二维液相色谱-质谱法研究犬肾小管上皮细胞脂质组成及马兜铃酸(Ⅰ)对其影响
2016-08-02聂洪港刘冉冉杨悠悠刘虎威
聂洪港,刘冉冉,杨悠悠,刘虎威,白 玉
(1.北京大学分析测试中心,北京 100871;2.北京分子科学国家实验室,生物有机与分子工程教育部重点实验室,北京大学化学与分子工程学院分析化学研究所,北京 100871;3.中粮营养健康研究院,营养健康与食品安全北京市重点实验室,北京 102209)
二维液相色谱-质谱法研究犬肾小管上皮细胞脂质组成及马兜铃酸(Ⅰ)对其影响
聂洪港1,刘冉冉2,杨悠悠3,刘虎威2,白玉2
(1.北京大学分析测试中心,北京100871;2.北京分子科学国家实验室,生物有机与分子工程教育部重点实验室,北京大学化学与分子工程学院分析化学研究所,北京100871;3.中粮营养健康研究院,营养健康与食品安全北京市重点实验室,北京102209)
摘要:采用在线正反相二维液相色谱-质谱联用技术研究了犬肾小管上皮细胞脂质组成及马兜铃酸(Ⅰ)对其影响。二维色谱的第一维用于分离不同种类脂质,第二维用于分离同类脂质的不同分子,进而利用高分辨质谱对脂质分子进行检测。该方法减少了共流出,降低了电离抑制,提高了灵敏度与准确性。借助精确质荷比检索数据库、高分辨二级质谱和当量碳数与保留时间规律等方法检测了犬肾小管上皮细胞中13类脂质的1 416个脂质分子。选取11种外源性脂质标准品进行方法验证,方法的线性关系、检测限、重复性均满足检测要求。在此基础上,考察了犬肾小管上皮细胞暴露于马兜铃酸(Ⅰ)后的脂质变化情况,对改变含量2~4倍的15个脂质分子进行了鉴定。该实验结果可为马兜铃酸的毒理、病理研究和相关疾病的临床诊断提供丰富的信息,并展现了二维液相色谱-质谱法在脂质组学研究中广阔的应用前景。
关键词:二维液相色谱-质谱;犬肾小管上皮细胞;马兜铃酸;脂质组学
网络出版时间:2016-07-05;网络出版地址:http:∥www.cnki.net/kcms/detail/11.2979.TH.20160705.1012.002.html
马兜铃酸(aristolochic acid,AA)是从马兜铃属植物中提取出的一系列硝基苯蒽环类羧酸,主要成分是马兜铃酸(Ⅰ)和马兜铃酸(Ⅱ),其结构示于图1。古人用马兜铃属植物治疗蛇咬伤,现代医学则利用AA的抗炎性能治疗关节炎、痛风、风湿以及脓疮等[1-2]。但有研究证明,AA是造成马兜铃酸肾病的原因,该病是长期摄入马兜铃属植物后发生的一种快速进行性纤维化间质肾炎[3-4],其病理表现有肾脏体积缩小、部分两侧肾脏不对称、外形不规整,常伴有肾小管萎缩和肾小管消失等[5]。该病变以皮质浅层受累最为明显,越往皮质深层病变越轻。探求马兜铃酸肾病的药理、毒理和作用机制一直是该领域的研究热点,代谢组学在相关研究中发挥了重要作用[6-7]。
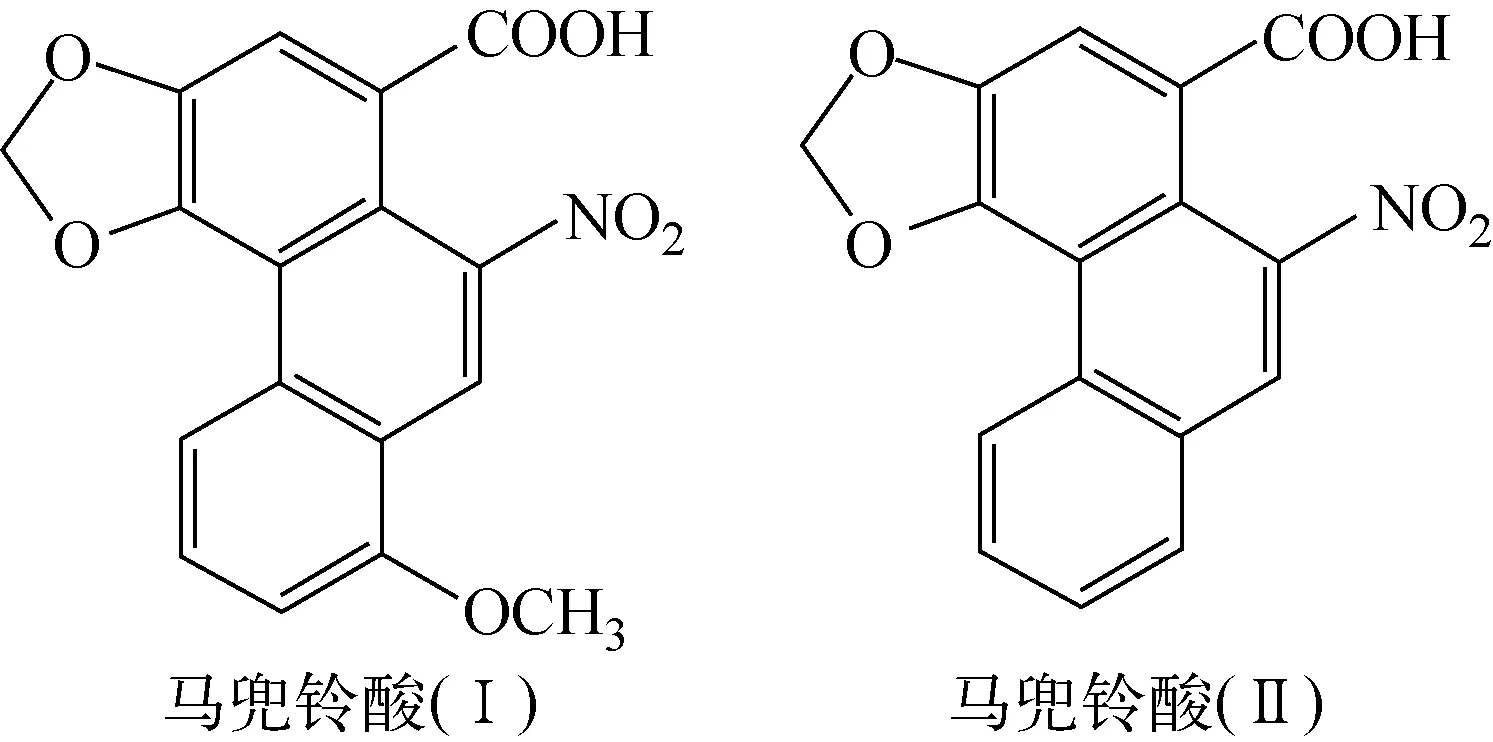
图1 马兜铃酸(Ⅰ)和马兜铃酸(Ⅱ)的结构式[8]
脂质组学是代谢组学最为活跃的分支之一,它通过系统全面地分析生物体中脂质分子的种属、代谢情况,以及与其他分子的相互作用,揭示其在生命活动中对膜的构建、信号传导、调控转录和翻译等过程的影响,阐明脂质及其代谢与细胞、器官和机体的生理、病理之间的关系[9-11]。色谱、质谱及相关联用技术是脂质组学研究最主要的分析技术[12-13]。其中,在线正反相二维液相色谱-质谱联用技术可实现在一次进样中先分离不同种类脂质,再分离鉴定同类脂质中的不同分子,可最大限度地减少共流出、降低电离抑制、增加低丰度脂质分子信息、减少假阳性的检测结果、提升脂质分子鉴定的准确性和信息量,已被应用于多种样品的脂质组学研究[14-19]。
