Preparation of Cr-MnOx/Cordierite and Their Properties for Catalytic Oxidation of 1,2-Dichlorobenzene
2016-07-12ZHANGWenruiTANGAidongXUEJianliang
ZHANG Wen-rui, TANG Ai-dong, XUE Jian-liang
1.College of Chemical and Environmental Engineering, Shandong University of Science and Technology, Qingdao 266590, China 2.School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
Preparation of Cr-MnOx/Cordierite and Their Properties for Catalytic Oxidation of 1,2-Dichlorobenzene
ZHANG Wen-rui1, TANG Ai-dong2, XUE Jian-liang1
1.College of Chemical and Environmental Engineering, Shandong University of Science and Technology, Qingdao 266590, China 2.School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
Cr-MnOx/cordierite composites were prepared by Sol-gel, Impregnation, Co-precipitation and Rheological phase reaction method.Various technologies including X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive spectrometer (EDS), thermogravimetry/differential scanning calorimetry (TG/DCS), and temperature-programmed reduction (TPR) were used to characterize the structure and morphology properties of the synthesized composites.The catalytic ability test of 1,2-dichlorobenzene (o-DCB) over the catalysts was conducted in a fixed-bed flow reactor with a gas hourly space velocity (GHSV) of 30,000 h-1 to investigate the catalytic performance of the prepared composites.The results indicated that the combined Cr2O3and Mn2O3phases supported on cordierite possessed a special ball-shaped and better redox property in the catalyst prepared by the Co-precipitation method with a Cr/Mn atomic ratio of 2∶5, which was conducive to the increase of the synergistic effect and subsequently enhancement of the catalytic performance.Furthermore, it exhibited better stability within 60 h, which indicates a good prospect for industrial applications.
1,2-dichlorobenzene; Cr-MnOx/Cordierite composites; Co-precipitation method; Catalytic oxidation
Introduction
Incineration of wastes is expected to be more appealing as it reduces the mass and volume of wastes and produces energy[1].However, the combustion processes have a huge drawback that many organic byproducts, such as dioxins, could be produced originated from incomplete combustion.They are all environmentally persistent organic pollutants (POPs), which are very toxic as well as carcinogenic and poses a permanent sanitary risk to ecosystem and human health[2-5].Therefore, in order to fix the problem of the atmospheric mission of these chlorinated volatile organic compounds (Cl-VOCs), strict emission limits have been imposed by environmental legislation.Lots of techniques have been developed for solving this trouble of atmospheric release of Cl-VOCs, such as thermal incineration, adsorption and so on[6].The temperature of thermal incineration is higher than 850 ℃, and thermal treatment is quite expensive and may also lead emission of by-products, such as dioxins, dibenzofurans, etc.Due to incomplete combustion[7].For the adsorption technique, a special newly kind of carbonaceous materials[8]made of Jatropha curcas seeds by simple thermo-chemical activation with NaOH was applied for Cl-VOCs removal recently.Despite the success of adsorption and thermal incineration, there is still a need for research on techniques, which are both economically more favorable and truly destroy the pollutants rather than merely remove them for recycling elsewhere in nature.The catalytic oxidation of Cl-VOC to COx, H2O and HCl/Cl2appears very promising in this context[1].The major advantages of catalytic are that the reaction can be efficiently performed at relatively low temperatures and little pollutants can be treated efficiently.Therefore, an effective catalyst is the key to flue gas treatment.
Generally, there are two categories of catalysts for VOCs abatement: supported noble metals[9-10]and transition metal oxides[11-15].However, noble metal-based catalysts are not particularly suitable for the oxidation of VOCs because of their quick deactivation and the formation of poly chlorinated compounds during the catalytic combustion[16-17].Among transition metal oxides, the chromium[18], manganese[19]and copper oxides[20-21]are estimated to be the most active catalysts for the oxidation of Cl-VOCs.In recent years, numerous efforts have been devoted to the investigations about the Cr-Mn mixed oxide catalysts, which have been widely used in selective catalytic reduction of NOxwith NH3[22-23].However, few results about the Cr-Mn mixed oxide catalysts oxidation of dioxins have been reported.Furthermore, to prolong the useful life and catalytic activity of catalyst, the active catalysts were always based on carrier likes TiO2.Recently, porous cordierite, which was composited of 2MgO·2Al2O3·5SiO2, has attracted considerable attentions as a carrier since its very low thermal expansion coefficient characteristic as an attribute of excellent thermal shock resistance[24-27], super mechanical strength and its contribution to the dispersion of active substance.Nevertheless, the composition of Cr-MnOxdeposited on cordierite has been rarely reported.
