槲皮素糖苷类化合物的合成及其对α-葡萄糖苷酶的抑制活性
2016-08-16文中行胡占兴陈洪菊张利梅梁光义徐必学
文中行,胡占兴,袁 洁,陈洪菊,张利梅,梁光义*,徐必学*
(1.贵州大学 药学院,贵州 贵阳 550025;2.贵州省中国科学院天然产物化学重点实验室,贵州 贵阳 550002)
槲皮素糖苷类化合物的合成及其对α-葡萄糖苷酶的抑制活性
文中行1,2,胡占兴2,袁洁1,2,陈洪菊2,张利梅2,梁光义2*,徐必学2*
(1.贵州大学 药学院,贵州 贵阳 550025;2.贵州省中国科学院天然产物化学重点实验室,贵州 贵阳 550002)
以芦丁为原料合成了6个槲皮素糖苷类化合物,并经MS,1H NMR及13C NMR 确证其结构。以阿卡波糖为阳性对照测试目标产物对α-葡萄糖苷酶的抑制活性,发现当该类化合物中糖基上的羟基被苯甲酰化后能增强其对α-葡萄糖苷酶的抑制活性,其中V-b和V-c的IC50各为19.4 μmol/L,21.5 μmol/L,而未被苯甲酰化的化合物VI-b和VI-c的抑制活性相对较低。这将为该类化合物在降糖活性方面的深入研究提供参考。
槲皮素糖苷;抑制活性;α-葡萄糖苷酶
α-葡萄糖苷酶的抑制表达为治疗II型糖尿病的一个重要方法,至今,尽管有很多有效的α-葡萄糖苷酶抑制剂,但他们的副作用不容忽视。例如,阿卡波糖可能会引起胃肠胀气,肠鸣音,腹泻,腹痛等不良反应[1]。因此寻找更有效更安全的α-葡萄糖苷酶抑制剂是药学工作者的一个重要任务。近年来,一些研究者发现很多黄酮糖苷类天然产物具有较好的降糖作用。例如6,8-二-C-葡萄糖基芹菜素 (1)[2],4′-甲氧基-6-C-(6-乙酰氧基-葡萄糖基)-8-C-α-L-阿拉伯糖基芹菜素 (2)[3],8-C-葡萄糖基芹菜素 (3)[4],3′-O--D-葡萄糖基木犀草素 (4) 及3-O--D-葡萄糖基槲皮素 (5)[5]对α-葡萄糖苷酶抑制活性要强于阳性对照阿卡波糖。另外,本课题组的前期研究工作发现3,5,5′-三-甲氧基-7-O--D-葡萄糖基槲皮素 (6)也具有明显的α-葡萄糖苷酶抑制活性,此外槲皮素3,7位羟基的反应活性较高,但选择性较差,基于此,我们设计了以芦丁为起始原料合成一系列3位羟基被烷基化,7位羟基被葡萄糖基化的槲皮素葡萄糖苷类化合物,并测试他们对α-葡萄糖苷酶的体外抑制活性。
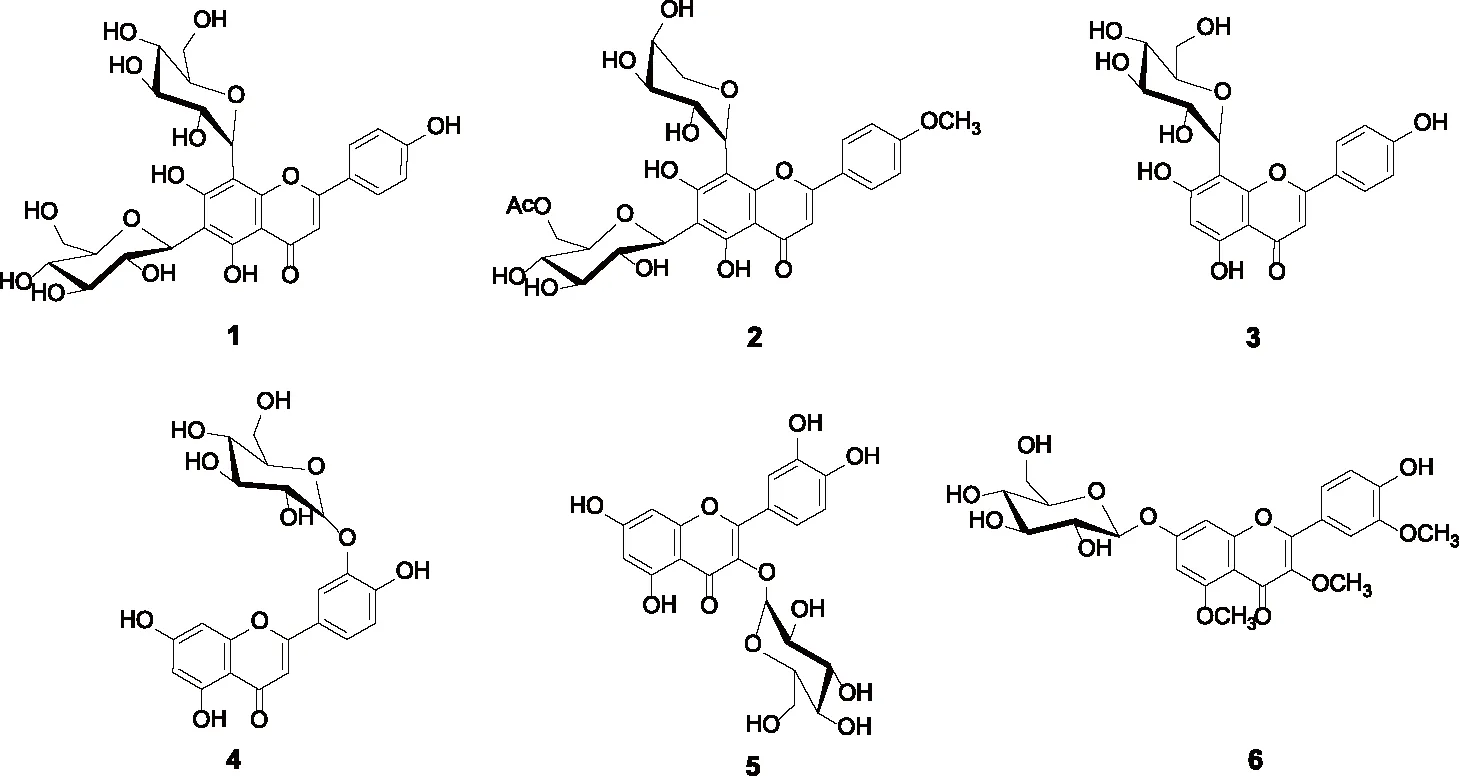
图 1 6个槲皮素葡萄糖苷类化合物的结构Fig.1 Structures of six flavonoid glycosides
1 仪器与试剂
HP-5793质谱仪(美国Hewlett-Packard公司),INOVA-400 MHz型超导核磁共振仪(TMS为内标,美国Varian公司),WNMR-I 500 MHZ核磁共振仪(TMS为内标,中国WIPM公司),柱色谱硅胶(300 ~ 400目)及GF254高效薄层板(青岛海洋化工厂),α-葡萄糖苷酶、牛血清白蛋白(BSA)及对硝基苯-α-葡萄糖昔(PNPG)(美国Sigma公司),阿卡波糖(德国Bayer公司),550型酶标仪(美国Bio-Rad公司),单道、多道移液器(德国Eppendorf公司),96微孔板(德国Greiner公司)。其余试剂若无特别说明均为市售分析纯或化学纯产品。