本工作拟采用二维液相色谱-质谱联用方法检测肾小管上皮(Madin-Darby canine kidney, MDCK)细胞中磷脂、脂肪酸、鞘脂等脂质成分,并将该细胞暴露于AA(Ⅰ)中,考察上述脂质化合物含量的变化情况,以期从脂质组学角度为AA(Ⅰ)的肾毒理学研究和马兜铃酸肾病的临床诊断提供依据。
1实验部分
1.1主要仪器与装置
自建二维液相色谱系统:第一维采用美国Agilent公司的1100液相色谱仪,配有四元泵、在线脱气机、自动进样器、柱温箱;第二维采用美国Agilent公司的1200液相色谱仪,配有二元泵和在线脱气机;两维色谱之间的接口包括美国IDEX Health & Science公司的两位六通阀,英国Edwards 公司的E2M2真空泵和自制的电热水浴锅。Agilent6530四极杆飞行时间质谱仪:美国Agilent公司产品,配有喷射流离子聚焦技术电喷雾离子源。
仪器控制及数据采集由美国Agilent公司MassHunter Data Acquisition B.02.00完成;定性、定量及统计学分析分别由美国Agilent公司MassHunter Qualitative Analysis B.02.00、MassHunter Quantitative Analysis B.03.01和Mass Profiler Professional 2.0完成。
1.2主要材料与试剂
马兜铃酸(Ⅰ)标准品:由中国药品生物制品检定所提供;磷脂标准品:sn-(1-heptade-canoyl-2-hydroxy)-glycerol-3-phospho-sn-3′-(1′,2′-heptadecanoyl)-glycerol,Hemi BMP(17∶0);1-(10Z-heptadecenoyl)-sn-glycero-3-phospho-(1′-rac-glycerol) (sodium salt),LPG(17∶1);1,2-dimyristoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (sodium salt),PG(14∶0/14∶0);N-(dodecanoyl)-heptadecasphing-4-enine-1-phosphoethanolamine,Sphingosyl PE(d17∶1/12∶0);1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine,PE(14∶0/14∶0); 1-(10Z-heptadecenoyl)-sn-glycero-3-phosphoethanolamine,LPE(17∶0);1,2-dimyristoyl-sn-glycero-3-phospho-L-serine (sodium salt),PS(14∶0/14∶0);N-heptadecanoyl-D-erythro-sphingosylphosphorylcholine,SM (d18∶1/17∶0);1-heptadecanoyl-sn-glycero-3-phosphocholine,LPC(17∶0);1,2-dimyristoleoyl-sn-glycero-3-phosphocholine,PC (14∶0/14∶0):美国Avanti Polar Lipid公司产品;十七烷酸(heptadecanoic acid)标准品FFA(17∶0)、质谱纯甲酸铵、细胞培养级二甲基亚砜:均为美国Sigma-Aldrich公司产品;正己烷、乙醇、甲醇和氯仿:均为色谱纯,美国Dikma公司产品;实验用纯净水:中国娃哈哈公司产品;MDCK细胞:美国American Type Culture Collection (ATCC)公司产品;DMEM高糖培养基、10%胎牛血清、1%谷胺酰胺、1%青霉素/链霉素和胰酶/EDTA消化液:均为美国Thermo-Fisher公司产品。