The characteristics of metal-oxide supported catalysts depends on their nature, size, shape, surface area[15].And the interaction between active ingredients and catalyst carrier is a critical factor in determining catalytic activity and selectivity.According to synthetic approaches to metal oxide catalysts, their activity can be changed and thermal treatments can cause the morphological changes of metal oxide catalysts occurring from sintering process.So the preparation method of catalysts has a great importance.It is shown that many effective methods, including the sol-gel, the citric acid, co-precipitation, impregnation, hydrothermal/solvothermal reaction and hydrolysis, should be mentioned, as the most wildly used methods in the present.The Cr-MnOxcomposites prepared by the citric acid method were studied for the low-temperature selective catalytic reduction of NOxwith ammonia by Chen et al[23].It was found that the Cr (0.4)-MnOxshowed the highest activity and yielded 98.5% NO conversion at 120 ℃.However, there is no detailed comparative study regarding the influence of the preparation methods on the physicochemical properties of Cr-MnOx/cordierite catalyst and their performance in 1,2-dichlorobenzene oxidation.
Herein, in this study, the comparisons of the activity and characterization of the Cr-MnOx/cordierite prepared by Sol-gel, Impregnation, Co-precipitation and Rheological phase reaction method were carried out.Then, a room-temperature mixed co-precipitation route was developed to form cordierite containing Cr-MnOxcomposites with a mass ratio of Cr∶Mn from 1∶5 to 4∶5.The samples were characterized in detail by XRD, SEM, EDS, TG-DSC and TPR and catalytic activity.In addition, the structure and morphology properties of the composites were investigated.The main goal of this study is to present an optimal route to prepare Cr-MnOx/cordierite catalysts with high catalytic activity.
1 Experimental
1.1 Catalysts Synthesis
1.1.1 Impregnation method (Im)
The catalyst was prepared by incipient wetness impregnation of a cordierite with manganese nitrate and chrome nitrate.An amount of 20 mL of distilled water was added to a 250 mL beaker containing Mn(NO3)250% solution (7.2 mL) and Cr(NO3)2·9H2O (2.478 g) with stirring, the mixture was stirred at 80 ℃ until the water was evaporated.The paste obtained was dried overnight at 105 ℃ and was crushed into fine powder named Cr-MnOxprecursors.Catalyst powders were dispersed in deionized water with mass ratio of 1∶40, and then they were loaded inside the pore of cordierite honeycombs by impregnation.Finally, the Cr-MnOx/cordierite catalyst was dried overnight at 105 ℃ and calcined at 500 ℃ for 6 h in air.
1.1.2 Sol-gel method (Sg)
Mn(NO3)250% solution (7.2 mL), Cr(NO3)2·9H2O (2.478 g) and citric acid were mixed and dissolved in deionized water.The molar ratio of citric acid to the metal components (the total mole of chromium and manganese) was 0.3.The mixture was stirred at room temperature for 1 h until a blue sol was formed.Then, the sol was stirred at 80 ℃ until the water was evaporated.The obtained dried paste was crushed into fine Cr-MnOxpowder.A cordierite honeycomb was introduced into the Cr-MnOxsolution (the mass ratio of Cr-MnOxpowder and deionized water was 1∶40).All Cr-MnOxmixture was loaded inside the pore of cordierite honeycombs via impregnation.Finally, the Cr-MnOx/cordierite catalyst was dried overnight at 105 ℃ and calcined at 500 ℃ for 6 h in air.
