791-796.
[26]Bu Q M,Bai X F,Zhu J J,et al.Accumulation and distribution of salt in leaves of Atriplex triangularis under salt stress. Chinese Journal of Applied&Environmental Biology,2007,13(2):192-195.
[27]Wei X H,Wang L M,Long R J,et al.Effects of exogenous nitric oxide,salicylic acid and hydrogen peroxide on free amino acid and soluble protein contents in tobacco leaves.Journal of Plant Physiology and Molecular Biology,2006,32(2):257-260.
[28]Wang L,Chen Q,Wu K H,et al.Physiological mechanisms of pretreatment with hydrogen peroxide enhancing the capacity of the sensitive black soybean resistance to Al stress.Acta Botanica Boreali-Occidentalia Sinica,2013,33(2):336-342.
[29]Agarwal S,Sairam R K,Srivastava G C,et al.Changes in antioxidant enzymes activity and oxidative stress by abscisic acid and salicylic acid in wheat genotypes.Biologia Plantarum,2005,49(4):541-550.
[30]Vandenabeele S,Vanderauwera S,Vuylsteke M,et al.Catalase deficiency drastically affects gene expression induced by high light in Arabidopsis thaliana.The Plant Journal,2004,39(1):45-58.
[31]Asada K.Ascorbate peroxidase-a hydrogen peroxide-scavenging enzyme in plants.Physiologia Plantarum,1992,85(2):235-241.
[32]Abdul W,Mubaraka P,Sadia G,et al.Pretreatment of seed with H2O2improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins.Journal of Plant Physiology,2007,164(3):283-294.
[33]Robert B,David O W.Arabidopsis OXS2 is a transcription factor in the oxidative stress response,Abstract of annual meeting of the American Society of Plant Biologists,July 24-28,2004,Orlando,FL,USA[EB/OL].http://abstracts.aspb. org/pb2004/public/M04/9154.html,2015-03-01.
[7]刘忠静,郭延奎,林少航,等.外源过氧化氢对干旱胁迫下温室黄瓜叶绿体超微结构和抗氧化酶的影响.园艺学报,2009,36(8):1140-1146.
[8]白晓娟,刘丽娟,张春华,等.H2O2预处理对不同水稻品种Cd耐性的影响.中国水稻科学,2010,24(4):391-397.
[11]张波,张怀刚.外源H2O2对小麦幼苗耐盐性的调节作用.西北植物学报,2007,27(12):2491-2495.
[12]谷文英,莫平华,杨江山,等.外源一氧化氮和过氧化氢调节菊苣盐适应性.生态学杂志,2014,33(1):89-97.
[16]刘凤歧,刘杰淋,朱瑞芬,等.4种燕麦对NaCl胁迫的生理响应及耐盐性评价.草业学报,2015,24(1):183-189.
[17]李合生.植物生理生化实验原理和技术[M].北京:高等教育出版社,2000.
[18]陈建勋,王晓峰.植物生理学实验指导[M].广州:华南理工大学出版社,2002.
[22]朱晓军,梁永超,杨劲松,等.钙对盐胁迫下水稻幼苗抗氧化酶和膜脂过氧化作用的影响.土壤学报,2005,42(3):453-459.
[23]邱宗波,孙立,李金亭,等.外源过氧化氢对小麦水分胁迫伤害的防护作用研究.植物研究,2010,30(3):294-298.
[25]宋东亮,沈君辉,李来庚.高等植物细胞壁中纤维素的合成.植物生理学报,2008,44(4):791-796.
[26]卜庆梅,柏新富,朱建军,等.盐胁迫条件下三解滨藜叶片中盐分的积累与分配.应用与环境生物学报,2007,13(2):192-195.
[27]魏小红,王利民,龙瑞军,等.外源一氧化氮、水杨酸和过氧化氢对烟草叶片游离氨基酸和可溶性蛋白含量的影响.植物生理与分子生物学学报,2006,32(2):257-260.
[28]王琳,陈奇,武孔焕,等.过氧化氢预处理增强敏感型黑豆抗铝能力的生理机制.西北植物学报,2013,33(2):336-342.
The physiological mechanisms through which exogenous H2O2increases the resistance of Avena nuda to salt stress
LIU Jian-Xin*,WANG Jin-Cheng,WANG Rui-Juan,JIA Hai-Yan
College of Life Science and Technology,Longdong University,University Provincial Key Laboratory for Protection and Utilization of Longdong Bio-resources in Gansu Province,Qingyang 745000,China
Soil salinity is a major limiting factor for plant growth and productivity globally.Hydrogen peroxide (H2O2)is an important signaling molecule in plants that regulates many important physiological and biochemical processes and induces tolerance to different stresses,including salt stress.A study has been undertaken in order to further understand the operation of these regulatory mechanisms in oat seedlings(Avena nuda).A new oat cultivar,‘Dingyou No.6',was selected to investigate,using greenhouse nutrient solution cultivation,the effects of exogenous H2O2on plant growth,osmotic adjustment substances accumulation and active oxygen metabolism in seedlings under salt stress.The results showed that 150 mmol/L NaCl exposure significantly inhibited seedling growth.It enhanced the production of free amino acid and proline and decreased the contents of glutathione(GSH)and soluble sugar in leaves.Foliar spraying of 0.010 mmol/L H2O2significantly alleviated the inhibitory effect of NaCl stress on seedling growth.Exogenous H2O2increased the contents of soluble protein,soluble sugar and proline,and decreased free amino acid content in leaves.Under 150 mmol/L NaCl stress,superoxide dismutase(SOD),catalase(CAT),peroxidase(POD)and ascorbate peroxidase(APX)ac-tivities all increased,along with excessive production of O2·-,H2O2and malondialdehyde(MDA)in seedling leaves.Spraying the stressed seedlings with 0.010 mmol/L H2O2treatments significantly increased the activities of SOD,CAT,POD and APX and GSH content,but decreased O2·-production rate and the contents of H2O2and MDA in leaves.These results indicate that exogenous H2O2could enhance anti-oxidative ability and decrease membrane lipid peroxidation injury in oat seedlings under NaCl stress.Exogenous H2O2enhanced seedlings'salinity tolerance by regulating osmotic adjustment substances accumulation and active oxygen metabolism in plant leaves.
salt stress;H2O2;oat(Avena nuda);reactive oxygen metabolism;osmotic adjustment substances
10.11686/cyxb2015128
2015-03-10;改回日期:2015-05-14
甘肃省庆阳市科技计划项目(KZ2014-19)资助。
刘建新(1964-),男,甘肃通渭人,教授,本科。
Corresponding author.E-mail:liujx1964@163.com