1.3样品制备
1.3.1MDCK细胞培养MDCK细胞采用DMEM高糖培养基培养,内含10%胎牛血清、1%谷胺酰胺、1%青霉素/链霉素,于5%CO2、恒湿和37 ℃条件下培养,待90%融合后,用胰酶/EDTA消化液消化,制成单细胞悬液;调节细胞密度为每毫升5×105个,以每孔1 mL接种于6孔细胞培养板,24 h后弃去培养液,用PBS清洗2次;向给药组的每个孔内分别加入200 nmol AA(Ⅰ),与未给药对照组一同置于5%CO2、37 ℃培养箱中,继续培养24 h;24 h后,给药组的部分细胞死亡,悬浮在培养基里,部分仍贴壁;收集每个样品的培养液,并用胰蛋白酶消化仍然贴壁的细胞,将收集的贴壁细胞分别用血球计数板计数。
1.3.2MDCK细胞脂质的提取采用经改进的Folch方法[20]提取MDCK细胞脂质。首先从给药组和对照组各选取8份细胞样品,每份样品含有约5×105个活细胞,向其中加入10 mL Folch溶剂(氯仿-甲醇溶液,2∶1,V/V),以3 000 r/min离心10 min,取下层溶液,氮气吹干,复溶于1 mL环己烷-异丙醇溶液(7∶3,V/V)中,待色谱-质谱分析。
1.3.3方法验证样品的制备 随机选取10份细胞样品(每份含约5×105个活细胞),向1号样品中加入11种脂质标准品各0.001 μg,而2~10号样品中11种脂质标准品的加入量分别为0.002、0.01、0.02、0.1、0.2、1、2、10、20 μg。采用1.3.2节方法,得到10个方法验证用样品,其单一内标浓度分别为0.001、0.002、0.01、0.02、0.1、0.2、1、2、10、20 mg/L。
1.4实验条件
1.4.1色谱条件第一维色谱柱为Agilent Rx-SIL色谱柱(2.1 mm×150 mm×5 μm),柱温25 ℃,进样量20 μL;流动相:A1为含有5 mmol/L甲酸铵的环己烷-异丙醇-水溶液(30∶70∶2,V/V/V),B1为含有5 mmol/L甲酸铵的甲醇-水溶液(100∶2,V/V),流速0.1 mL/min。
第二维色谱柱为Agilent Eclipse Plus C8色谱柱(2.1 mm×10 mm×3.5 μm),柱温40 ℃;流动相:A2为含有5 mmol/L甲酸铵的甲醇-水溶液(50∶50,V/V),B2为含有5 mmol/L甲酸铵的甲醇,流速0.3 mL/min。
接口水浴锅温度50 ℃,在真空泵的作用下,一维色谱流出的流动相在接口处气化并被真空泵抽出,被分析物质沉积在样品环中,当六通阀切换时,其被转移至第二维色谱系统进行进一步的分离。第一维和第二维液相色谱梯度洗脱条件和六通阀的转换时间列于表1。
1.4.2质谱条件负离子模式;鞘气温度350 ℃,鞘气流速8 L/min;喷雾气压力138 kPa;干燥气温度300 ℃,干燥气流速5 L/min;毛细管入口电压3 500 V;碎裂电压190 V;锥孔1电压65 V;质谱采集速率1.02 spectra/s,采集范围为m/z100~2 000;在目标二级质谱模式中,母离子质量窗口m/z1.3,碰撞能量40 V。
2结果与讨论