2 合成路线及方法
2.1合成路线
本实验以芦丁为起始原料,经过7至8步反应合成V和VI类槲皮素葡萄糖苷化合物,合成路线如图2所示。
2.2化合物的合成
2.2.13′,4′,7-三-苄氧基槲皮素(I)[6]
称取无水芦丁(50 g,0.08 mol)置于 1000 mL 反应瓶中,加入 600 mL 无水 DMF,搅拌溶解后加入无水 K2CO3(56 g,0.41 mol)和溴苄(36.9 mL,0.3 mol)。室温反应 2.5 d后,将反应液倒入冰水中,用浓盐酸调节 pH 至 6,析出大量沉淀,过滤,得到黄色粘稠物,粗品不需纯化直接用600 mL乙醇加热溶解,再加入200 mL浓盐酸,于70℃加热3 h,冷却至室温,倒入冰水中,过滤析出的沉淀,水洗至中性,干燥,得 46.8 g 土黄色粉末 I,两步反应总产率 80%。ESI-MSm/z: 595.2 [M + Na]+;1H NMR (400 MHz,DMSO-d6)δ(ppm): 12.48 (s,1H),7.81 (d,J= 1.9 Hz,1H),7.84 (dd,J= 8.8,1.9 Hz,1H),7.30 - 7.55 (m,15H),7.25 (d,J= 8.8 Hz,1H),6.86 (d,J= 1.9 Hz,1H),6.47 (d,J= 1.9 Hz,1H),5.24 (s,4H),5.21 (s,2H)。
2.2.2中间体 II-a ~ II-c总的合成方法[6]
称取 I(10 g,17.5 mmol)置于 500 mL 反应瓶中,加入 150 mL 无水 DMF,搅拌溶解后加入无水K2CO3(4.3 g,31.5 mmol)和 CH3CH2I(CH3CH2CH2I,(CH3)2CHCH2I)(1.2 eq.),室温反应 8 h,反应

图2 V和VI类槲皮素葡萄糖苷化合物的合成路线Fig.2 Synthetic route of quercetin glycosides V and VI
反应试剂与条件: (1) BnBr,K2CO3,DMF,25℃,2.5 d; (2) ethanol,concentrated hydrochloric acid,70℃,3 h; (3) R1I,K2CO3,DMF,25℃,8 h; (4) H2,10% Pd/C,THF,25℃,15 h; (5) dichlorodiphenylmethane,diphenylether,175℃,1 h; (6)α-D-glucopyranosyl bromide tetrabenzoate,K2CO3,TBAB,CHCl3/DMF/H2O,25℃,48 h; (7) 10% Pd/C,H2,CH3OH/THF/H2O,45℃,48 h; (8) CH3ONa,CH3OH/CH2Cl2,25℃,30 min.