1.1.3 Co-precipitation method (Co)
For this method, Mn(NO3)250% solution (7.2 mL) and Cr(NO3)2·9H2O (2.478 g) were dissolved completely in 20 mL water, and then saturated solution of ammonium carbonate was gradually added with thorough stirring until the pH of mixture was 10.The suspension mixture was stirred at 80 ℃ until the water was evaporated.The paste obtained was dried overnight at 105 ℃ and was crushed into fine powder.Catalyst powders were dispersed in deionized water with mass ratio of 1∶40.After that, a cordierite honeycombs was brought into for impregnating the Cr-MnOxmixture.Finally, the Cr-MnOx/cordierite catalyst was dried overnight at 105 ℃ and calcined at 500 ℃ for 6 h in air.At the same time, the different [Cr]/[Mn] atomic ratio (1∶5, 2∶5, 3∶5 and 4∶5) of Cr-MnOx/cordierite catalysts were prepared by the same procedure.
1.1.4 Rheological phase reaction method (Rh)
For this method, Cr(NO3)2·9H2O (2.478 g) and oxalic acid (2.478 g) were mixed in the crucible.The mixture was thoroughly ground for 40 min, and then Mn(NO3)250% solution (7.2 mL) was stirred into the mixture until it turned into a rheological phase.This was placed into a stainless steel pressure reactor with a Teflon inner liner, sealed and put in a baking oven at 100 ℃ for 8 h.Then, the mixture was evaporated until the water drying.The paste obtained was dried overnight at 105 ℃ and was crushed into fine powder.Catalyst powders were dispersed in deionized water with mass ratio of 1∶40.A cordierite honeycomb was pulled into the product for impregnating the Cr-MnOxmixture.At last, the Cr-MnOx/cordierite catalyst was dried overnight at 105 ℃ and calcined at 500 ℃ for 6 h in air.
1.2 Characterization of catalyst
The phase structure of catalysts were performed on a D8-ADVANCE X-ray diffract meter (XRD) with Cu Kα radiation (Cu Kα=0.154 06 nm).Scanning electron microscopy (SEM) and electron diffraction spectra (EDS) were used to investigate the morphology of catalysts and the dispersion of the elements with a JEOL S-4800 electron microscope.Thermal decomposition of catalyst precursors were monitored by TG-DSC at a heating rate of 10 ℃·min-1up to 900 ℃.The temperature-programmed reduction (H2-TPR) experiment was carried out for every 50 mg of catalysts from 30 to 900 ℃ with a Gas Chromatograph (GC 1690) equipped with thermal conductivity detector (TCD) and silica packed column.The TPR runs were carried out with a linear heating rate (10 ℃·min-1) in a flow of 10% H2in argon with a flow rate of 40 mL·min-1.The hydrogen consumption was measured quantitatively by a thermal conductivity detector.
1.3 Catalytic activity measurement
Catalytic activity was carried out in a self-designed apparatus at atmospheric pressure of 150~300 ℃ (Fig.1).Because dioxins are very toxic and hard to handle, laboratory studies are usually employ model compounds such as 1,2-dichlorobenzene (o-DCB) to predict the dioxide destruction behavior of different catalysts.

Fig.1 Schematic diagram of the experimental apparatus
Cr-MnOx/cordierite catalyst was placed in stainless steel tubes with 40 cm of inner diameter.O-DCB containing feed stream to the reactor was carried by air and controlled by a flow controller with a typical GHSV (gas hourly space velocity) of 30 000 h-1.The concentration of o-DCB in air was 3% and the O2/o-DCB molar ratio was 6.8∶1 before reaction.Oxygen was excess to insure complete oxidation of o-DCB.Acetone was used as the absorption solution, the o-DCB concentration difference between bottle 1 and bottle 2 was conducted as the evaluation standard of catalytic performance.The final products of CO, CO2and HCl during o-DCB catalytic oxidation have been detected by gas chromatography and absorbed by exhaust collection device.After 60 min reaction at each temperature, quantitative analysis of o-DCB was performed using a gas chromatography (GC-2010) equipped with flame ionization detector (FID).The type of column used for separation is GDX-101 and the pressure for column, H2, N2and O2were 0.08, 0.05, 0.3, and 0.2 MPa, respectively.During the o-DCB analysis the temperature of the column and detector of GC with nitrogen as carrier gas were set up as 140 and 260 ℃, respectively.o-DCB conversion efficiency was obtained by the following equation:
o-DCB conversion=([o-DCB]bottle1-
[o-DCB]bottle2)/[o-DCB]bottle1×100%
(1)
2 Results and Discussion
2.1 Overview of the catalysts
The o-DCB conversion over the Cr-MnOx/cordierite catalysts prepared by different methods is presented in Fig.2.Pure cordierite honeycomb was also presented for comparison.It was clear that pure cordierite honeycomb showed no catalytic activity.Cr-MnOx/cordierite prepared by Co method performed a best catalytic capacity in all catalysts.For this catalyst, more than 55% and 80% o-DCB could be removed above 200 and 250 ℃ respectively, and the o-DCB conversions reached almost 90% at 300 ℃ with a GHSV of 30 000 h-1.However, the conversion efficiencies of o-DCB over others Cr-MnOx/cordierite catalysts prepared by Im, Sg and Rh method were below obviously compared with Cr-MnOx/cordierite prepared by Co method under the same condition.