2.1MDCK细胞脂质的分离与检测
MDCK细胞中的脂质提取物在第一维色谱中按其极性差异实现分离,共检测到13类脂质:游离脂肪酸(FFA),磷脂酰甘油(PG),降解磷脂酰甘油(LPG),磷脂酰肌醇(PI),降解磷脂酰肌醇(LPI),磷脂酰乙醇胺(PE), 降解磷脂酰乙醇胺(LPE),磷脂酰丝氨酸(PS),降解磷脂酰丝氨酸(LPS),磷脂酰胆碱(PC),降解磷脂酰胆碱(LPC),鞘磷脂(SM)和心磷脂(CL)。将第一维色谱分为5段(第1段含FFA;第2段含PI、PG、LPG;第3段含PE、CL;第4段含LPI、LPE、PS、LPS;第5段含PC、LPC、SM),分别转移至第二维反相色谱进行进一步的分离,结果示于图2。
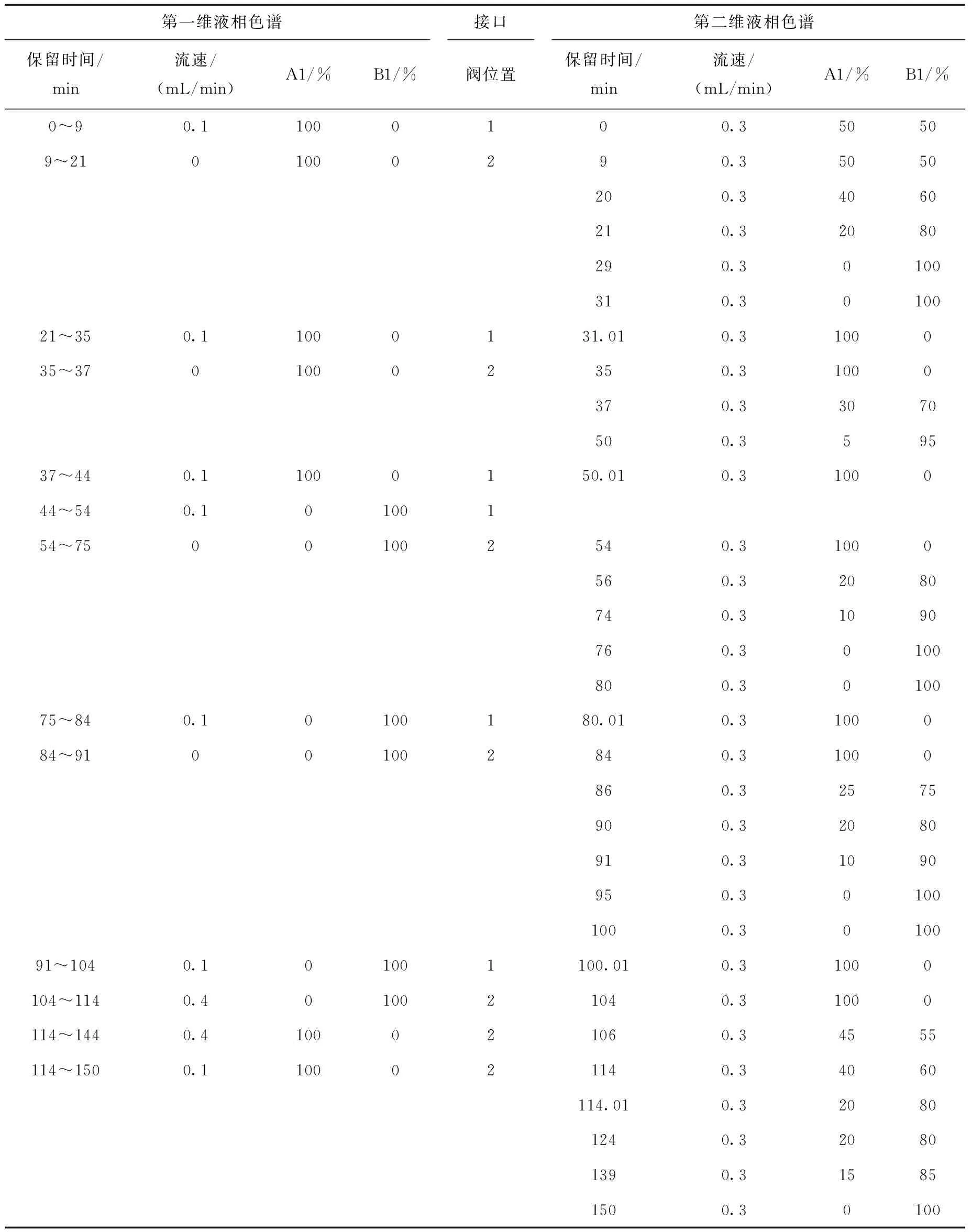
表1 二维液相色谱梯度洗脱程序
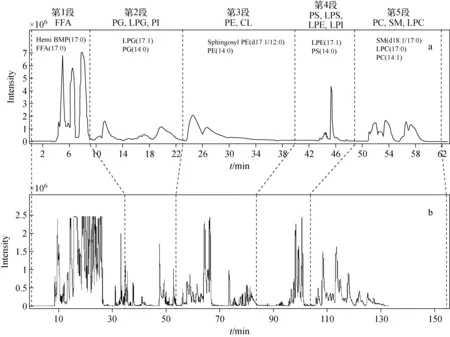
图2 MDCK细胞脂质的一维(a)和二维(b)液相色谱基峰色谱图
将下面3种方式相结合对脂质化合物进行定性:1) 根据精确质量数检索脂质数据库,推测分子式;2) 根据脂质二级质谱特征碎片离子解析脂质分子结构。根据文献报道[21-29]及本实验室前期工作经验[14-19],除FFA外,其余12类脂质化合物在丰度满足二级质谱分析的前提下,均可得到具有特征性的二级离子,适用于脂类化合物的结构解析,主要脂质化合物的离子碎片列于表2;3) 根据同类脂质分子中支链的当量碳数与其保留时间的关系。
对于大多数含量相对较高的非脂肪酸类脂质化合物,可以采用精确质荷比、化合物特征碎裂途径和保留时间共同定性;而低丰度的脂质分子和脂肪酸类化合物通常因为无法得到稳定可靠的二级质谱信息,则需通过精确质荷比和保留时间进行定性,下面将分别介绍。
首先,将一级质谱检测得到的准分子离子质荷比输入LIPIDMAPS网站(http:∥www.lipidmaps.org/tools/ms/LMSD_search_mass_options.php)的在线数据库“Search the LMSD for lipids with a given mass (m/z) value”进行搜索,数据库软件会列出可能的脂类化合物。Q-TOF MS在有参比离子校正的情况下,质量误差可控制在±0.002以下;而在没有参比离子进行校正的二级质谱中,质量误差可达±0.005以下。与理论计算值相比,相对质量误差在±0.005以上则认为未能达到定性要求,因此本实验设定的质量窗口是±0.005。例如,在第3段检测到某一分析物离子的质荷比为766.540 6,将这一数值输入在线数据库进行检索。因为在第3段流出的脂质分子属于PE类化合物,而在数据库检索结果中既符合PE类脂质,又符合质量偏差小于±0.005的只有C43H77NO8P-PE(38∶4) (理论质量数为766.539 2),所以基本可以确定该脂质是两脂肪酸支链上含有38个碳原子和4个不饱和双键的PE分子丢失一个H+形成的准分子离子,同时可确定这一化合物的分子式。若要确定脂肪酸链的具体信息,还需要对该化合物的二级质谱进行解析。在结构分析的过程中,采用targeted MS/MS模式选取母离子(m/z766.54),以40 V的碎裂能量对母离子进行碎裂,采集碎片离子信息,得到其二级质谱图,示于图3。