Investigation of the Interaction between Perfluorododecanoic Acid and Human Serum Albumin by Multi-Spectroscopic and Molecular Modeling Techniques
2016-07-12HUTaoyingWANGYirunZHOUShanshanLIUYing
HU Tao-ying, WANG Yi-run, ZHOU Shan-shan,2, LIU Ying,2*
1. College of Life and Environmental Sciences, Minzu University of China, Beijing 100081, China
2. Beijing Engineering Research Center of Food Environment and Public Health, Minzu University of China, Beijing 100081, China
Investigation of the Interaction between Perfluorododecanoic Acid and Human Serum Albumin by Multi-Spectroscopic and Molecular Modeling Techniques
HU Tao-ying1, WANG Yi-run1, ZHOU Shan-shan1,2, LIU Ying1,2*
1. College of Life and Environmental Sciences, Minzu University of China, Beijing 100081, China
2. Beijing Engineering Research Center of Food Environment and Public Health, Minzu University of China, Beijing 100081, China
Perfluorododecanoic acid (PFDoA) is the most toxic emerging environmental contaminant among the 8~12 carbon chain perfluoroalkyl acids (PFAAs). A large amount of knowledge in the field of environmental PFAAs has been accumulated so far, while we are still just at the beginning of research into the interaction between PFDoA and human serum albumin (HSA). The goal of this study was to comprehensively determinate the binding mechanism of PFDoA with HSA by using fluorescence quenching technique in combination with molecular modeling and circular dichroism (CD) spectroscopy under the simulative physiological conditions. The quenching of HSA fluorescence by PFDoA was found to be a result of the combination of dynamic quenching and the formation of PFDoA-HSA complex. The calculated binding distance (r=3.65 nm) indicated that the non-radioactive energy transfer came into being in the interaction between PFDoA (acceptor) and HSA (donor). By performing displacement measurements, the specific binding of PFDoA in the vicinity of site I of HSA was clarified. Furthermore, the binding details between PFDoA and HSA were further confirmed by molecular docking studies, which revealed that PFDoA was bound at subdomain IIA by multiple interactions, such as the interaction between O1 of PFDoA with Arg 257 and Ser 287 predominately through polar force. And the best calculated docking energy is -25.87 kJ·mol-1, this high negative value indicated that the PFDoA molecule exhibited large binding affinity towards HSA. The effects of PFDoA on the conformation of HSA were analyzed by synchronous fluorescence spectra and three-dimensional fluorescence spectra, and the results exhibited that the hydrophobicity of the microenvironment around tryptophan residue was increased and the conformation of HSA was altered after binding PFDoA. The CD spectra quantitatively calculated the protein secondary structure, which suggested a loss of helical stability after the PFDoA-HSA complex formation. The binding research presented in this paper enriches our knowledge of the interaction dynamics of perfluoroalkyl acids to the HSA and reveals the chemical essence of the interaction between biomacromolecule and ligand.
Perfluorododecanoic acid; Human serum albumin; Fluorescence spectroscopy; Molecular modeling; Circular dichroism
Introduction
Protein-ligand interactions play a key role in the distribution and transportation of small molecules in the biological systems and processes. One plasma protein that has been extensively studied during such work is human serum albumin (HSA), it is responsible for distributing and metabolizing many endogenous and exogenous ligands, e.g. toxicants, fatty acids, drugs, dyes and polymers, frequently by the formation of non-covalent complexes, thereby affecting the metabolism and excretion of various substances and influencing the characteristics of their physiological effects[1]. Therefore, the study on the interaction of various ligands with HSA is of imperative and essential importance for life sciences, chemistry, and clinical medicine.
