熊果酸抑制胃癌细胞COX-2表达的信号转导研究
2016-08-10周逸婵朱国琴李剑萍李晓林孙为豪
朱 悦,周逸婵,朱国琴,李剑萍,焦 政,李晓林,邵 耘,孙为豪
(1.南京医科大学第一附属医院老年消化科,江苏 南京 210029;2.盐城市第一人民医院肿瘤科,江苏 盐城 224005)
熊果酸抑制胃癌细胞COX-2表达的信号转导研究
朱悦1,周逸婵1,朱国琴1,李剑萍2,焦政1,李晓林1,邵耘1,孙为豪1
(1.南京医科大学第一附属医院老年消化科,江苏 南京210029;2.盐城市第一人民医院肿瘤科,江苏 盐城224005)
目的进一步明确熊果酸(ursolic acid,UA)抑制胃癌细胞环氧化酶-2(cycloxygenase-2,COX-2)表达的信号转导通路。方法人胃腺癌细胞株SGC-7901和MKN-45常规培养于RPMI-1640培养液中,细胞长至亚单层后分别加抗氧化剂N-乙酰-L半胱氨酸(NAC)、单磷酸腺苷激活的蛋白激酶(AMP-activated protein kinase, AMPK)激活剂5-氨基咪唑-4-甲酰胺核苷酸(AICAR)、AMPK抑制剂compound C和信号转导与转录活化因子3(signal transducer and activator of transcription 3, STAT3)抑制剂WP1066预处理后再加UA连续培养24 h,Western blot检测AMPK、STAT3磷酸化水平和COX-2蛋白表达。结果抗氧化剂NAC和AMPK抑制剂compound C有效地阻断了UA抑制STAT3磷酸化和COX-2表达的作用,AMPK激活剂AICAR抑制STAT3磷酸化和COX-2表达,AICAR和UA联合作用大于单用,STAT3抑制剂WP1066对UA诱导的AMPK磷酸化无明显影响,WP1066单独或联合UA均可抑制STAT3磷酸化和COX-2表达且联合作用大于单用。结论UA通过ROS/AMPK/STAT3信号转导通路抑制胃癌细胞COX-2表达。
熊果酸;胃癌;活性氧;单磷酸腺苷激活的蛋白激酶;信号转导与转录活化因子3;环氧化酶-2