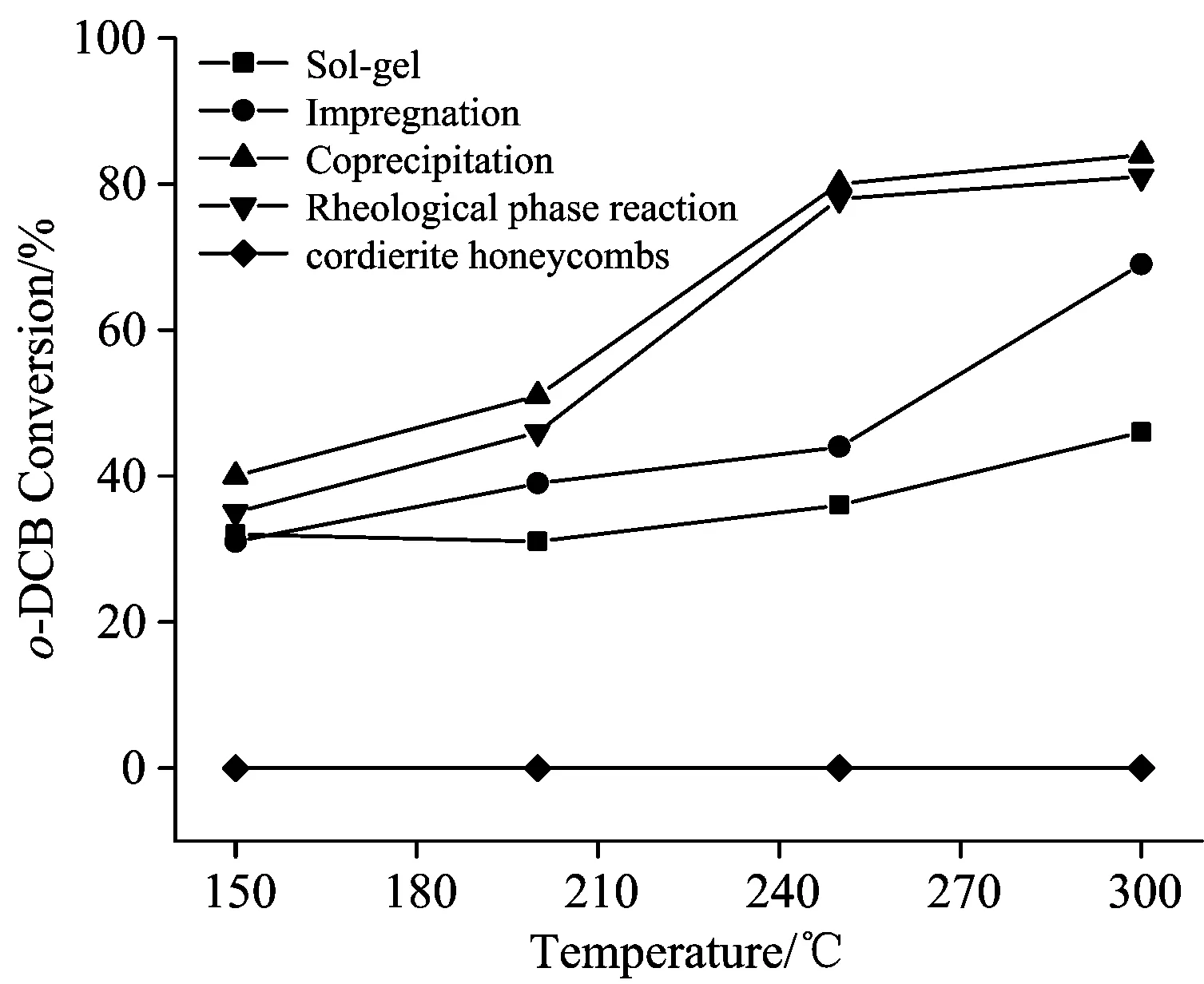
Fig.2 The o-DCB conversion over the Cr-MnOx/cordierite (Cr∶Mn=1∶5) catalysts prepared by different method

Fig.3 The o-DCB conversion over the Cr-MnOx/cordierite catalysts prepared by Co-precipitation method with different ratio of Cr/Mn
Then, the Cr content had been evaluated for Cr-MnOx/cordierite prepared by Co-precipitation method.From the Fig.3, the variation of the ratio of Cr/Mn had different effects on the catalytic activity for this method.The o-DCB conversion increased with the increase of Cr loading until the atomic ratio of Cr/Mn reached 2∶5, and then the further increase of Cr loading amount would lead to the decrease of o-DCB conversion due to the sintering effect.This effect will cause a decrease in the number of surface metal atoms per unit mass of metal and therefore decreases the number of active sites of the catalyst[28].