Perfluorododecanoic acid (PFDoA) belongs to perfluoroalkyl acids (PFAAs) which are a class of highly stable compounds used widely in commercial and industrial applications as fire retardants, food packaging, polymer additives and water- and stain-resistant materials[2]. They have strong C-F bonds which enable them to resist biological and chemical degradation, leading to their wide global distribution, bioaccumulation and various toxicities. Interestingly, unlike other persistent organic pollutants, PFAAs do not preferentially accumulate in lipids and fatty tissue but rather in liver, kidney and serum[3]. PFDoA has been identified in water, soil, wildlife and humans, and animal experiments have demonstrated that PFDoA is the most toxic among the 8~12 carbon chain PFAAs[4]. There was only one report to study the specific site between PFDoA and HSA by fluorescence spectroscopy[5]. However, this study is insufficient in terms of the quenching mechanism of fluorescence, conformation and specific binding in detail, which are of great importance for perfectly demonstrating the interaction of PFDoA with HSA.
The aim of this study was to learn the binding mechanism of PFDoA with HSA by using fluorescence, circular dichroism (CD) and molecular modeling. Special emphasis was to laid on determining the conformation and secondary structure changes of HSA by synchronous fluorescence, three-dimensional and CD spectra. Another object of this paper was to identify the PFDoA specific binding sites (site I or site II) on the HSA molecule through displacement experiments and molecular modeling, which can offer a molecular level explanation with the ability to estimate the participation of specific chemical groups of PFDoA and PFDoA-HSA interactions in complex stabilization. Finally, this work should give more understanding on realizing the transport and metabolism process of PFDoA and the chemical essence of the interaction between biomacromolecule and ligand.
1 Materials and methods
1.1 Materials
HSA (Sigma, USA) working solution was prepared with the concentration of 2.0×10-5mol·L-1. PFDoA (Shanghai Aijie Biological Technology Co., Ltd., China) was dissolved and diluted to 5.0×10-4mol·L-1with ultrapure water. The stock solutions of phenylbutazone and ibuprofen were prepared to 5.0×10-4mol·L-1. All above solutions were kept in the dark at 0~4 ℃. Phosphate buffer solution (pH 7.40) and 1.0 mol·L-1NaCl solution were used. All reagents were of analytical reagent grade and Millipore-Q ultrapure water was used throughout the experiment.
F-4500 spectrophotometer (Hitachi, Japan), V-550 spectrophotometer (JASCO, Japan), J-815 CD spectrometer (JASCO, Japan).
1.2 Methods
Fluorescence spectroscopy, UV-Vis spectroscopy, elimination of the inner filter effects and molecular modeling are seen literature [6].
The CD measurements were obtained over a wavelength range of 190~250 nm at 0.2 nm intervals using a 0.1 cm cell at room temperature. Each spectrum was the average of three successive scans and finally averaged for plots and analyses. In order to calculate the composition of secondary structure of the protein, CONTIN program was used to analyze CD spectra.
2 Results and discussions
2.1 Analysis of fluorescence quenching of HSA by PFDoA
Generally, the fluorescence of HSA comes from tryptophan (Trp), tyrosine (Tyr) and phenylalanine (Phe) residues, while the intrinsic fluorescence of HSA is almost attributed to Trp alone which is located in the 214 position. The fluorescence spectra of HSA in the presence of different concentrations of PFDoA are illustrated in Fig.1. As shown in Fig.1, the fluorescence intensity of HSA remarkably decreased with the addition of PFDoA at the excitation wavelength of 280 nm, and a slight blue shift (from 352 to 346 nm) was also observed for the maximum emission wavelength, indicating that the microenvironment around HSA changed after adding PFDoA and the formation of PFDoA-HSA complex occurred.
Fluorescence quenching is usually classified as static quenching (formation of a ground-state complex) and dynamic quenching (collisional encounters). Generally, they can often be distinguished by their different dependence on temperature: the quenching constants decrease with increasing temperature for the static quenching, but the reversed effect is observed for the dynamic quenching[7]. To elucidate the quenching mechanism, the fluorescence quenching data were analyzed with the Stern-Volmer equation[8]
(1)
whereF,F0,KSV,kq,τ0and [Q] are seen literature [8]. Fig.2 displayed the Stern-Volmer plots for the quenching of HSA by PFDoA at three different temperatures. TheKSVandkqat different temperatures are listed in Table 1. As evident from Fig.2 and Table 1, theKSVvalues enhanced with increasing temperature, indicating that the predominant quenching process was dynamic quenching mechanism. However, the static quenching effect of complex formation could not be completely precluded in the present study. On the one hand, spectroscopic studies above have confirmed the formation of PFDoA-HSA complex. On the other hand, thekq(×1012L·mol-1·s-1) was greater than the maximum scattering collision quenching constant (2.0×1010L·mol-1·s-1)[9], suggesting a static quenching. Therefore, in conclusion, the fluorescence quenching mechanism of HSA by PFDoA was a combination of dynamic quenching with ground complex formation.