胃癌是影响我国人民生命健康最严重的恶性肿瘤之一,是癌症死亡的主要原因。根治性手术是目前治疗胃癌的最有效方法,但手术不能完全避免局部复发和远处转移的可能,防止肿瘤复发和转移的辅助治疗越来越受到国内外学者的重视。熊果酸(ursolic acid, UA)是广泛存在于白花蛇舌草、女贞子、乌梅和夏枯草等天然植物中的一种五环三萜类化合物,具有抑制肿瘤细胞增殖、诱导细胞凋亡、抗血管生成、抗促癌、抗突变和调节氧化应激等作用,近年来已成为肿瘤化学预防研究的热点[1-3]。临床前研究显示,UA在转基因肿瘤动物模型和人癌裸鼠移植模型中的抑瘤作用明显[3-4],具有良好的临床应用前景。我们的前期研究发现,UA促进胃癌细胞内活性氧(reactive oxygen species, ROS)生成,诱导单磷酸腺苷激活的蛋白激酶(AMP-activated protein kinase, AMPK)磷酸化,抑制信号转导与转录活化因子3(signal transducer and activator of transcription 3, STAT3)磷酸化和环氧化酶-2(cycloxygenase-2,COX-2)表达[5-6]。UA通过下调COX-2表达而抑制胃癌细胞增殖、诱导凋亡[6-7]。然而,UA抑制胃癌细胞COX-2表达的信号转导通路尚不清楚。本研究选择2株不同分化程度但COX-2高表达的SGC-7901和MKN-45胃癌细胞株作为研究对象,观察ROS/AMPK/STAT3信号转导通路在UA影响SGC-7901和MKN-45胃癌细胞株COX-2表达中的作用,进一步探讨UA抑制胃癌细胞COX-2表达的具体机制。
1 材料与方法
1.1药物和试剂UA、AMPK激活剂5-氨基咪唑-4-甲酰胺核苷酸(AICAR)、二甲基亚砜(DMSO)、碘化丙啶(PI)、苯甲磺酰氟(phenylmethanesulfonyl fluoride,PMSF) 和抑肽酶为美国Sigma-Aldrich公司产品;AMPK抑制剂compound C和STAT3抑制剂WP1066为德国Merck公司产品;RPMI 1640培养液和胎牛血清为美国Gibco BRL公司产品;蛋白质定量BCA试剂盒为美国Pierce公司产品;抗氧化剂N-乙酰-L半胱氨酸(NAC)为江苏碧云天生物技术公司产品;兔抗人AMPK和磷酸化AMPK(p-AMPK)、乙酰辅酶A羧化酶(ACC)和磷酸化ACC(p-ACC)、STAT3和 磷酸化STAT3(p-STAT3)、COX-2和β-actin单克隆抗体为美国Cell Signaling Technology公司产品;辣根过氧化物酶标记的山羊抗兔IgG抗体为美国Bioworld Technology公司产品。PVDF膜为美国Millipore公司产品;ECL发光试剂盒为英国Amersham公司产品;其它试剂为国产分析纯级。