液用乙酸乙酯和水萃取,有机层依次用 0.1 M 稀盐酸和水洗涤,无水硫酸镁干燥,浓缩至干,残留物经硅胶柱层析纯化,得到中间体 II-a(II-b,II-c)。
3-乙氧基-3′,4′,7-三-苄氧基槲皮素(II-a):黄色粉末,产率 81%;ESI-MSm/z: 623.2 [M + Na]+;1H NMR (400 MHz,DMSO-d6)δ(ppm): 12.51 (s,1H),7.75 (d,J= 2.3 Hz,1H),7.60 (dd,J= 8.7,2.3 Hz,1H),7.49 - 7.26 (m,15H),7.13 (d,J= 8.6 Hz,1H),6.73 (d,J= 2.1 Hz,1H),6.16 (d,J= 2.2 Hz,1H),5.16 (s,2H),5.15 (s,2H),5.13 (s,2H),3.80 (q,J= 6.8 Hz,2H),1.24 (t,J= 6.8 Hz,3H).3-丙氧基-3′,4′,7-三-苄氧基槲皮素(II-b):黄色粉末,产率 83%;ESI-MSm/z: 637.2 [M + Na]+;1H NMR (400 MHz,CDCl3)δ(ppm): 12.68 (s,1H),7.77 (d,J= 1.9 Hz,1H),7.67 (dd,J= 8.6,2.0 Hz,1H),7.51 - 7.44 (m,5H),7.44 - 7.29 (m,10H),7.02 (d,J= 8.6 Hz,1H),6.46 (d,J= 2.1 Hz,1H),6.42 (d,J= 2.1 Hz,1H),5.26 (s,2H),5.23 (s,2H),5.13 (s,2H),3.90 (t,J= 6.9 Hz,2H),1.72 - 1.61 (m,2H),0.91 (t,J= 7.4 Hz,3H)。
3-异丁氧基-3′,4′,7-三-苄氧基槲皮素(II-c):黄色粉末,产率80%;ESI-MSm/z: 651.2 [M + Na]+;1H NMR (400 MHz,CDCl3)δ(ppm): 12.68 (s,1H),7.72 (d,J= 2.0 Hz,1H),7.67 (dd,J= 8.6,2.0 Hz,1H),7.52 - 7.29 (m,15H),7.02 (d,J= 8.6 Hz,1H),6.46 (d,J= 2.1 Hz,1H),6.42 (d,J= 2.1 Hz,1H),5.25 (s,2H),5.22 (s,2H),5.13 (s,2H),3.72 (d,J= 6.7 Hz,2H),2.08 - 1.93 (m,1H),0.95 (d,J= 6.7 Hz ,6H)。
2.2.3中间体III-a ~ III-c 总的合成方法[7]
称取II-a(II-b,II-c)(9 mmol)置于500 mL反应瓶中,加入无水乙醇和四氢呋喃各100 mL,加热搅拌溶解后加10% Pd/C(于II-a质量的20%),并以氢气置换反应体系中的空气,用氢气球密封反应体系,室温反应过夜,冷却、过滤,滤液浓缩至干,所得产物无需纯化,直接用于下一步反应。
将上一步所得产物置于250 mL反应瓶中,氩气保护下,加入80 mL无水二苯基醚,搅拌溶解后加入二氯二苯甲烷(2.6 mL,0.135 mol),175℃反应1 h后,反应液分散于氯仿和饱和碳酸氢钠水溶液中,萃取,有机相用水洗涤,无水硫酸镁干燥,浓缩至干,残留物经硅胶柱纯化,得III-a(III-b,III-c)。
3-乙氧基-3′,4′-O-二苯基甲叉槲皮素(III-a):土黄色粉末,两步产率 56%;ESI-MSm/z: 517.1 [M + Na]+;1H NMR (400 MHz,DMSO-d6)δ(ppm): 12.60 (s,1H),7.72 (dd,J= 10.4,1.9 Hz,1H),7.71 (d,J= 2.2 Hz,1H),7.58 - 7.52 (m,J= 7.7,1.5 Hz,3H),7.49 - 7.43 (m,6H),7.23 (d,J= 8.2 Hz,1H),6.46 (d,J= 1.9 Hz,1H),6.20 (d,J= 1.9 Hz,1H),4.02 (q,J= 7.0 Hz,2H),1.21 (t,J= 7.0 Hz,3H);13C NMR (100 MHz,DMSO-d6)δ(ppm): 178.29,164.41,161.36,156.58,155.12,148.59,146.86,139.41,137.30,129.77,128.85,125.93,124.44,124.18,117.46,109.12,108.59,104.42,98.81,94.03,68.19,15.46。