2.2 Characterization of the catalysts

Fig.4 XRD patterns of the (a) Cr-MnOx/cordierite catalysts; (b) Cr-MnOxcatalysts prepared by different methods and (c) Cr-MnOxcatalysts prepared by Co-precipitation method with different ratio of Cr/Mn
The XRD patterns of Cr-MnOx/cordierite catalysts prepared by different methods are shown in Fig.4(a).The strong peak of cordierite support was detected in all samples.Also, several diffraction peaks appeared were attributed to Mn2O3(PDF card 41-1442) in the four samples.There were no peaks corresponded to Cr2O3or other chrome oxides because it’s covered by strong support peaks of cordierite.So, the Cr-MnOxcatalysts without support prepared by different methods are detected and the XRD patterns of them are shown in Fig.4 (b).From the XRD, the characteristic diffraction peak of Mn2O3(PDF card 41-1442) was observed in all samples.For Cr-MnOxprepared by impregnation method, some new peaks assigned to the MnO2phases were formed except Mn2O3.At the same time, Cr2O3(PDF card 38-1479) were perceived in three Cr-MnOxcatalysts except Cr-MnOxcatalyst prepared by Sg method, and there was no other new phase or solid solution.As can been seen, the intensity of Mn2O3in Cr-MnOxcatalysts prepared by Co-precipitation method (Mn2O3%=27.5%) was stronger than other three Cr-MnOxcatalysts prepared by Im (Mn2O3%=15.7%), Sg (Mn2O3%=18.8%) and Rh method (Mn2O3%=24.5%).In addition, The XRD patterns of Cr-MnOxcatalysts prepared by Co-precipitation method with different ratio of Cr/Mn are shown in Fig.4(c).It can be seen that the peaks of Mn2O3and Cr2O3were decreased and increased respectively with the increasing of ratio of Cr/Mn.Combined with the results of o-DCB conversion efficiency (Fig.3), we can speculate that the high content Mn2O3and proper Cr2O3of Cr-MnOx/cordierite prepared by Co-precipitation method (the ratio of Cr/Mn was 2∶5) played a major role on the high activity.
TG/DSC analysis of Cr-MnOx(Cr∶Mn=2∶5) prepared by Co-precipitation method was performed under air flow and the results are shown in Fig.5.The weight loss before 150 ℃ was due to decomposition of physically adsorbed water and surface impurities.The Mn2+and Cr3+hydroxides has been formed in the PH of 5 and 8 respectively, and the Mn(OH)2and Cr(OH)3has been formed in this system.The endothermic peak located at 201.35 ℃ accompanied with a highly weight loss on TG curve was caused by the formation of manganese oxides, referring to the decomposition of Mn(OH)2.The curve also exhibited a broad endothermic peak at 507.87 ℃ with a slightly loss on TG curve,attributing to the formation of chrome oxides as the decomposition of Cr(OH)3.There was Mn2O3and Cr2O3in the Cr-MnOxcatalyst according to XRD data.Conclusion would be drawn that Mn2O3and Cr2O3existed in Cr-MnOxcatalyst prepared by Co-precipitation method is responsible to its high catalytic activity.