Fig.1 Fluorescence emission spectra of PFDoA-HSA system
cHSA=2.0×10-6mol·L-1;cPFDoA(×10-6mol·L-1)(1~7): 0, 1.6, 3.2, 4.8, 6.4, 8.0, 9.6; curve 8:cHSA=0,cPFDoA=1.6×10-6mol·L-1;T=298 K
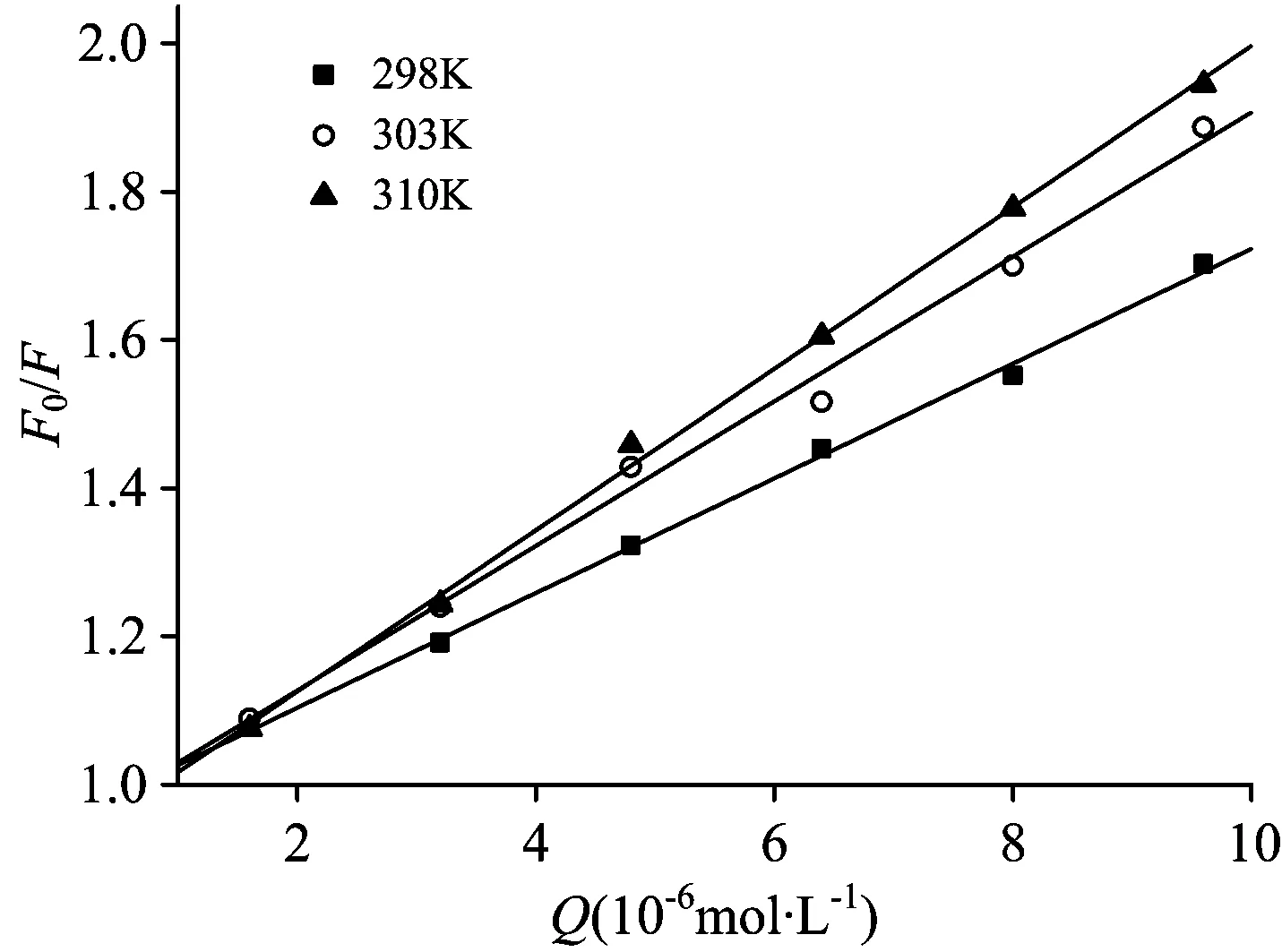
Fig.2 The Stern-Volmer plots for PFDoA-HSA system
cPFDoA(×10-6mol·L-1)(1~7): 0, 1.6, 3.2, 4.8, 6.4, 8.0, 9.6;cHSA=2.0×10-6mol·L-1
Table 1 Stern-Volmer quenching constants for interaction of PFDoA with HSA at different temperatures

pHT/KKSV/(×104L·mol-1)kq/(×1012L·mol-1·s-1)RSD2987 747 740 99910 01127 403039 759 750 99660 026931010 8910 890 99900 0162
2.2 Energy transfer from HSA to PFDoA
According to Föster non-radioactive energy transfer theory, the energy transfer efficiencyEis related not only to the distancerbetween the donor and acceptor, but also to the critical energy transfer distanceR0(when the transfer efficiency equals 50%)[10]
(2)
(3)
(4)
whereK2,N,φ,J,F(λ), andε(λ) are seen literature [10]. The overlap of the fluorescence emission spectra of HSA and the UV-Vis absorption spectra of PFDoA is represented in Fig.3. In the present case,K2=2/3,N=1.336 andφ=0.15 for HSA. From Eqs. (2)—(4), we can calculate thatR0=2.55 nm,E=0.10,J=9.93×10-15cm3·L·mol-1, andr=3.65 nm. The specific binding distance was smaller than 8 nm, which indicated that energy transfer between PFDoA and HSA can occur with high possibility. Furthermore, the larger distancer, compared to that ofR0, revealed that a static-type quenching mechanism occurs to a larger extent.

Fig.3 Overlapping between the emission spectrum of HSAaand absorption spectrum of PFDoAb
cHSA=cPFDoA=2.0×10-6mol·L-1
2.3 Identification of the specific binding site
To identify the binding site of PFDoA on HSA, the displacement experiments were carried out using the site markers of phenylbutazone for site Ⅰ and ibuprofen for site Ⅱ. The ratio of PFDoA to HSA was kept at 5∶1 to keep nonspecific binding markers to a minimum. The changes induced by the site markers are presented in Fig.4. The fluorescence of the complex was remarkably affected in presence of phenylbutazone, but remained invariant with ibuprofen. The observations demonstrated that phenylbutazone displaced PFDoA from the binding site, while ibuprofen had a little effect on the binding of PFDoA to HSA. Hence, it can be concluded that PFDoA was likely to be bound to site Ⅰ in the subdomain IIA of HSA.