3-丙氧基-3′,4′-O-二苯基甲叉槲皮素(III-b):土黄色粉末,两步产率 58%;ESI-MSm/z: 531.1 [M + Na]+;1H NMR (400 MHz,DMSO-d6)δ(ppm): 12.59 (s,1H),7.69 - 7.65 (m,2H),7.57 - 7.53 (m,4H),7.48 - 7.42 (m,7H),7.22 (d,J= 8.8 Hz,1H),6.44 (d,J= 2.1 Hz,1H),6.19 (d,J= 2.1 Hz,1H),3.88 (t,J= 6.5 Hz,2H),1.66 - 1.52 (m,2H),0.83 (t,J= 7.4 Hz,3H);13C NMR (100 MHz,DMSO-d6)δ(ppm): 178.21,164.37,161.36,156.57,155.19,148.57,146.78,139.38,137.44,129.76,128.82,125.91,124.34,124.17,117.42,109.06,108.78,104.45,98.79,94.00,74.00,22.89,10.51。
3-异丁氧基-3′,4′-O-二苯基甲叉槲皮素(III-c):土黄色粉末,两步产率 55%;ESI-MSm/z: 545.1 [M + Na]+;1H NMR (400 MHz,DMSO-d6)δ(ppm): 12.59 (s,1H),7.65 - 7.60 (m,2H),7.57 - 7.51 (m,4H),7.48 - 7.41 (m,6H),7.20 (d,J= 8.3 Hz,1H),6.43 (d,J= 2.1 Hz,1H),6.18 (d,J= 2.1 Hz,1H),3.68 (d,J= 6.4 Hz,2H),1.92 - 1.80 (m,1H),0.84 (d,J= 6.7 Hz,6H);13C NMR (100 MHz,DMSO-d6) δ (ppm): 178.15,164.37,161.39,156.59,155.28,148.59,146.74,139.38,137.58,129.76,128.82,125.92,124.27,124.18,117.41,109.02,108.99,104.51,98.81,94.00,78.64,28.56,19.13。
2.2.4中间体IV-a ~ IV-c总的合成方法[8]
取III-a(III-b,III-c)(0.416 mmol)、TBAB(14 mg,0.042 mmol)和K2CO3(0.403 g,2.91 mmol)混合于50 mL反应瓶中,加入5 mL DMF和5 mL水,室温搅拌15 min,缓慢加入用10 mL氯仿溶解的 2,3,4,6-四-O-苯甲酰基-α-D-溴代吡喃葡萄糖(1.1 g,1.66 mmol),室温搅拌48 h。反应液用稀盐酸调pH至2-3,乙酸乙酯萃取,有机层以水洗涤,无水硫酸镁干燥,浓缩至干,残留物经硅胶柱纯化,得IV-a(IV-b,IV-c)。
3-乙氧基-3′,4′-O-二苯基甲叉-7-O-β-D-苯甲酰基葡萄糖基槲皮素(IV-a):黄色油状物,产率 52%;ESI-MSm/z: 1095.3 [M + Na]+;1H NMR (400 MHz,CDCl3)δ(ppm): 12.65 (s,1H),8.00 - 7.93 (m,6H),7.90 - 7.85 (m,2H),7.66 (dd,J= 8.4,1.8 Hz,1H),7.62 - 7.57 (m,5H),7.55 - 7.50 (m,2H),7.47 (t,J= 7.5 Hz,1H),7.42 - 7.37 (m,9H),7.36 - 7.29 (m,4H),7.26 - 7.20 (m,3H),7.19 - 7.13 (m,2H),6.95 (d,J= 8.4 Hz,1H),6.53 (d,J= 2.2 Hz,1H),6.49 (d,J= 2.2 Hz,1H),6.00 (t,J= 9.2 Hz,1H),5.81 (dd,J= 9.1,7.4 Hz,1H),5.74 (t,J= 9.3 Hz,1H),5.57 (d,J= 7.3 Hz,1H),4.74 (d,J= 9.7 Hz,1H),4.50 - 4.40 (m,2H),4.11 - 4.01 (m,2H),1.33 (t,J= 7.1 Hz,3H);13C NMR (100 MHz,CDCl3)δ(ppm): 179.46,166.58,166.15,165.65,165.43,162.68,162.28,156.66,149.88,147.94,140.23,140.19,138.52,134.11,133.97,133.89,133.56,130.37,130.31,130.28,130.07,129.83,129.66,129.49,129.29,129.09,129.02,128.97,128.93,128.84,128.78,128.68,126.67,125.75,124.63,124.36,109.15,108.97,107.73,99.88,98.65,95.49,77.68,73.43,73.02,71.86,69.69,69.14,63.49.