1.2细胞和培养人胃腺癌中分化细胞株SGC-7901购自中国科学院上海生科院细胞资源中心,人胃腺癌低分化细胞株MKN-45购自南京凯基生物科技发展有限公司。SGC-7901和MKN-45细胞常规传代培养于含10%胎牛血清、100 kU·L-1青霉素和100 mg·L-1链霉素的RPMI 1640培养液中,37℃、5% CO2及饱和湿度的二氧化碳培养箱中培养生长。隔天换液,3 d传代1次。
1.3药物配制UA、AICAR、compound C和WP1066先以DMSO溶解,NAC用超纯水溶解,而后均以RPMI 1640培养液稀释至所需浓度,DMSO在培养液中的浓度不超过0.1%,0.22 μm的微孔滤膜过滤除菌后4℃保存备用。
1.4实验分组将传代后处于对数生长期的细胞分为对照(control)组、UA(30 μmol·L-1)组、抗氧化剂(NAC, 5 mmol·L-1)组、NAC+UA组、AMPK激活剂(AICAR, 0.5 mmol·L-1)组、AICAR+UA组、AMPK抑制剂(compound C, 2.5 μmol·L-1)组、compound C+UA组、STAT3抑制剂(WP1066, 5 μmol·L-1)组、WP1066+UA组。NAC+UA组NAC预处理30 min后UA再干预培养24 h;AICAR+UA组AICAR预处理2 h后UA再干预培养24 h;compound C+UA组compound C预处理1 h后UA再干预培养24 h;WP1066+UA组WP1066预处理1 h后UA再干预培养24 h。
1.5细胞蛋白提取和Western blot检测各组干预培养后的细胞用预冷的PBS洗涤3次,以100 μL细胞裂解液(PBS内含:Nonidet P-40 1%,脱氧胆酸钠5 g·L-1,SDS 1 g·L-1,PMSF 0.1 g·L-1和抑肽酶10 mg·L-1)4℃处理60 min。细胞裂解物经11 000×g4℃离心10 min后取上清,BCA试剂盒测定其蛋白浓度。