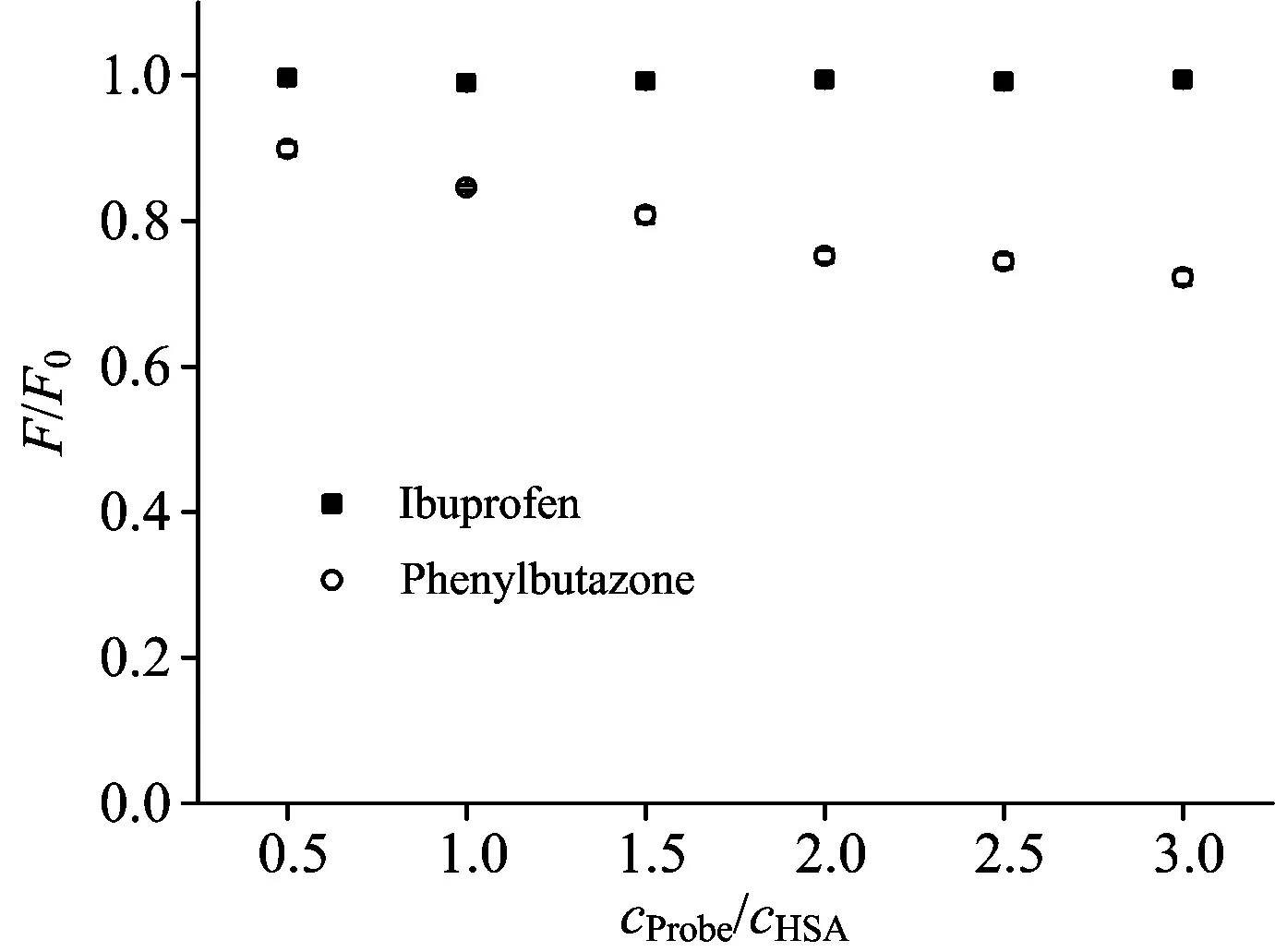
Fig.4 Effects of site markers on the fluorescence of PFDoA-HSA

Fig.5 (a) The binding site of PFDoA on HSA. HSA is shown in cartoon and PFDoA is represented using spheres. (b) Enlarged binding mode between PFDoA and HSA. HSA is shown in cartoon, the interacting side chains of HSA are displayed in surface mode and PFDoA is represented using balls and sticks. (c) Molecular modeling of the interaction between PFDoA and HSA. The atoms of PFDoA are blue
To further study the specific binding mode of PFDoA on HSA, we had run a docking program to simulate the binding mode between PFDoA and HSA. The crystal structure of HSA was taken from the Protein Data Bank (entry codes 2BXN). The best energy ranked result is shown in Fig.5, and the calculated docking energy is -25.87 kJ·mol-1. The inside wall of the pocket of subdomain IIA was formed by hydrophobic side chains, whereas the entrance to the pocket was surrounded by positively charged residues consisting of Lys 199, Phe 211, Trp 214, Ser 287 and Ala 291 [Fig.5(c)]. Thus we concluded that PFDoA was able to fit well within the hydrophobic cavity of subdomain IIA (site Ⅰ), which was consistent with the results observed in the displacement experiments. Table 2 showed the existence of polar force, hydrophobic interaction and halogen-bond between PFDoA and HSA. For example, O1 of PFDoA interacted with Arg 257 and Ser 287 through polar force.