3-丙氧基-3′,4′-O-二苯基甲叉-7-O-β-D-苯甲酰基葡萄糖基槲皮素(IV-b):黄色油状物,产率 63%;ESI-MSm/z: 1109.3 [M + Na]+;1H NMR (400 MHz,CDCl3)δ(ppm): 12.66 (s,1H),7.99 - 7.93 (m,6H),7.90 - 7.86 (m,2H),7.65 - 7.57 (m,6H),7.55 - 7.44 (m,3H),7.42 - 7.37 (m,9H),7.37 - 7.30 (m,4H),7.25 - 7.21 (m,2H),6.95 (d,J= 8.3 Hz,1H),6.53 (d,J= 2.2 Hz,1H),6.50 (d,J= 2.2 Hz,1H),6.01 (t,J= 9.2 Hz,1H),5.82 (dd,J= 9.2,7.4 Hz,1H),5.74 (t,J= 9.4 Hz,1H),5.57 (d,J= 7.4 Hz,1H),4.74 (d,J= 9.5 Hz,1H),4.50 - 4.40 (m,2H),3.98 - 3.87 (m,2H),1.79 - 1.68 (m,2H),0.95 (t,J= 7.4 Hz,3H);13C NMR (100 MHz,CDCl3)δ(ppm): 178.93,166.08,165.66,165.16,164.94,162.20,161.78,156.18,149.37,147.41,139.73,139.70,138.22,133.62,133.47,133.40,133.07,129.88,129.82,129.79,129.59,129.33,129.18,128.80,128.60,128.53,128.48,128.44,128.37,128.34,128.29,126.19,124.08,123.90,108.79,108.46,107.29,99.37,98.17,95.00,74.55,72.94,72.53,71.37,69.20,63.01,23.30,10.38。
3-异丁氧基-3′,4′-O-二苯基甲叉-7-O-β-D-苯甲酰基葡萄糖基槲皮素(IV-c): 黄色油状物,产率 52%;ESI-MSm/z: 1123.3 [M + Na]+;1H NMR (500 MHz,CDCl3)δ(ppm): 8.00 - 7.92 (m,7H),7.90 - 7.84 (m,2H),7.61 - 7.44 (m,10H),7.41 - 7.30 (m,15H),7.24 (s,2H),6.94 (d,J= 10.0 Hz,1H),6.53 (s,1H),6.50 (s,1H),6.00 (t,J= 8.8 Hz,1H),5.81 (t,J= 7.6 Hz,1H),5.74 (t,J= 9.2 Hz,1H),5.56 (d,J= 6.2 Hz,1H),4.73 (d,J= 11.5 Hz,1H),4.52 - 4.38 (m,2H),3.80 - 3.63 (m,2H),2.09 - 1.97 (m,1H),0.96 (d,J= 5.7 Hz,6H);13C NMR (125 MHz,CDCl3)δ(ppm): 178.91,166.11,165.69,165.18,164.97,162.25,161.80,156.22,149.40,147.40,139.75,139.72,138.40,133.63,133.49,133.42,133.09,129.90,129.85,129.81,129.62,129.35,129.21,128.84,128.63,128.57,128.50,128.46,128.39,128.35,128.32,126.22,124.04,123.95,117.89,108.94,108.43,107.37,99.38,98.23,95.03,79.24,72.97,72.56,71.40,69.23,63.04,28.99,19.18。
2.2.5目标产物V-a ~ V-c总的合成方法[9]
取 IV-a(IV-b,IV-c)(0.523 mmol)于250 mL反应瓶中,加入80 mL甲醇、20 mL四氢呋喃和1 mL水溶解,加入10% Pd/C(于IV-a质量的20%),并以氢气置换反应体系中的空气后,以氢气球密封反应体系,45℃反应48 h,过滤去除钯碳,减压回收溶剂至干,所得残留物经硅胶柱层析纯化,得V-a(V-b,V-c)。
3-乙氧基-7-O-β-D-苯甲酰基葡萄糖基槲皮素(V-a):黄色油状物,产率 51%;ESI-MSm/z: 931.3 [M + Na]+;1H NMR (400 MHz,CDCl3)δ(ppm): 12.54 (s,1H),7.99-7.86 (m,8H),7.75 (s,1H),7.55 - 7.41 (m,5H),7.40 - 7.23 (m,10H),7.02 - 6.94 (m,1H),6.46 (d,J= 1.9 Hz,1H),6.45 (d,J= 2.0 Hz,1H),6.03 (t,J= 9.2 Hz,1H),5.81 (dd,J= 8.9,7.3 Hz,1H),5.77 (t,J= 8.8 Hz,1H),5.54 (d,J= 7.3 Hz,1H),4.74 (dd,J= 12.0,2.5 Hz,1H),4.55 - 4.39 (m,2H),4.01 (q,J= 6.9 Hz,2H),1.28 (t,J= 7.0 Hz,3H);13C NMR (100 MHz,CDCl3)δ(ppm): 179.17,166.58,166.06,165.39,165.26,162.05,161.95,157.28,156.31,147.58,143.72,137.84,133.82,133.74,133.67,133.39,130.02,129.98,129.78,129.22,128.87,128.64,128.57,128.51,122.66,122.41,115.83,115.32,107.22,99.60,98.26,95.35,77.36,72.85,72.78,71.61,69.46,69.03,63.26。