常规进行SDS-PAGE电泳后转印至PVDF膜,室温封闭2 h,分别加入一抗(APMK抗体、p-AMPK抗体、ACC抗体、p-ACC抗体、STAT3抗体、p-STAT3抗体、COX-2抗体和β-actin抗体),4℃孵育过夜,辣根过氧化物酶标记的山羊抗兔IgG抗体为第二抗体,4℃孵育2 h,ECL发光,使用ImageJ(National Institute of Health, Bethesda, MD)图像分析软件对蛋白电泳带的灰度进行半定量分析。
2 结果
2.1UA和抗氧化剂NAC对胃癌细胞AMPK、STAT3磷酸化和COX-2表达的影响Western blot检查结果显示,抗氧化剂NAC有效抑制UA诱导的AMPK磷酸化,ACC是AMPK的下游直接效应靶蛋白,p-ACC被认为是观察AMPK被激活的最佳指标[8](Fig 1),UA抑制STAT3磷酸化和COX-2表达的作用被NAC所逆转(Fig 2)。
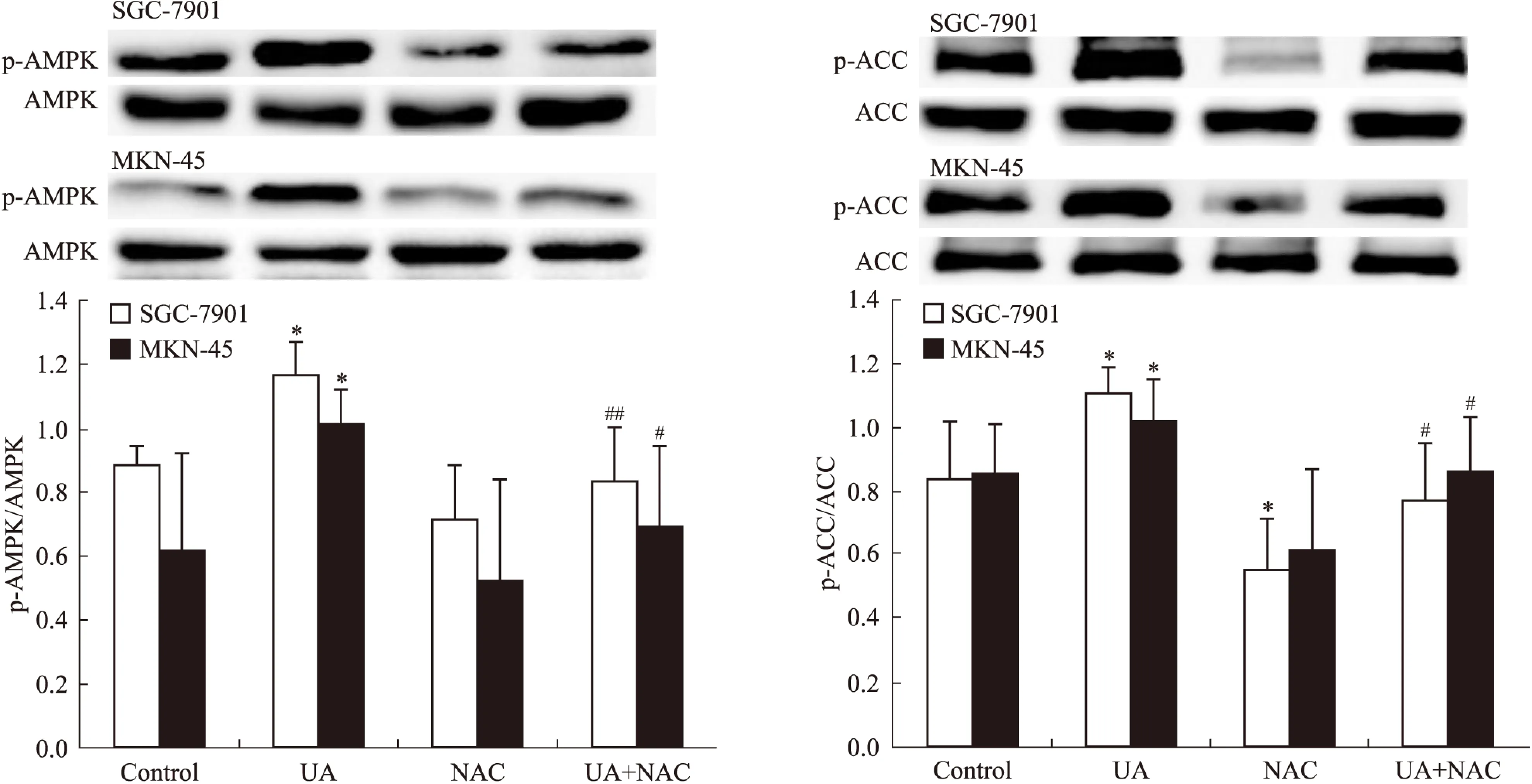
Fig 1 Effects of NAC on ursolic acid(UA)-induced AMPK and ACC phosphorylation in gastric cancer cells


Fig 2 Effects of NAC on UA-inhibited STAT3 phosphorylation and COX-2 expression in gastric cancer cells
2.2UA和AMPK激活剂AICAR对胃癌细胞AMPK、STAT3磷酸化和COX-2表达的影响Western blot检查结果显示,AMPK激活剂AICAR单用或联合UA均可诱导SGC-7901和MKN-45细胞AMPK磷酸化(Fig 3),抑制STAT3磷酸化和COX-2表达,联合作用大于单药作用(Fig 4)。
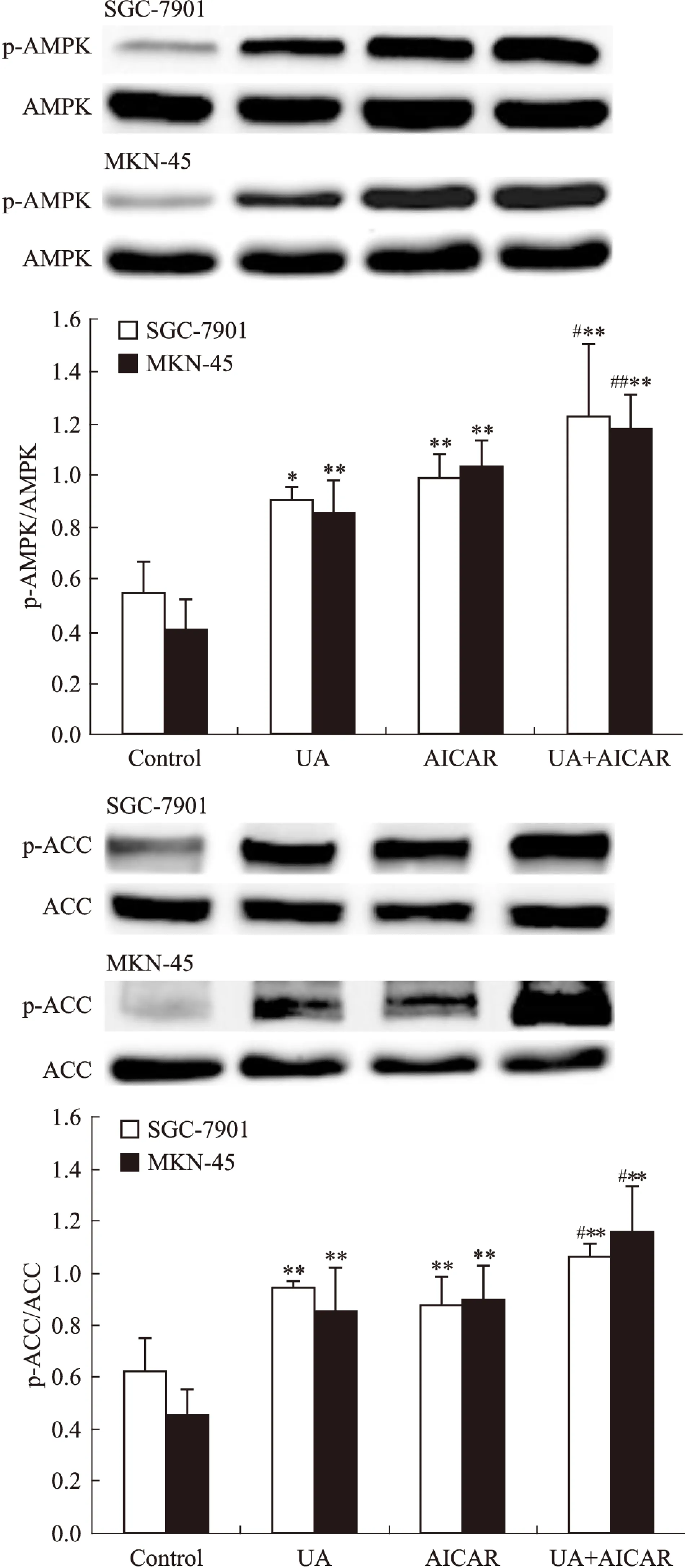
Fig 3 Effects of AMPK activator AICAR(5-amino-4-imidazolecarboxamide riboside-1-b-D-ribofuranoside) on UA-induced AMPK and ACC phosphorylation in gastric cancer cells

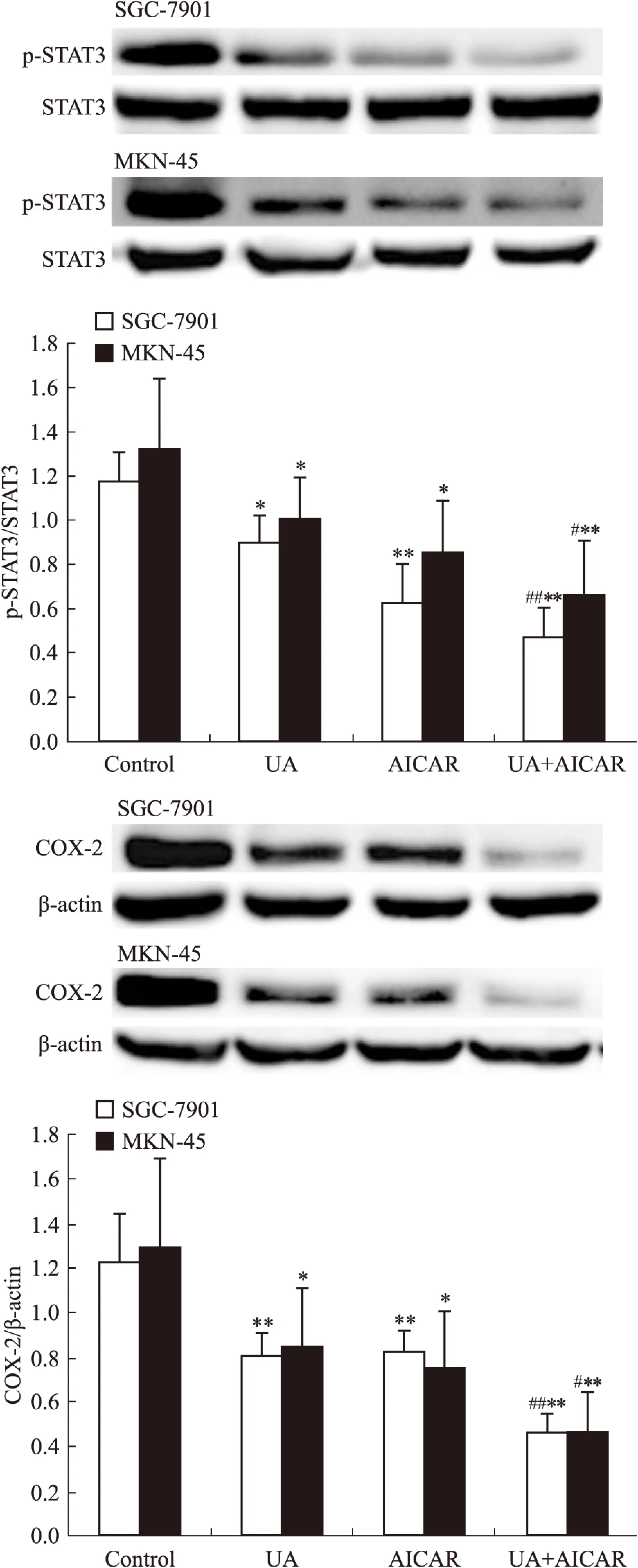