图中,m/z303.232 9和m/z283.263 4离子分别是20∶4和18∶0脂肪酸根的离子;m/z480.309 7 和m/z462.298 7离子分别对应于PE(38∶4)负离子以烯酮和脂肪酸的形式中性丢失一个20∶4脂肪酰基侧链后形成的离子;m/z196.036 6和m/z140.011 2离子是所有PE分子的特征碎片离子;m/z196.036 6代表PE准分子离子的两个脂肪酰基侧链,其中一个以脂肪酸的形式,另一个以烯酮的形式全部中性丢失后产生的特征离子;m/z140.011 2离子则是乙醇胺磷酸负离子。这些碎片离子的信息与文献[21-29]报道一致,因此可推断出这一分子的两个脂肪酰基的组成是18∶0和20∶4,脂质分子可确定为PE(18∶0/20∶4)。大部分脂质分子的碎裂机理已被详细研究过,根据这些脂质分子的特征碎裂路径和产生的特征离子,可以对产生稳定可靠二级质谱的高丰度化合物进行定性分析。

表2 主要脂质化合物的离子碎片
由于脂肪酸类化合物和其他丰度相对较低的脂质化合物很难得到稳定的二级质谱信息,因此,本实验采用在数据库中搜索检测到的准分子离子质荷比以确定其可能的分子信息,并结合保留时间进行辅助定性。实验发现,同类脂质分子在反相色谱分离中的保留时间与其支链的当量碳数相关,支链上含有相同碳原子个数的同类脂质分子随着支链上碳碳双键个数的增加疏水性减弱,在反相色谱柱上的保留时间缩短,这与文献[14]发现的规律一致。利用这个规律,可以对无法得到二级质谱信息的脂类化合物进行辅助定性,这比仅使用精确的准分子离子质量对化合物定性更加准确。
通过上述3种方式对MDCK细胞脂质提取物的二维液相色谱-质谱结果进行分析,在13类脂质中共检测到1 416个脂质分子,结果列于表3。其中,PC和PE是检出脂质分子个数最多的两类脂质化合物,占总个数的68%;其他11类脂质分子仅占总个数的32%。
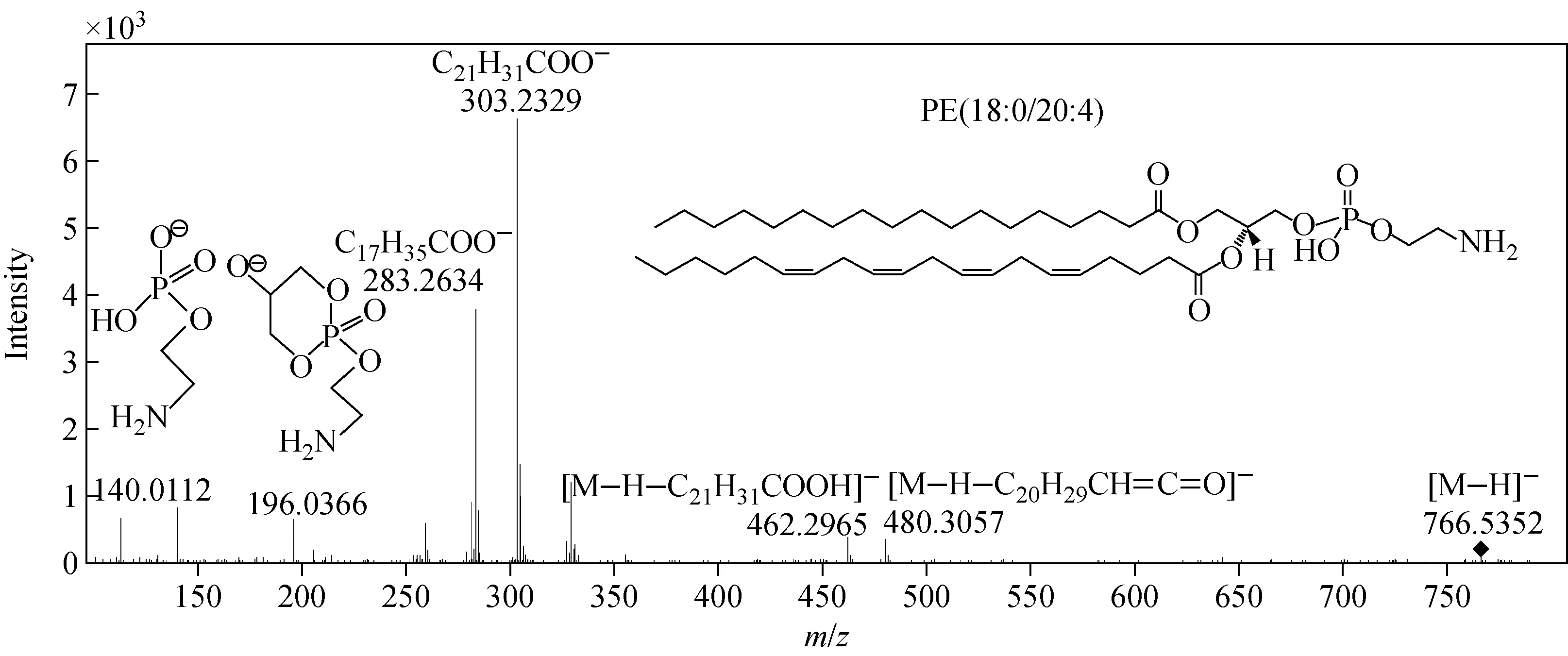
图3 负离子模式下,母离子m/z 766.54的二级质谱图

脂质种类PIPGPCPEPSLPILPGLPCLPELPSCLSMFFA总数脂质分子个数8810490473771375644195530541416
2.2方法验证
从11类脂质中各选取1个外源性脂质标准品,使其保留时间较均匀地分布于第一维色谱中,5段中每段含有2~3个脂质标准品:第1段含FFA(17∶0)和Hemi BMP(17∶0);第2段含LPG(17∶1)和PG(14∶0/14∶0);第3段含PE(14∶0/14∶0)和Sphingosyl PE(d17∶1/12∶0);第4段含LPE(17∶0)和PS(14∶0/14∶0);第5段含 SM (d18∶1/17∶0)、LPC(17∶0)和PC (14∶0/14∶0)。采用这11个脂质标准品,按2.4节方法配制10个浓度水平的验证样品,每个浓度水平连续检测3次,且每个浓度水平之间插入一次空白溶剂的分析。计算时对测试标准品的萃取离子色谱峰进行积分,得到该化合物相应浓度的峰面积,将每个浓度水平测得的3次峰面积取平均值与相应的浓度建立线性方程。计算得到的11个脂质标准品的线性方程、线性范围、线性回归系数R2、检测限(LOD)、相对标准偏差(RSD)等,列于表4。可知,11种验证标准品的线性回归系数均大于0.991 7,表明11种标准品在相应的范围内质谱信号响应和浓度具有良好的线性关系。除PS(14∶0/14∶0) 的LOD为5 μg/L外,其余10种脂质标准的LOD都达到2 μg/L,峰面积和保留时间的相对标准偏差分别小于7.7%和0.11%(n=6),这说明本方法可满足定量比较分析的需要,适用于脂质组学的相关研究。
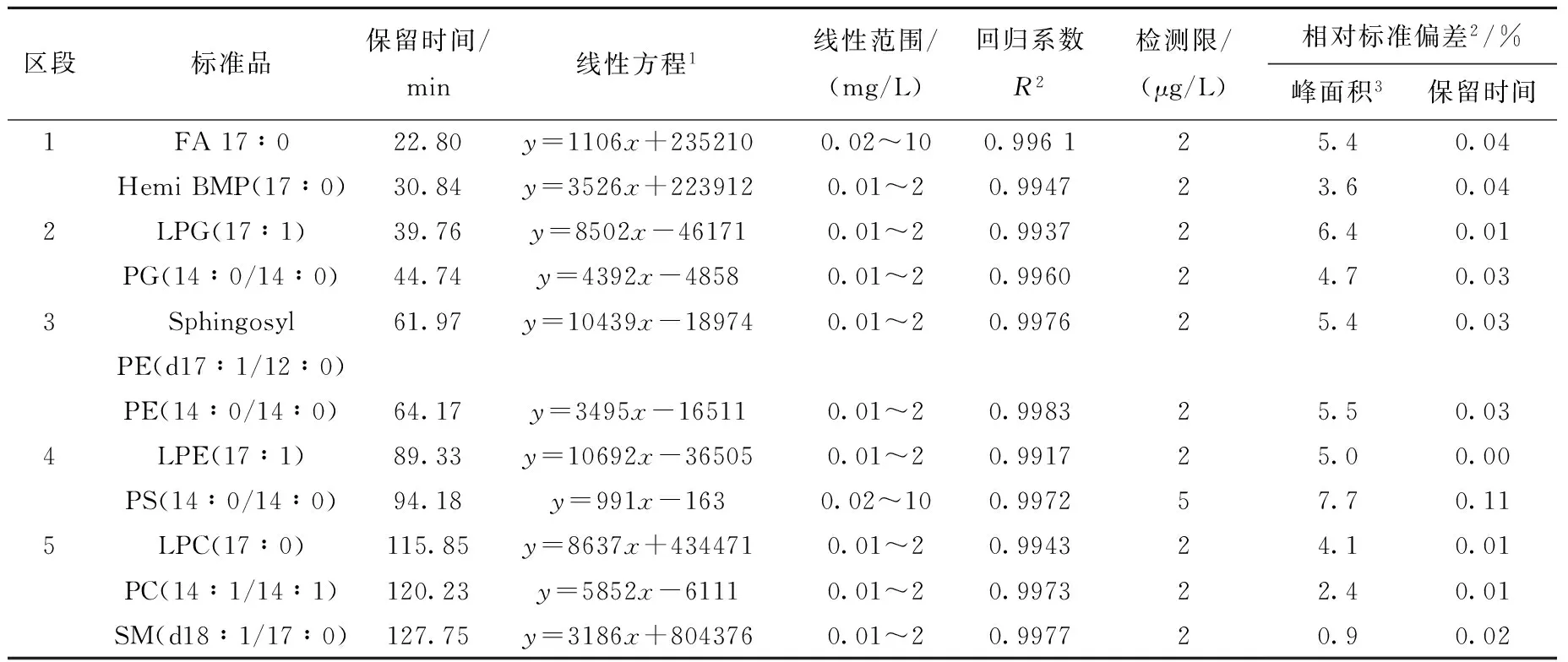
表4 11种标准品的方法验证结果