3-丙氧基-7-O-β-D-苯甲酰基葡萄糖基槲皮素(V-b):黄色油状物,产率 49%;ESI-MSm/z: 945.3 [M + Na]+;1H NMR (400 MHz,CDCl3)δ(ppm): 12.58 (s,1H),7.99 - 7.86 (m,8H),7.69 (s,1H),7.54 - 7.48 (m,3H),7.47 - 7.39 (m,2H),7.38 - 7.27 (m,8H),6.97 (d,J= 8.4 Hz,1H),6.44 (s,2H),6.02 (t,J= 9.2 Hz,1H),5.81 (dd,J= 8.8,7.2 Hz,1H),5.79 - 5.74 (m,1H),5.53 (d,J= 7.3 Hz,1H),4.74 (dd,J= 12.0,2.6 Hz,1H),4.54 - 4.39 (m,2H),3.89 (t,J= 6.9 Hz,2H),1.75 - 1.65 (m,2H),0.88 (t,J= 7.4 Hz,3H);13C NMR (100 MHz,CDCl3)δ(ppm): 178.99,166.43,165.91,165.25,165.12,161.96,161.78,156.97,156.17,147.30,143.52,137.98,133.66,133.58,133.51,133.24,129.87,129.83,129.64,129.10,128.74,128.49,128.42,128.37,122.55,122.37,115.74,115.15,107.15,99.42,98.16,95.20,77.26,77.01,76.76,74.81,72.73,72.65,71.47,69.33,63.13,23.22,10.29。
3-异丁氧基-7-O-β-D-苯甲酰基葡萄糖基槲皮素(V-c):黄色油状物,产率 84%;ESI-MSm/z: 959.3 [M + Na]+;1H NMR (500 MHz,CDCl3)δ(ppm): 12.66 (s,1H),8.00 - 7.92 (m,7H),7.90 - 7.84 (m,2H),7.61 - 7.44 (m,10H),7.41 - 7.30 (m,15H),7.24 (s,2H),6.94 (d,J= 10.0 Hz,1H),6.53 (s,1H),6.50 (s,1H),6.00 (t,J= 8.8 Hz,1H),5.81 (t,J= 7.6 Hz,1H),5.74 (t,J= 9.2 Hz,1H),5.56 (d,J= 6.2 Hz,1H),4.73 (d,J= 11.5 Hz,1H),4.52 - 4.38 (m,2H),3.80 - 3.63 (m,2H),2.09 - 1.97 (m,1H),0.91 (d,J= 6.7 Hz,6H);13C NMR (100 MHz,CDCl3)δ(ppm): 179.05,166.62,166.01,165.41,165.29,162.10,161.83,156.97,156.26,147.29,143.63,138.36,133.82,133.75,133.66,133.42,130.02,129.98,129.96,129.79,129.19,128.83,128.64,128.60,128.56,128.53,122.68,122.64,115.90,115.25,107.32,99.42,98.26,95.31,79.63,72.86,72.75,71.59,69.43,63.27,29.05,19.33。
2.2.6目标产物VI-a ~ VI-c总的合成方法[8]
取V-a(V-b,V-c)(0.089 mmol)于10 mL反应瓶中,加入4 mL甲醇和1 mL二氯甲烷溶解,缓慢加入甲醇钠(0.41 mg,0.27 mmol),室温搅拌30 min。搅拌下加入阳离子交换树脂调节pH至5-6,过滤,浓缩滤液,所得残留物经硅胶柱纯化,得VI-a(VI-b,VI-c)。
3-乙氧基-7-O-β-D-葡萄糖基槲皮素(VI-a):淡黄色粉末,产率 92%;ESI-MSm/z: 515.2 [M + Na]+;1H NMR (400 MHz,DMSO-d6)δ(ppm): 9.83 (s,1H),9.40 (br,1H),8.29 (br,1H),7.60 (d,J= 2.2 Hz,1H),7.47 (dd,J= 8.5,2.2 Hz,1H),6.90 (d,J= 8.5 Hz,1H),6.75 (d,J= 2.1 Hz,1H),6.42 (d,J= 2.1 Hz,1H),5.41 (br,1H),5.13 (br,1H),5.06 (d,J= 7.3 Hz,2H),4.61 (br,1H),4.11 (br,1H),4.01 (q,J= 7.0 Hz,2H),3.73 - 3.65 (m,1H),3.49 - 3.45 (m,1H),3.30 - 3.23 (m,2H),3.17 (s,1H),1.26 (t,J= 7.0 Hz,3H);13C NMR (100 MHz,DMSO-d6)δ(ppm): 178.33,162.90,160.95,156.60,156.03,148.86,145.21,136.85,120.91,120.79,115.73,115.67,105.84,99.86,99.21,94.48,79.21,77.18,76.42,73.15,69.57,67.81,60.65,48.66,15.37。