Table 2 The distances and driving forces between the PFDoA atoms and the atoms of residues obtained by molecular docking
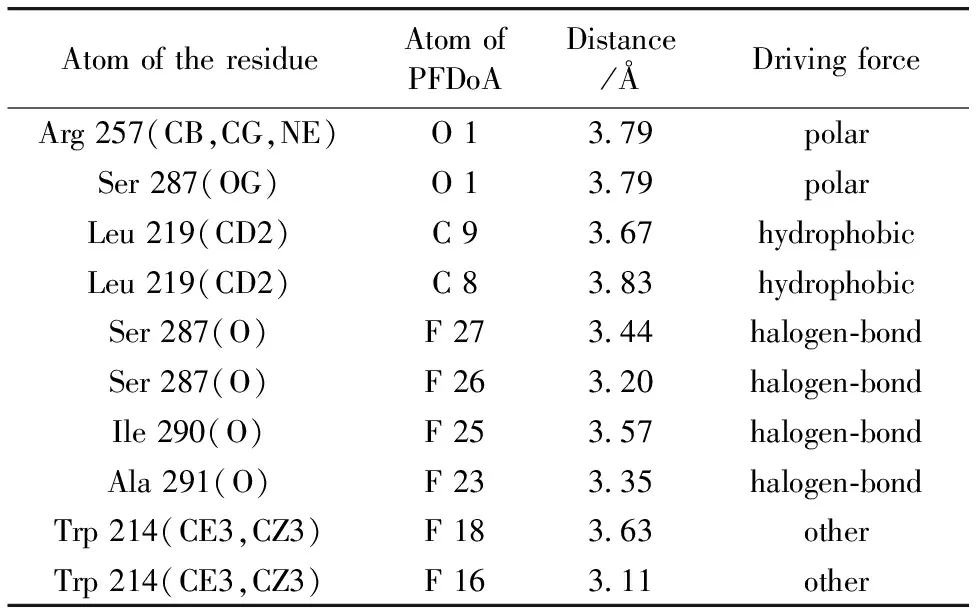
AtomoftheresidueAtomofPFDoADistance/ÅDrivingforceArg257(CB,CG,NE)O13 79polarSer287(OG)O13 79polarLeu219(CD2)C93 67hydrophobicLeu219(CD2)C83 83hydrophobicSer287(O)F273 44halogen⁃bondSer287(O)F263 20halogen⁃bondIle290(O)F253 57halogen⁃bondAla291(O)F233 35halogen⁃bondTrp214(CE3,CZ3)F183 63otherTrp214(CE3,CZ3)F163 11other
2.4 Conformation investigation
Synchronous fluorescence is a kind of simple and sensitive method to measure the fluorescence quenching. It can provide the characteristic information of polarity change around the chromophore micro-environment, and has several advantages, such as spectral simplification, reduction of the spectral bandwidth, and avoidance of different perturbing effects[11]. The synchronous fluorescence spectra of Tyr residues (Δλ=15 nm) and Trp residues (Δλ=60 nm) in HSA with addition of PFDoA were observed, as shown in Fig.6. It is apparent from Fig.6(a) that the emission peaks did not shift over the investigated concentration range with a little rise in fluorescence intensity, which indicated that PFDoA had little effect on the microenvironment of the Tyr residues in HSA. In Fig.6(b), the emission peaks of the Trp residues showed obvious reduction of fluorescence intensity with a slight blue shift (2 nm) upon the addition of PFDoA. This phenomenon expressed the conformational changes of the Trp residues, around which the polarity was decreased and the hydrophobicity was increased. These results confirmed that conformational and micro-environmental of HSA were changed in the presence of PFDoA, and the fluorescence quenching of HSA was mainly contributed to Trp residues.