Fig 4 Effects of AMPK activator AICAR on UA-inhibited STAT3 phosphorylation and COX-2 expression in gastric cancer cells
2.3UA和AMPK抑制剂compound C对胃癌细胞AMPK、STAT3磷酸化和COX-2表达的影响AMPK抑制剂compound C有效抑制UA诱导的AMPK磷酸化(Fig 5),UA抑制STAT3磷酸化和COX-2表达的作用被compound C所逆转(Fig 6)。

Fig 5 Effects of AMPK inhibitor compound C on UA-induced AMPK and ACC phosphorylation in gastric cancer cells


Fig 6 Effects of AMPK inhibitor Compound C on UA-inhibited STAT3 phosphorylation and COX-2 expression in gastric cancer cells
2.4UA和STAT3抑制剂WP1066对胃癌细胞AMPK、STAT3磷酸化和COX-2表达的影响STAT3抑制剂WP1066对UA诱导的AMPK磷酸化无明显影响(Fig 7),WP1066单用或联合UA均可抑制SGC-7901和MKN-45细胞STAT3磷酸化和COX-2表达,且联合作用大于单药作用(Fig 8)。
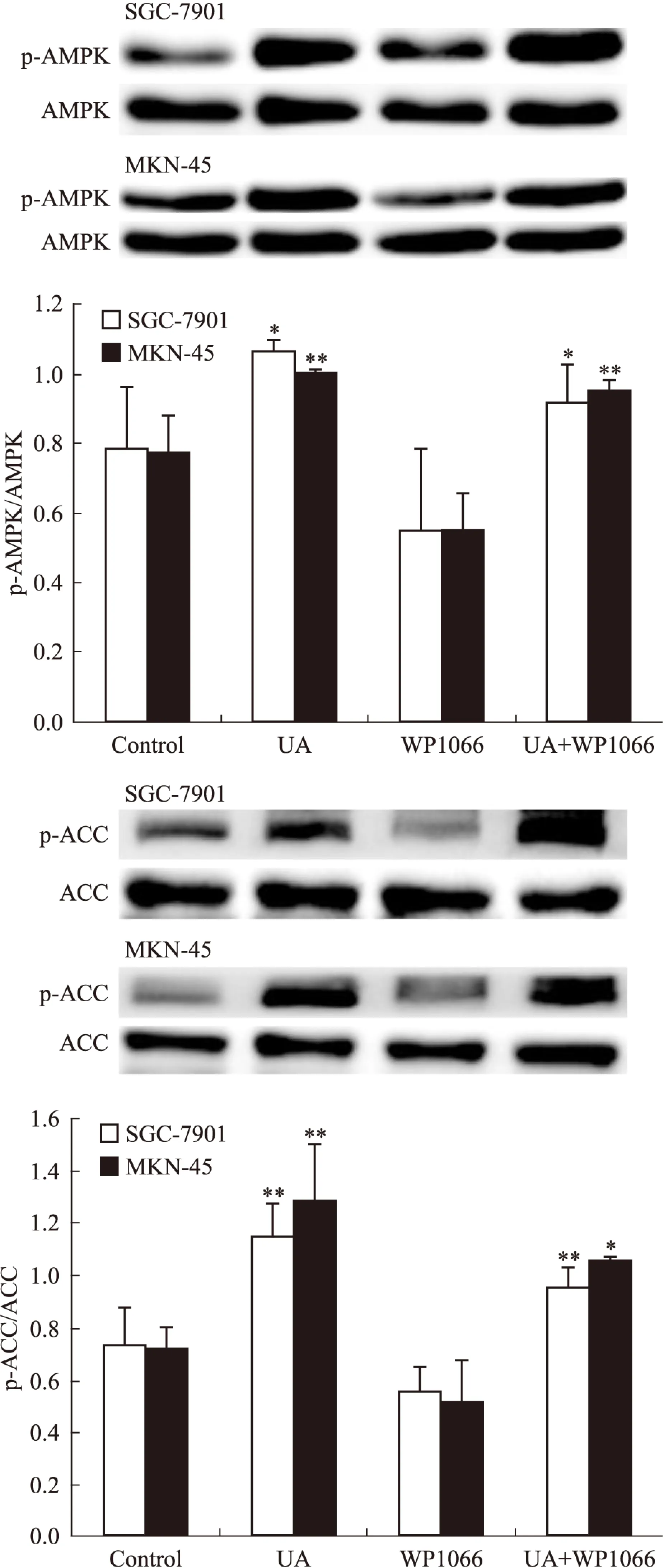
Fig 7 Effects of STAT3 inhibitor WP1066 on UA-induced AMPK and ACC phosphorylation in gastric cancer cells

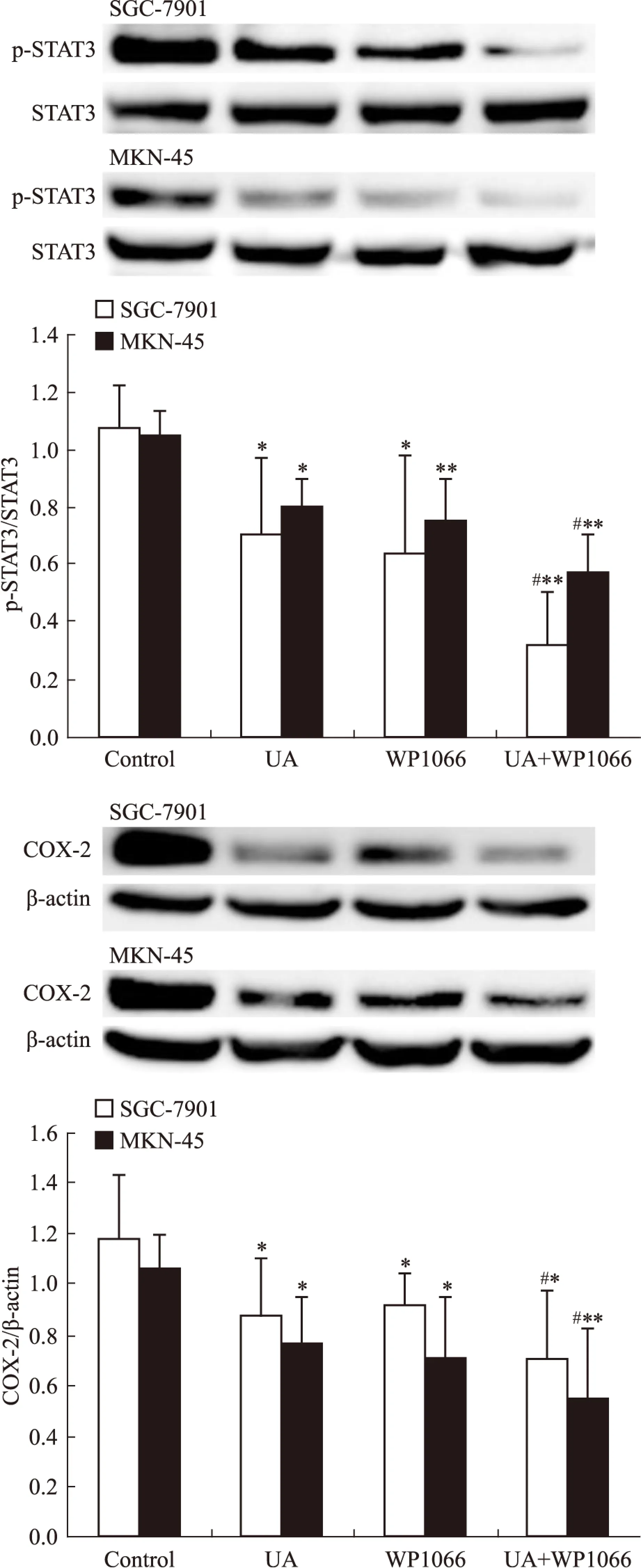
Fig 8 Effects of STAT3 inhibitor WP1066 on UA-inhibited STAT3 phosphorylation and COX-2 expression in gastric cancer cells
3 讨论