3-丙氧基-7-O-β-D-葡萄糖基槲皮素(VI-b):淡黄色粉末,产率 63%;ESI-MSm/z: 529.2 [M + Na]+;1H NMR (400 MHz,DMSO-d6)δ(ppm): 12.72 (s,1H),8.29 (s,1H),7.55 (d,J= 2.2 Hz,1H),7.45 (dd,J= 8.5,2.2 Hz,1H),6.89 (d,J= 8.5 Hz,1H),6.74 (d,J= 1.9 Hz,1H),6.42 (d,J= 2.0 Hz,1H),5.05 (d,J= 7.4 Hz,2H),4.63 (s,1H),3.89 (t,J= 6.6 Hz,2H),3.72 - 3.64 (m,1H),3.51 - 3.48 (m,2H),3.29 - 3.22 (m,2H),3.18 - 3.12 (m,1H),1.65 (h,J= 7.3 Hz,2H),0.89 (t,J= 7.4 Hz,3H);13C NMR (100 MHz,DMSO-d6)δ(ppm): 178.29,162.91,160.98,156.68,156.07,148.88,145.24,137.06,120.88,115.84,115.64,105.91,99.89,99.24,94.51,79.23,77.20,76.44,73.73,73.17,69.61,60.67,48.69,22.85,10.46。
3-异丁氧基-7-O-β-D-葡萄糖基槲皮素(VI-c):淡黄色粉末,产率 68%;ESI-MSm/z: 543.3 [M + Na]+;1H NMR (400 MHz,DMSO-d6)δ(ppm): 12.67 (s,1H),7.45 (d,J= 2.1 Hz,1H),7.37 (dd,J= 8.4,2.1 Hz,1H),6.84 (d,J= 8.4 Hz,1H),6.69 (d,J= 1.3 Hz,1H),6.37 (d,J= 1.5 Hz,1H),5.40 (br,1H),5.08 (br,1H),5.00 (d,J= 7.3 Hz,2H),4.60 (br,1H),3.64 (d,J= 6.5 Hz,3H),3.56 (s,2H),3.28 - 3.16 (m,3H),3.11 (t,J= 8.8 Hz,1H),1.96 - 1.80 (m,1H),0.85 (d,J= 6.7 Hz,6H).13C NMR (100 MHz,DMSO-d6)δ(ppm): 178.28,162.93,161.04,156.80,156.13,148.87,145.27,137.29,121.05,120.89,115.98,115.63,106.00,99.93,99.29,94.56,78.47,77.24,76.47,73.21,69.66,60.71,28.57,19.22。
2.3α-葡萄糖苷酶抑制活性测试
按照杨付梅等[10]的方法,将20 μL 0.1 mol/L的含有0.2 U/mLα-葡萄糖苷酶及 0.1% 牛血清白蛋白的磷酸盐缓冲液用50 μL 0.1 mol/L的磷酸盐缓冲液稀释后,加入10 μL 0.1 mol/L的含有1 μmol/L 的目标化合物的磷酸盐缓冲液,37℃ 孵育10 min,然后加入20 μL 0.1 mol/L的有含10 mmol/L的对硝基苯-α-葡萄糖昔的磷酸盐缓冲液,37℃反应15 min,加入反应终止剂后测定405 nm OD值,阿卡波糖(Acarbose)为阳性对照药物。

图1 目标产物的结构

CompoundR3R3'R4'R5RIC50(μmol/L)V-a-CH2CH3-H-H-H-Bz56.5V-bCH2CH2CH3-H-H-H-Bz19.4V-cCH2CH(CH3)2-H-H-H-Bz21.5VI-a-CH2CH3-H-H-H-H>100VI-b-CH2CH2CH3-H-H-H-H>100VI-cCH2CH(CH3)2-H-H-H-H>100Acarbose2.5×10-3
3 结果与讨论
从以上活性测试结果可以看出,在所筛选的6个样品中,当化合物中糖基上的羟基游离时,所得衍生物活性相对较低,见VI-a、VI-b和VI-c;而当糖基上的羟基苯甲酰化后,所得衍生物V-a、V-b和V-c的抑制活性则大为提高,特别是V-b和V-c显示出较好的抑制活性,其IC50各为19.4 μmol/L,21.5 μmol/L,以上结果表明苯甲酰基会增强其抑制活性。该类化合物3位上的羟基被丙基取代所得衍生物与异丁基取代所得衍生物的活性相当,而当3位上的羟基被乙基取代后所得衍生物的活性明显降低。这将为该类化合物在降糖活性方面的深入研究提供参考。
槲皮素酚羟基的相对活性总体表现为:7 > 3 ≈ 4′ > 3′ >> 5。5-羟基活性最弱是其与羰基形成分子内氢键所致[11]。在本研究中,以槲皮素为起始原料来合成目标化合物是难以实现的,所以选择芦丁为起始原料。先对芦丁 7,4′,3′ 位的三个羟基用苄基保护,然后将 3 位上的芸香糖基水解,再对 3-羟基进行一系列烷基化修饰,最后将三个苄基脱掉,并对 3′,4′-羟基选择性保护后即可实现对 7-羟基进行糖基化修饰。该合成路线条件简单,原料易得,且糖基化收率较高。
[1]Hollander P. Safety Profile of Acarbose,anα-Glucosidase Inhibitor [J].Drugs,1992,44(3): 47 - 53.