Fig.6 Synchronous fluorescence spectra of PFDoA-HSA system at Δλ=15 nm (a) and Δλ=60 nm (b)
cHSA=2.0×10-6mol·L-1;cPFDoA(×10-6mol·L-1)(1~7): 0, 1.6, 3.2, 4.8, 6.4, 8.0 and 9.6;T=298 K


Fig.7 Three-dimensional fluorescence contour spectra (a and b) and three-dimensional fluorescence spectra (c and d) of HSA before and after adding PFDoA
2.5 Investigation on changes of HSA secondary structure
To ascertain the possible influence of PFDoA binding on the secondary structure of HSA, CD measurements were carried out in the presence of different PFDoA concentrations (Fig.8). The α-helix contents were calculated from mean residue ellipticity (MRE) values at 209 nm using the following two equations[14]
(5)
(6)
wherecp, n (n=585,forHSA), l,MRE209, 4 000,and33 000areseenliterature[14].ThequantitativeanalysisresultsofthesecondarystructureinHSAareshowedinTable3.Theα-helixdecreasedfrom47.3%to41.2%withthegraduallyincreasingconcentrationofPFDoA,whichshowedthattheinteractionbetweenPFDoAandHSAledtoachangeoftheprotein’ssecondarystructure,withthelossofhelicalstability.Furthermore,theCDspectraofHSAinthepresenceandabsenceofPFDoAwereobservedtobesimilarinshape,indicatingthatthestructureofHSAwasalsopredominantlyα-helix even after binding to PFDoA[15]. Therefore, we concluded that the binding of PFDoA to HSA induced some secondary structural changes in protein.

Fig.8 The CD spectra of HSA in the absence and presence of PFDoA
cHSA=2.0×10-6mol·L-1; molar ratiosnPFDoA∶nHSAfrom 1 to 3∶0∶1, 10∶1, 20∶1

Table 3 Secondary structure of HSA in the absence and presence of PFDoA determined by CONTIN
3 Conclusions
This task portrays an integrated experimental and computational modeling approach of the complexation of PFDoA with HSA at simulative physiological conditions (pH 7.40). PFDoA quenched the intrinsic fluorescence of HSA through a combined process of dynamic and static quenching mechanisms. The distance (r) of 3.65 nm between the donor and acceptor suggested that there was a high possibility of energy transfer from Trp-214 to PFDoA. The displacement experiments indicated that PFDoA was bound to subdomain IIA which was the same as site Ⅰ. The molecular modeling further confirmed the specific binding site of PFDoA on HSA. The conformation and microenvironment of HSA were changed after the addition of PFDoA through synchronous fluorescence and three-dimensional fluorescence spectra. As further revealed by CD spectra, the presence of PFDoA resulted in reduction ofα-helix but augment of random, which displayed that PFDoA induced the unfolding of the polypeptide of HSA. The binding study of PFDoA with HSA is of great importance in pharmacy, pharmacology and biochemistry and it will not only help understand the transportation and distribution of PFDoA in blood but also elucidate the mechanism.
[1] Memarpoor-Yazdi M, Mahaki H. J. Lumin.,2013, 136: 150.
[2] Stahl L L, Snyder B D, Olsen A R, et al. Sci. Total Environ.,2014, 499: 185.
[3] Zafeiraki E, Costopoulou D, Vassiliadou I, et al. Chemosphere, 2014, 94: 169.
[4] Kennedy Jr. G L, Butenhoff J L, Olsen G W, et al. Crit. Rev. Toxicol.,2004, 34(4): 351.
[5] Chen Y M, Gao L H. Arch. Toxicol.,2009, 83(3): 255.
[6] Dong C Y, Ma S Y, Liu Y. Spectrochim. Acta A, 2013, 103: 179.