ROS是氧在机体代谢过程中产生的中间产物及其衍生物,参与细胞增殖、分化、转化和凋亡以及细胞内重要信号途径的转导[9],近年来研究表明,抗肿瘤药物诱导细胞凋亡与其引起肿瘤细胞内ROS水平增加密切相关[10-12]。AMPK是细胞能量代谢的主要调节器[13],激活AMPK可诱导胃癌和胰腺癌等肿瘤细胞发生凋亡[14-15],UA和白藜芦醇(resveratrol)的抗肿瘤作用与ROS依赖的AMPK活化有关[5,16-17]。
AMPK除参与细胞内的能量代谢调节外还调控一些基因的转录和表达[18],本研究结果显示,UA诱导AMPK磷酸化,抑制STAT3磷酸化和COX-2表达;抗氧化剂NAC能逆转UA对STAT3磷酸化和COX-2表达的抑制作用,提示ROS是UA抑制STAT3活性和COX-2表达的重要介质[5]。AMPK激活剂AICAR是AMP类似物,明显抑制STAT3磷酸化和COX-2表达,UA与AICAR联合使用对STAT3磷酸化和COX-2表达的抑制作用和单用UA组相比差异有显著性,提示UA联合AICAR后AMPK得到进一步的激活。而AMPK抑制剂compound C则逆转UA对STAT3磷酸化和COX-2表达的抑制作用,进一步证明UA通过AMPK信号通路抑制胃癌细胞STAT3活化和COX-2表达。
我们前期研究发现,JAK2/STAT3和PI3K/Akt信号途径能诱导胃癌细胞COX-2的表达[19],而UA能阻断STAT3通路抑制多发性骨髓瘤细胞增殖[20]。STAT3在各种类型的人胃癌细胞株和胃癌组织中都有较高的活性,JAK/STAT信号转导途径可能在胃癌的发生、发展中起重要的作用[21]。因此,我们推测UA通过ROS/AMPK/STAT3通路下调COX-2表达。当然,由于细胞内信号转导机制十分复杂,UA抑制胃癌细胞COX-2表达尚不排除有其它通路的存在,也不排除不同通路之间存在关联(如cross-talk等),要全面了解其机制需大量而深入的研究。
[1]Kalani K, Yadav D K, Khan F, et al. Pharmacophore, QSAR, and ADME based semisynthesis andinvitroevaluation of ursolic acid analogs for anticancer activity[J].JMolModel, 2012, 18(7):3389-413.