注:1.y为萃取离子峰的峰面积,x为标准品浓度(μg/L);2.标准品浓度0.1 mg/L,n=6;3.萃取离子色谱图的峰面积
2.3马兜铃酸(Ⅰ)对MDCK细胞中脂质含量的影响
采用Mass Profiler Professional软件对给药组和对照组各8份细胞脂质提取物的二维液相色谱-质谱结果进行统计学分析,主成分分析(PCA)结果示于图4。可见,16个样品被分成2组,该软件给出了区分这2组数据做出主要贡献的化合物列表,通过对这些化合物设定阈值,即峰面积的绝对倍数变化大于2且p值小于0.05,共找到2组中峰面积存在显著差异的15个脂质分子,结果列于表5。
采用四极杆飞行时间质谱对上述15个离子进行二级碎裂,由碎片离子推测可能的碎裂路径,进一步确认其可能的分子组成与结构信息。以LPE(O-16∶1)为例,其二级质谱图和碎裂路径示于图5。
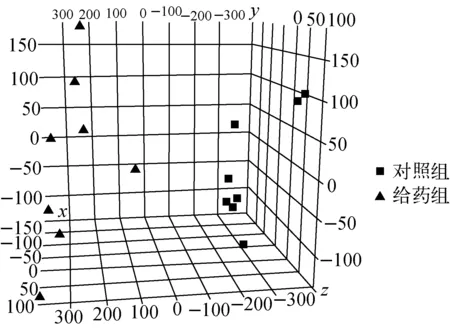
图4 用AA(Ⅰ)处理的MDCK细胞和对照组数据的主成分分析图
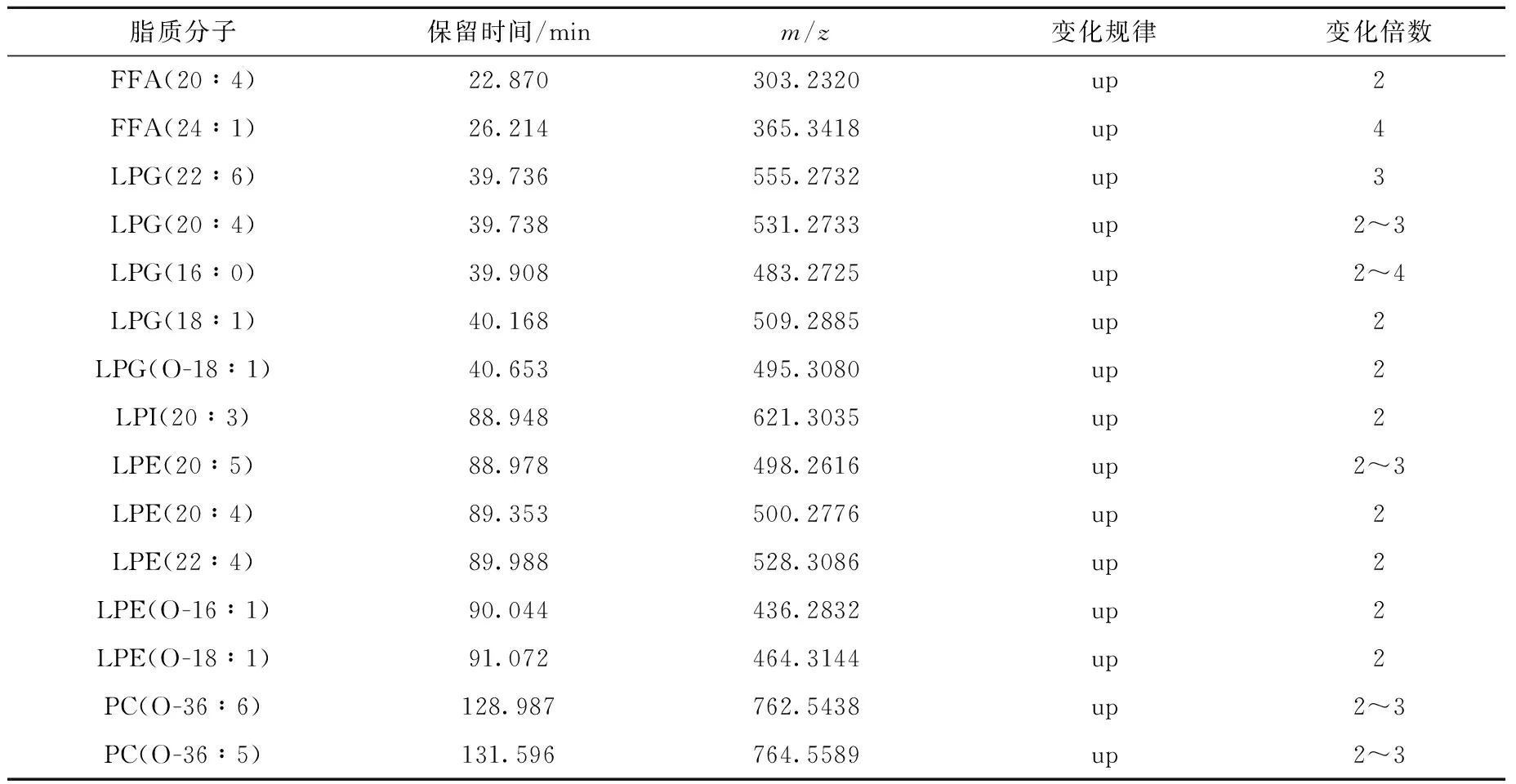
脂质分子保留时间/minm/z变化规律变化倍数FFA(20∶4)22.870303.2320up2FFA(24∶1)26.214365.3418up4LPG(22∶6)39.736555.2732up3LPG(20∶4)39.738531.2733up2~3LPG(16∶0)39.908483.2725up2~4LPG(18∶1)40.168509.2885up2LPG(O-18∶1)40.653495.3080up2LPI(20∶3)88.948621.3035up2LPE(20∶5)88.978498.2616up2~3LPE(20∶4)89.353500.2776up2LPE(22∶4)89.988528.3086up2LPE(O-16∶1)90.044436.2832up2LPE(O-18∶1)91.072464.3144up2PC(O-36∶6)128.987762.5438up2~3PC(O-36∶5)131.596764.5589up2~3
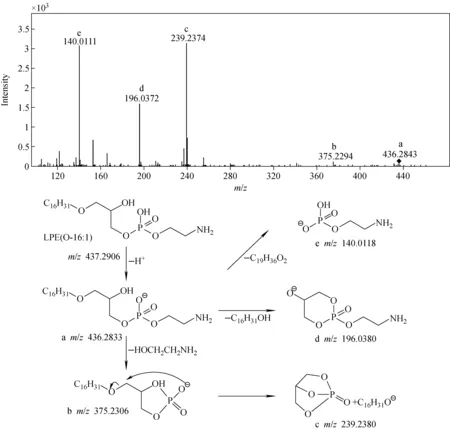
图5 LPE(O-16∶1)的二级质谱图和碎裂路径
15个脂质分子的含量在给药组中有明显变化,这可能同AA(Ⅰ)与MDCK细胞的DNA形成加合物,其转录成的蛋白对脂质合成的调控能力降低有关;也可能是AA直接与相关蛋白作用,改变其活性,致使脂质合成不受控,脂质的大量合成占用了过多的能量,造成了细胞凋亡,或超量的脂质影响了细胞的正常代谢使其凋亡。上述推断仍需进一步研究的验证。给药前后含量发生明显变化的脂质分子可能会由细胞渗入尿液,使其在尿液中的含量增加,这有望为临床诊断马兜铃酸肾病提供一种无损检测的方法。
4总结与展望
本工作采用二维液相色谱-质谱法分析了MDCK细胞中13类脂质共1 416种分子种属,并比较了这些脂质在MDCK暴露于AA(Ⅰ)前后含量的变化,为毒理学研究和潜在临床诊断方法提供了技术手段。二维液相色谱-质谱法在生物脂质轮廓分析,尤其是低丰度脂质分析中,具有灵敏度高、定性准确、信息丰富等特点,有望在脂质组学研究中发挥更加重要的作用。
参考文献:
[1]RÜCKER V G, CHUNG B S. Aristolochic acids from Aristolochia manshuriensis (author’s transl)[J]. Planta Medica, 1975, 27(1): 68-71.