[2]Md. N I,Ishrat J I,Hyun A J,etal. Vicenin 2 isolated from Artemisia capillaris exhibited potent anti-glycation properties [J].FoodChemToxicol,2014(69):55 - 62.
[3]Shahira M E,Maha M S. A new a-glucosidase inhibitor from Achillea fragrantissima (Forssk.) Sch. Bip. growing in Egypt [J].NatProdRes,2014,28 (11): 812 - 818.
[4]Chenyan gan,Li ping,Li peng,etal.α-Glucosidase Inhibitory Effect and Simultaneous Quantification of Three Major Flavonoid Glycosides in Microctis folium [J].Molecules,2013(18): 4221 - 4232.
[5]Nguyen P H,Dung V V,Zhao B T,etal.Antithrombotic and antidiabetic flavonoid glycosides from the grains of Sorghum bicolor (L.) Moench var. hwanggeumchal [J].ArchPharmRes,2014(37): 1394 - 1402.
[6]Lihua jun,Luan xin hui,Zhao yi min. Facile Synthesis of 3-O-Methylquercetin [J].ChinJOrgChem,2004,24(19): 1619 - 1621.
[7]Mohamed B,Stéphane L,Aziz A,etal. Hemisynthesis of all the O-monomethylated analogues of quercetin including the major metabolites,through selective protection of phenolic functions[J].Tetrahedron,2002(58):10001 - 10009.
[8]Gao qi,Lian gao yan,Lin feng.The first total synthesis of 7-O-β-D- glucopyranosyl-4′-O-α- L-rhamnopyranosyl apigenin via a hexanoyl ester-based protection strategy [J].CarbohydrRes,2009(344): 511 - 515.
[9]Mohammed K,Christian R.Regiospecific synthesis of quercetin O-β-D-glucosylated and O-β-D-glucuronidated isomers [J].Tetrahedron,2011(67): 4731 - 4741.
[10]Yangfu mei,Sun qian yun. Study on microassay for screeningα-glucosidase inhibitors by orthogonal matrix method [J].ChinPharmacolBull,2009,25(8),1113 - 1116 .
[11]Meiqing gang,Yuan wei cheng,Wang chun. Progress in the Synthesis of 3-Hydroxyflavones [J].ChinJOrgChem,2015,35(1): 70 - 84.
Synthesis of Quercetin Glycosides and Their Inhibitory Activities to α-Glucosidase
WENZhong-hang1,2,HUZhan-xing2,YUANJie1,2,CHENHong-ju2,ZHANGLi-mei2,LIANGGuang-yi2*,XUBi-xue2*
(1.SchoolofPharmaceuticalSciences,GuizhouUniversity,Guiyang,Guizhou550025,China; 2.TheKeyLaboratoryofChemistryforNaturalProductsofGuizhouProvinceandChineseAcademyofSciences,Guiyang,Guizhou550007,China)
Six quercetin glycosides were designed and synthesized andtheir structures were confirmed by1H NMR,13C NMR and MS. The inhibitory activities of those compounds to α-glucosidase were evaluatedinvitro.The benzoylated compounds V-b and V-c showed promising bioactivities with IC50of 19.4 μmol/L and 21.5 μmol/L,while the inhibitory activities of VI-b and VI-c were lower.These results suggested that the benzoyl substitutions on quercetin glycosides might enhance inhibitory activities. This research may provide a reference for the studies on the hypoglycemic activity of quercetin glycosides.
Quercetin glycosides; Inhibitory activity; α-Glucosidase
1008-0457(2016)03-0018-07国际DOI编码:10.15958/j.cnki.sdnyswxb.2016.03.003
2016-02-29;修回日期:2016-04-15
贵州省优秀青年科技人才培养对象专项资金[黔科合人字(2015)24号]。
梁光义(1952-),教授,博士生导师,主要研究方向:天然药物化学,E-mail: guangyi_liang@126.com;徐必学(1976-),博士,副研究员,主要研究方向:天然药物化学,E-mail: bixue_xu@126.com。
R914
A