[2]Zhao C, Yin S, Dong Y, et al. Autophagy-dependent EIF2AK3 activation compromises ursolic acid-induced apoptosis through upregulation of MCL1 in MCF-7 human breast cancer cells[J].Autophagy, 2013, 9(2):196-207.
[3]Shanmugam M K, Manu K A, Ong T H, et al. Inhibition of CXCR4/CXCL12 signaling axis by ursolic acid leads to suppression of metastasis in transgenic adenocarcinoma of mouse prostate model[J].IntJCancer, 2011, 129(7):1552-63.
[4]Prasad S, Yadav V R, Sung B, et al. Ursolic acid inhibits growth and metastasis of human colorectal cancer in an orthotopic nude mouse model by targeting multiple cell signaling pathways: chemosensitization with capecitabine[J].ClinCancerRes, 2012, 18(18):4942-53.
[5]庞姗姗,朱悦,周逸婵,等. 熊果酸通过ROS/AMPK/STAT3信号通路抑制胃癌细胞COX-2表达[J]. 江苏医药,2015, 41(12):1368-70.
[5]Pang S S, Zhu Y, Zhou Y C, et al. Ursolic acid inhibiting COX-2 expression in gastric cancer cell through ROS/AMPK/STAT3 signal pathway[J].JiangsuMedJ, 2015, 41(12):1368-70.
[6]唐丹,李剑萍,郑锡凤,等. 熊果酸通过STAT3通路调控胃癌细胞增殖和凋亡[J]. 中国药理学通报,2012, 28(2):179-84.
[6]Tang D, Li J P, Zheng X F, et al. Regulation of ursolic acid on proliferation and apoptosis of gastric cancer cells via STAT3 signaling pathway[J].ChinPharmacolBull, 2012, 28(2):179-84.
[7]Zhang H, Li X, Ding J, et al. Delivery of ursolic acid(UA) in polymeric nanoparticles effectively promotes the apoptosis of gastric cancer cells through enhanced inhibition of cyclooxygenase 2(COX-2)[J].IntJPharm, 2013, 441(1-2):261-8.
[8]Shaw R J. Glucose metabolism and cancer[J].CurrOpinCellBiol,2006,18(6):598-608.
[9]Ray P D, Huang B W, Tsuji Y. Reactive oxygen species(ROS) homeostasis and redox regulation in cellular signaling[J].CellSignal, 2012, 24(5):981-90.
[10]Yi B,Liu D,He M,et al. Role of the ROS/AMPK signaling pathway in tetramethylpyrazine-induced apoptosis in gastric cancer cells[J].OncolLett,2013, 6(2):583-9.
[11]Li X, Zheng D, Lu X, et al. Enhanced cytotoxicity and activation of ROS-dependent c-Jun Np-terminal kinase and caspase-3 by low doses of tetrandrine-loaded nanoparticles in Lovo cells-A possible Trojan strategy against cancer[J].EurJPharmBiopharm, 2010, 75(3):334-40.
[12]Li X, Lu X, Xu H, et al. Paclitaxel/tetrandrine coloaded nanoparticles effectively promote the apoptosis of gastric cancer cells based on "oxidation therapy"[J].MolPharm, 2012, 9(2):222-9.
[13]Hardie D G. AMPK: positive and negative regulation, and its role in whole-body energy homeostasis[J].CurrOpinCellBiol, 2015, 33:1-7.
[14]Saitoh M, Nagai K, Nakagawa K, et al. Adenosine induces apoptosis in the human gastric cancer cells via an intrinsic pathway relevant to activation of AMP-activated protein kinase[J].BiochemPharmacol, 2004, 67(10):2005-11.