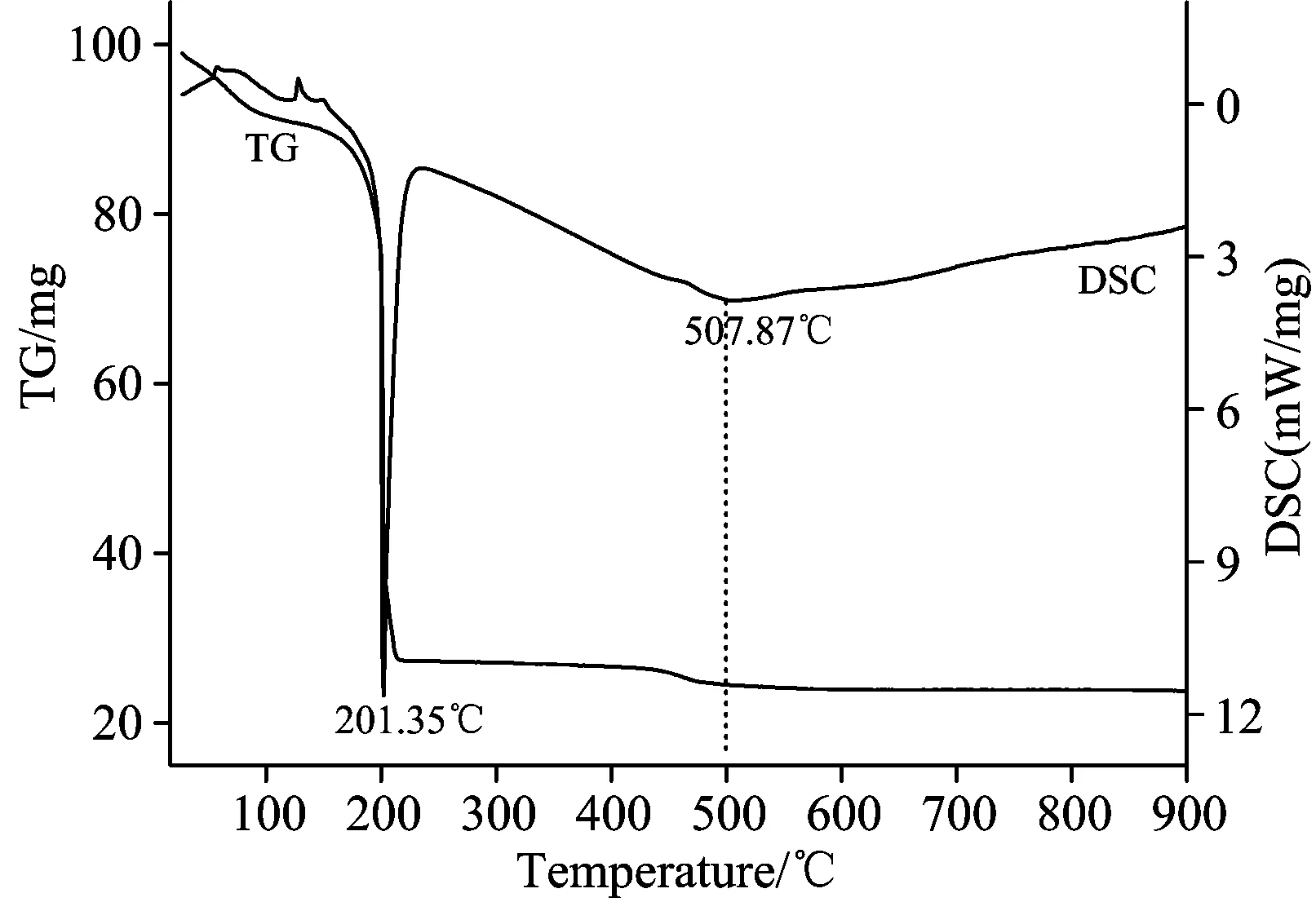
Fig.5 TG/DSC curves of Cr-MnOx (Cr∶Mn=2∶5) precursor prepared by Co-precipitation method

Fig.6 SEM images of Cr-MnOx catalysts prepared by different methods
Fig.6 is SEM images of four typical catalysts showing their morphology.It indicated that there were significant disparities in the morphology of four catalysts.In Fig.6(a), it can be seen the particles of Cr-MnOxcatalyst prepared by Co-precipitation method had comparatively regular ball-shape with asymmetrical grain size.It was worth noting that ball-shape catalyst was composited by needle nanowire with sizes from 30 to 1 000 nm resulting in its fluffy appearance and less density.Some bigger ball-like particles seem to be accumulations of the smaller ones.In Fig.6(b), the primary grain size of Cr-MnOxcatalyst prepared by Rheological phase reaction method was about 20 nm, and the particle size distribution was relatively uniform.In Fig.6(c) and (d), the morphology of Cr-MnOxcatalyst prepared by Sol-gel method was similar to that of prepared by Impregnation method.It is clear that there were mainly the lump-shaped particles with the better crystallinity, and some small particles attached on its surface.From the results of SEM, it can be concluded that the particle size of Cr-MnOxcatalyst prepared by Co-precipitation and Rheological phase reaction method was smaller than that of the other catalysts.Experimental results revealed that, the order of the particle size of Cr-MnOxcatalyst in the four samples was the reverse as the order of their catalytic activity (the order of the particle size: Sg≈Im>Co>Rh; the order of activity: Co>Rh>Im>Sg).It is believed that the Cr-MnOxactive phase in small catalyst particle could interact with reactants better and fully, which is beneficial to the transfer of electron between the interface of reactants and Cr-MnOx.Though the grain size of catalyst prepared by Rh was smaller than catalyst prepared by Co method, the agglomeration of catalysts prepared by Rh was more serious due to calcination than by Co method.This will bring a bad effect on catalytic activity (see Fig.1).Therefore, the excellent catalytic activity of Cr-MnOxcatalyst prepared by Co-precipitation method was mainly due to its special ball-shape particles