[7] Zhang J, Yan Q S, Liu J P, et al. J. Lumin.,2013, 134: 747.
[8] Deng F Y, Dong C Y, Liu Y. Mol. Biosyst.,2012, 8(5): 1446.
[9] Markarian S A, Aznauryan M G. Mol. Biol. Rep.,2012, 39(7): 7559.
[10] Naik P N, Chimatadar S A, Nandibewoor S T. Spectrochim. Acta A, 2009, 73(5): 841.
[11] Hu Y J, Liu Y, Wang J B, et al. J. Pharm. Biomed. Anal.,2004, 36(4): 915.
[12] Sun H W, Wu Y J, Xia X H, et al. J. Lumin.,2013, 134: 580.
[13] Roy A S, Tripathy D R, Chatterjee A, et al. Spectrochim. Acta A, 2013, 102: 393.
[14] Matei I, Hillebrand M. J. Pharm. Biomed. Anal.,2010, 51(3): 768.
[15] Cheng Z J. Spectrochim. Acta A, 2012, 93: 321.
*通讯联系人
O657.3
A
多光谱和分子模拟技术研究全氟十二酸与人血清白蛋白的相互作用
胡涛英1,王艺润1,周珊珊1,2,刘 颖1,2*
1. 中央民族大学生命与环境科学学院,北京 100081
2. 中央民族大学北京市食品环境与健康工程技术研究中心,北京 100081
全氟十二酸(PFDoA)是8~12个碳链的全氟烷酸(PFAAs)中毒性最强的新型环境污染物。已有大量研究表明PFAAs在环境中广泛积累,但对PFDoA与HSA的相互作用还处于起步阶段。本研究力争在模拟生理条件下,采用荧光猝灭法、分子模拟技术和圆二色谱确定HSA与PFDoA的相互作用机理。研究结果表明,PFDoA对HSA的猝灭是动态猝灭与形成PFDoA-HSA基态复合物引起的猝灭共同作用的结果。计算得到的结合距离(r=3.65 nm)表明,PFDoA(受体)与HSA(供体)之间的相互作用发生了非辐射能量转移。取代反应结果表明,PFDoA键合在HSA的site Ⅰ位点上。分子对接进一步研究了PFDoA与HSA作用的详细结合情况,表明PFDoA通过多种作用力结合在HSA的亚域IIA内,例如,PFDoA上的O 1原子主要通过极性键与HSA上的Arg 257和Ser 287残基结合。计算得到的最优对接能量为-25.87 kJ·mol-1,表明PFDoA对HSA有较大的结合亲和力。同步荧光光谱和三维荧光光谱研究了PFDoA对HSA构象的影响,结果显示,与PFDoA结合后,色氨酸的微环境疏水性增加,HSA的构象也发生改变。PFDoA与HSA作用前后圆二色谱二级结构的定量分析结果表明,PFDoA-HSA复合物的形成使螺旋稳定性降低。该研究结果为全氟烷酸与HSA的动力学研究提供了理论依据和可靠数据,并揭示了生物大分子与配体相互作用的化学本质。
全氟十二酸; 人血清白蛋白; 荧光光谱; 分子模拟; 圆二色谱
2015-04-07,
2015-08-22)
Foundation item: The National Natural Science Foundation of China (21177163), 111 Project B08044, First-class University First Class Academic Program of Minzu University of China (YLDX01013), Coordinate Development of First-Class and First-Class University Discipline Construction Funds(10301-0150200604), The Academic Team Construction Project of Minzu University of China (2015MDTD25C&13C), First-class Universities and First-class Discipline Construction Transitional Funds Under Special Funding (10301-01404031, 2015), Graduate Student Scientific Research Innovation Project of Minzu University of China (K2014042),2015MDTD08C
10.3964/j.issn.1000-0593(2016)07-2330-07
Received: 2015-04-07; accepted: 2015-08-22
Biography: HU Tao-ying, (1989—), Master of College of Life and Environmental Science, Minzu University of China e-mal: hty0945020@163.com *Corresponding author e-mal: liuying4300@163.com