[15]Wang B, Wang X B, Chen L Y, et al. Belinostat-induced apoptosis and growth inhibition in pancreatic cancer cells involve activation of TAK1-AMPK signaling axis[J].BiochemBiophysResCommun, 2013, 437(1):1-6.
[16]Zheng Q Y, Jin F S, Yao C, et al. Ursolic acid-induced AMP-activated protein kinase(AMPK) activation contributes to growth inhibition and apoptosis in human bladder cancer T24 cells[J].BiochemBiophysResCommun, 2012, 419(4):741-7.
[17]Yuan Y, Xue X, Guo R B, et al. Resveratrol enhances the antitumor effects of temozolomide in glioblastoma via ROS-dependent AMPK-TSC-mTOR signaling pathway[J].CNSNeurosciTher, 2012, 18(7):536-46.
[18]McGee S L, Hargreaves M. AMPK and transcriptional regulation[J].FrontBiosci,2008, 13:3022-33.
[19]Xu W, Chen G S, Shao Y, et al. Gastrin acting on the cholecystokinin2 receptor induces cyclooxygenase-2 expression through JAK2/STAT3/PI3K/Akt pathway in human gastric cancer cells[J].CancerLett, 2013, 332(1):11-8.
[20]Pathak A K, Bhutani M, Nair A S, et al. Ursolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human multiple myeloma cells[J].MolCancerRes, 2007, 5(9):943-55.
[21]Huang W, Yu L F, Zhong J, et al. Stat3 is involved in angiotensin II-induced expression of MMP2 in gastric cancer cells[J].DigDisSci, 2009, 54(10):2056-62.
Signal transduction pathway of ursolic acid inhibiting COX-2 expression in gastric cancer cells
ZHU Yue1, ZHOU Yi-chan1, ZHU Guo-qin1, LI Jian-ping2,JIAO Zheng1, LI Xiao-lin1, SHAO Yun1, SUN Wei-hao1
(1.DeptofGeriatricGastroenterology,theFirstAffiliatedHospitalofNanjingMedicalUniversity,Nanjing210029,China;2.DeptofOncology,theFirstPeople′sHospitalofYanchengCity,YanchengJiangsu224005,China)
AimOur previous study has found that ursolic acid(UA) increased intracellular reactive oxygen species(ROS) production and adenosine monophosphate-activated protein kinase(AMPK) phosphorylation, inhibited signal transducer and activator of transcription 3(STAT3) phosphorylation and cyclooxygenase-2(COX-2) expression in gastric cancer cells. However, the molecular mechanism by which UA inhibits COX-2 expression in gastric cancer cells has not been fully clarified. In this study we aimed to further clarify the signal transduction pathways involved in the UA-mediated inhibition of COX-2 expression in gastric cancer cells.MethodsHuman gastric cancer cell lines SGC-7901 and MKN-45 were routinely cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum. Sub-confluent cell cultures were pre-treated with antioxidant N-acetylcysteine(NAC), AMPK activator 5-amino-4-imida- zolecarboxamide-riboside(AICAR), AMPK inhibitor compound C, or STAT3 inhibitor WP1066 and then treated with or without UA for 24 h. The expression of AMPK and phosphorylated AMPK(p-AMPK), STAT3 and phosphorylated STAT3(p-STAT3), as well as COX-2 was detected by Western blot analysis.ResultsAntioxidant NAC and AMPK inhibitor compound C blocked UA-induced inhibition of STAT3 phosphorylation and down-regulation of COX-2 expression in gastric cancer cells. Both AMPK activator AICAR and UA inhibited STAT3 phosphorylation and COX-2 expression; the combination of two drugs resulted in further reduction. STAT3 inhibitor WP1066 did not affect UA-induced AMPK phosphorylation, whereas it inhibited STAT3 phosphorylation and COX-2 expression. The inhibitory effects on the STAT3 phosphorylation and COX-2 expression were significantly enhanced when SGC-7901 and MKN-45 cells were treated simultaneously with WP1066 plus UA.ConclusionUA inhibits COX-2 expression in gastric cancer cells, which may be mediated through ROS/AMPK/STAT3 signal transduction pathway.
ursolic acid; gastric cancer; reactive oxygen species; adenosine monophosphate-activated protein kinase; signal transducer and activator of transcription 3; cyclooxygenase-2
2016-01-07,
2016-03-17
国家自然科学基金面上项目(No 81372659)
朱悦(1990-),女,硕士生,研究方向:胃癌防治,E-mail: 421892415@qq.com;孙为豪(1963-),男,教授,博士生导师,研究方向:胃肠道肿瘤的分子生物学,通讯作者,E-mail:swh@njmu.edu.cn
10.3969/j.issn.1001-1978.2016.07.009
A
1001-1978(2016)07-0925-08
R284.1;R345.4;R345.57;R735.202.2;R977.3;R977.6
网络出版时间:2016-6-20 11:49网络出版地址:http://www.cnki.net/kcms/detail/34.1086.R.20160620.1149.018.html