with high surface area.Combined with the analysis of TG-DSC, we can see that the Mn2+and Cr3+hydroxides has been formed in an alkaline environment.The primary particles of Mn2O3and Cr2O3generated from the decomposition of Mn(OH)2and Cr(OH)3separately.Plenty of primary particles agglomerated to form crystal nuclei, which covered by followed Mn2O3and Cr2O3.
Mn2++OH-→Mn(OH)2;
Cr3++OH-→Cr(OH)3
(2)
Mn(OH)2→Mn2O3;
Cr(OH)3→Cr2O3
(3)
Themechanismofformationofspecialball-shapeCr-MnOxhas not been understood yet.We will make effect to study this work next.
The H2-TPR patterns of the catalysts prepared by four methods with the same Cr∶Mn ratio (1∶5) are shown in Fig.7.The TPR profile of Cr-MnOx/cordierite catalyst prepared by Co-precipitation method was the combination of two physically mixed oxides.It can be seen that Cr2O3was reduced to CrO at 235 ℃[29-30].Moreover, two stages of MnOxtransformation were presented from 300 to 500 ℃, including Mn2O3shifting to Mn3O4followed by Mn3O4shifting to MnO[2].The other three Cr-MnOx/cordierite catalysts, however, showed nearly similar reduction behaviors, which were quite different from Cr-MnOx/cordierite catalyst prepared by Co-precipitation method.There was only the MnOxredox reaction in turn from 300 to 500 ℃ because the content of Cr was so little that the TPR cannot detect.In the profile of Cr-MnOx/cordierite catalysts prepared by Rheological phase reaction and Sol-gel method there were two peaks, corresponding to the reaction Mn2O3→Mn3O4, Mn3O4→MnO, respectively.With comparison with above catalysts, the profile of Cr-MnOx/cordierite catalyst prepared by Impregnation method showed a broad reduction peak.It also can be seen that the sequence of the area of peak was Co (1 853.89)>Rh(1 821.67)>Sg(1 225.57)>Im(333.68), implying that the consumed H2quantity was followed the order of Co>Rh>Sg>Im.It indicates that the Cr-MnOx/cordierite catalyst prepared by Co-precipitation method has better redox properties, therefore this catalysts display higher catalytic activity than the other three Cr-MnOx/cordierite catalysts prepared by Rheological phase reaction, Sol-gel and Impregnation method.The high surface area and the better redox properties enable catalyst’s best catalytic performance by the synergistic effect.