[2]PRIESTAP H A. Minor aristolochic acids from aristolochia-argentina and mass-spectral analysis of aristolochic acids[J]. Phytochemistry, 1987, 26(2): 519-529.
[3]STIBOROVA M, FREI E, ARLT V M, et al. Metabolic activation of carcinogenic aristolochic acid, a risk factor for balkan endemic nephropathy[J]. Mutation Research-Reviews in Mutation Research, 2008, 658(1/2): 55-67.
[4]DEBELLE F D, VANHERWEGHEM J LNORTIER J L. Aristolochic acid nephropathy: A worldwide problem[J]. Kidney International, 2008, 74(2): 158-169.
[5]POON S L, PANG S T, MCPHERSON J R, et al. Genome-wide mutational signatures of aristolochic acid and its application as a screening tool[J]. Science Translational Medicine, 2013, 5(197): 101.
[6]NIE W, LV Y, YAN L, et al. Prediction and characterisation of the system effects of aristolochic acid: A novel joint network analysis towards therapeutic and toxicological mechanisms[J]. Journal of Biological Chemistry, 2015, 5(51): 51 035-51 043.
[7]ZHAO Y Y, WANG H L, CHENG X L, et al. Metabolomics analysis reveals the association between lipid abnormalities and oxidative stress, inflammation, fibrosis, and nrf2 dysfunction in aristolochic acid-induced nephropathy[J]. Scientific Reports, 2015, 5: 12 936.
[8]ARLT V M, STIBOROVA MSCHMEISER H H. Aristolochic acid as a probable human cancer hazard in herbal remedies: A review[J]. Mutagenesis, 2002, 17(4): 265-277.
[9]HAN X L, GROSS R W. Global analyses of cellular lipidomes directly from crude extracts of biological samples by esi mass spectrometry: A bridge to lipidomics[J]. Journal of Lipid Research, 2003, 44(6): 1 071-1 079.
[10]WENK M R. The emerging field of lipidomics[J]. Nature Reviews Drug Discovery, 2005, 4(7): 594-610.
[11]WATSON A D. Lipidomics: A global approach to lipid analysis in biological systems[J]. Journal of Lipid Research, 2006, 47(10): 2 101-2 111.
[12]LI M, YANG L, BAI Y, et al. Analytical methods in lipidomics and their applications[J]. Analytical Chemistry, 2014, 86(1): 161-175.
[13]CAJKA T, FIEHN O. Comprehensive analysis of lipids in biological systems by liquid chromatography-mass spectrometry[J]. Trac Trends in Analytical Chemistry, 2014, 61: 192-206.
[14]NIE H G, LIU R R, YANG Y Y, et al. Lipid profiling of rat peritoneal surface layers by online normal- and reversed-phase 2d LC Q-TOF MS[J]. Journal of Lipid Research, 2010, 51(9): 2 833-2 844.
[15]LI M, FENG B S, LIANG Y, et al. Lipid profiling of human plasma from peritoneal dialysis patients using an improved 2D (np/rp) LC-QTOF MS method[J]. Analytical and Bioanalytical Chemistry, 2013, 405(21): 6 629-6 638.
[16]LI M, TONG X L, LV P, et al. A not-stop-flow online normal-/reversed-phase two-dimensional liquid chromatography-quadrupole time-of-flight mass spectrometry method for comprehensive lipid profiling of human plasma from atherosclerosis patients[J]. Journal of Chromatography A, 2014, 1 372: 110-119.
[17]TANG W, LI M, LU X H, et al. Phospholipids profiling and outcome of peritoneal dialysis patients[J]. Biomarkers, 2014, 19(6): 505-508.
[18]WENG R, SHEN S S, YANG L, et al. Lipidomic analysis ofp-chlorophenylalanine-treated mice using continuous-flow two-dimensional liquid chromatography/quadrupole time-of-flight mass spectrometry[J]. Rapid Communications in Mass Spectrometry, 2015, 29(16): 1 491-1 500.
[19]YANG L, CUI X G, ZHANG N N, et al. Comprehensive lipid profiling of plasma in patients with benign breast tumor and breast cancer reveals novel biomarkers[J]. Analytical and Bioanalytical Chemistry, 2015, 407(17): 5 065-5 077.
[20]FOLCH J, LEES M, SLOANE STANLEY G H. A simple method for the isolation and purification of total lipids from animal tissue[J]. Journal of Biological Chemistry, 1957, 226(1): 497-509.
[21]HSU F F, TURK J. Characterization of phosphatidylinositol, phosphatidylinositol-4-phosphate, and phosphatidylinositol-4,5-bisphosphate by electrospray ionization tandem mass spectrometry: A mechanistic study[J]. Journal of the American Society for Mass Spectrometry, 2000, 11(11): 986-999.
[22]HSU F F, TURK J. Studies on phosphatidylglycerol with triple quadrupole tandem mass spectrometry with electrospray ionization: Fragmentation processes and structural characterization[J]. Journal of the American Society for Mass Spectrometry, 2001, 12(9): 1 036-1 043.
[23]ZHANG X, REID G E. Multistage tandem mass spectrometry of anionic phosphatidylcholine lipid adducts reveals novel dissociation pathways[J]. International Journal of Mass Spectrometry, 2006, 252(3): 242-255.
[24]HSU F F, TURK J. Charge-remote and charge-driven fragmentation processes in diacyl glycerophosphoethanolamine upon low-energy collisional activation: A mechanistic proposal[J]. Journal of the American Society for Mass Spectrometry, 2000, 11(10): 892-899.
[25]HSU F F, TURK J. Charge-driven fragmentation processes in diacyl glycerophosphatidic acids upon low-energy collisional activation. A mechanistic proposal[J]. Journal of the American Society for Mass Spectrometry, 2000, 11(9): 797-803.
[26]HSU F F, TURK J. Studies on phosphatidylserine by tandem quadrupole and multiple stage quadrupole ion-trap mass spectrometry with electrospray ionization: Structural characterization and the fragmentation processes[J]. Journal of the American Society for Mass Spectrometry, 2005, 16(9): 1 510-1 522.
[27]HSU F F, TURK J. Differentiation of 1-o-alk-1′-enyl-2-acyl and 1-o-alkyl-2-acyl glycerophospholipids by multiple-stage linear ion-trap mass spectrometry with electrospray ionization[J]. Journal of the American Society for Mass Spectrometry, 2007, 18(11): 2 065-2 073.
[28]HSU F F, TURK J, RHOADES E R, et al. Structural characterization of cardiolipin by tandem quadrupole and multiple-stage quadrupole ion-trap mass spectrometry with electrospray ionization[J]. Journal of the American Society for Mass Spectrometry, 2005, 16(4): 491-504.
[29]HOUJOU T, YAMATANI K, NAKANISHI H, et al. Rapid and selective identification of molecular species in phosphatidylcholine and sphingomyelin by conditional neutral loss scanning and MS3[J]. Rapid Communications in Mass Spectrometry, 2004, 18(24): 3 123-3 130.
中图分类号:O657.63
文献标志码:A
文章编号:1004-2997(2016)04-0289-12
doi:10.7538/zpxb.youxian.2016.0037
收稿日期:2016-01-11;修回日期:2016-03-05
基金项目:国家自然科学基金项目(21175005,21405006,21527809)资助
作者简介:聂洪港(1980—),男(汉族),辽宁丹东人,博士,从事色谱、质谱仪器及检测技术研究。E-mail: hgnie@pku.edu.cn
通信作者:白玉(1976—),女(汉族),吉林九台人,副教授,从事生物分离与检测研究。E-mail: yu.bai@pku.edu.cn
Lipid Profiling of Madin-Darby Canine Kidney Cells and Its Lipid Changes Induced by Treatment of Aristolochic Acid (Ⅰ) Using Two Dimensional Liquid Chromatography-Mass Spectrometry
NIE Hong-gang1, LIU Ran-ran2, YANG You-you3, LIU Hu-wei2, BAI Yu2
(1.AnalyticalInstrumentationCenter,PekingUniversity,Beijing100871,China;2.BeijingNationalLaboratoryforMolecularSciences,KeyLaboratoryofBioorganicChemistryandMolecularEngineeringofMinistryofEducation,InstituteofAnalyticalChemistry,CollegeofChemistryandMolecularEngineering,PekingUniversity,Beijing100871,China;3.BeijingKeyLaboratoryofNutritionHealthandFoodSafety,ChineseOil&FoodstuffsCorporation(COFCO)NutritionandHealthResearchInstitute,Beijing102209,China)
Abstract:The mechanisms of lipids separation are as following: different lipid classes are separated by adsorption mechanisms and eluted out of the column in normal-phase liquid chromatography (NPLC), and individual molecular species are separated based on hydrophobicity in reversed-phase liquid chromatography (RPLC). In RPLC, the elution sequence of lipid molecules is determined by both the chain length and the degree of unsaturation in the fatty-acyl chains. To avoid co-elution of molecular species, an online, normal-phase and reversed-phase two-dimensional (2D) liquid chromatography (LC) quadrupole time-of-flight mass spectrometry (Q TOF-MS) system was developed for the lipid profiling of Madin-Darby canine kidney (MDCK) cells and the investigation of the lipid changes in MDCK cells treated with aristolochic acid (Ⅰ). Different lipid classes in MDCK cells were separated in the first dimension of the two-dimensional liquid chromatograph system and lipid molecular species were further separated in the second dimension followed by mass spectrometry detection, so that the ion suppression effects were reduced while the detection sensitivity was improved.
All lipids in MDCK cells were identified with high accuracy mass values measured by Agilent 6530 accurate mass Q TOF-MS. The abundant molecular species were confirmed by targeted MS/MS,meanwhile the retention time and low abundance lipid molecules were identified withm/zvalue and the retention time based on the correlation between the equivalent carbon number (ECN) and the retention time. The measured accurate masses were applied for preliminary identification using the online database with a mass tolerance of less than ±0.005 on the basis of the predicted elemental composition. 1 416 endogenous lipid species from 13 lipid classes were identified by accurate masses, tandem mass spectra and the retention time. 11 exogenous lipid standards from different classes, including FA 17∶0, Hemi BMP(17∶0), LPG(17∶1 ), PG(14∶0/14∶0), Sphingosyl PE(d17∶1/12∶0), PE(14∶0/14∶0), LPE(17∶1), PS(14∶0/14∶0), LPC(17∶0), PC(14∶1/14∶1), SM(d18∶1/17∶0), were selected to be separated in five fractions for the evaluation of this method. The linear regression coefficients (R2=0.991 7-0.998 3), the limit of detection (2-5 μg/L) and the relative standard deviation of peak area (0.9%-7.7%) and retention time (0.01%-0.11%) were all satisfactory.
To investigate the lipid changes in MDCK cells dosed with aristolochic acid (Ⅰ), 16 MDCK cell samples (each containing 5×105cells) were randomly separated into a dosed group (n=8) and a control group (n=8). All 16 samples were detected by the 2D LC/MS method. The dosed group and control group were alternately injected to reduce systemic error. During the sequence, one blank sample was injected after every three injections, and no significant carryover of lipids was observed. MS data of all 16 samples were extracted by Mass-Hunter Qualitative Analysis software and analyzed by Mass Profiler Professional software. Through setting threshold parameters, the software presented a list of potential biomarkers whose absolute fold-change of peak area was larger than 2 andpvalue less than 0.05. By the above-mentioned approach, 15 changed lipid species were confirmed, as their concentrations in the dosed group were 2-4 times of those in the control group. The results would contribute to the study on therapeutic and toxicological mechanisms of aristolochic acids and revealed that this two-dimensional liquid chromatography quadrupole time-of-flight mass spectrometry method was a promising tool for lipidomics research.
Key words:two-dimensional liquid chromatography mass spectrometry; Madin-Darby canine kidney (MDCK) cells; aristolochic acid; lipidomics