Fig.7 TPR profiles of the Cr-MnOx/cordierite catalysts prepared by different methods
2.3 The stability in catalytic activity of Cr-MnOx/cordierite catalyst prepared by Co-precipitation method
For practical use, the stability in catalytic activity is essential.Combined the above analysis, the Cr-MnOx/cordierite catalyst (Cr∶Mn=2∶5) prepared by Co-precipitation method was chosen to investigate the stability for the conversions of o-DCB.
Fig.8 presents the experimental results of the stability test as a function of time at 300 ℃.This test was carried out after the evaluation of activity of the fresh catalysts.The increasing activity can be achieved within 0.5~15 h, on which the conversions of o-DCB were about 77%, 79%, 84% and 84%.From 16 h to 24 h, the conversion was slowly decreased to 83% and 82%.And then, the stable activity was observed within 25~50 h, on which the conversion of o-DCB was kept about 79%.The conversion of o-DCB was decreased to 73% after reacting 53 h.

Fig.8 The stability test of Cr-MnOx/cordierite (Cr∶Mn=2∶5) catalyst prepared by Co-precipitation method, GHSV=30 000 h-1
To better illustrate the reason of decreased activity, we further investigated the change of the elements of catalyst before and after the stability test (the test time was 50 h).EDS spectra of Cr-MnOx/cordierite (Cr∶Mn=2∶5) catalyst prepared by Co-precipitation method before and after stability test are shown in Fig.9.Only a little difference can be observed for the two samples.For the catalyst before stability test, only Cr and Mn were detected, and the mass ratio of Cr/Mn (40%) was consistent with the Experimental design value.For the catalyst before stability test, a weak peak at 2.6 KeV showed the presence of chlorine.This result indicates that chlorinated species were retained on the surface of Cr-MnOx/cordierite catalyst during the o-DCB oxidation at a higher temperature for a long time.According to the results of stability test, the catalytic activity decreased with the increase of reaction time, it may be related to the accumulation of Cl on catalyst surface.That will hider the absorption of more o-DCB and reduce the number of active surface sites of catalysts[17].
In future, we will perform the works to enhance the catalytic stability activity of Cr-MnOx/cordierite preferentially.After then, mechanistic studies of o-DCB oxidation on Cr-MnOx/cordierite will be preceded.

Fig.9 EDS spectra of Cr-MnOx/cordierite (Cr∶Mn=2∶5) catalyst prepared by Co-precipitation method before and after stability test
3 Conclusion
Cr-MnOx/Cordierite composites were prepared by Sol-gel, Impregnation, Co-precipitation and Rheological phase reaction method, respectively.The catalytic tests on o-DCB removal ability for all catalysts were performed in a fixed-bed flow reactor.Experimental results showed that when the atomic ratio of Cr/Mn reached 1∶5, the activity of the catalysts prepared by Co-precipitation and Rheological phase reaction method reached their highest value, particularly the catalyst with the Cr/Mn atomic ratio of 2∶5 prepared by Co-precipitation method demonstrated higher catalytic activity than that of the other catalysts at temperature between 100 and 300 ℃.The combined Cr2O3and Mn2O3were the main phases in the Cr-MnOx/Cordierite catalyst, which were suggested by the XRD and TG-DSC.From the microstructure characterization by SEM and TPR, it could be known that catalyst with special ball-shaped and better redox properties contributed to a good catalytic performance for the catalyst prepared by the Co-precipitation method in the present study.It was also found that the Cr-MnOx/Cordierite resistance to Cl within 60 h, and exhibited better stability, which indicates a good prospect for industrial applications.
[1] Bertinchamps F, Gregoire C, Gaigneaux E M.Applied Catalysis B: Environmental, 2006, 66(1-2): 1.
[2] Liljelind P, Unsworth J, Maaskant O, et al.Chemosphere, 2001, 42(5-7): 615.
[3] Kulkarni P S, Crespo J G, Afonso C A M.Environment International, 2008, 34(1): 139.
[4] McKay G.Chemical Engineering Journal, 2002, 86(3): 343.
[5] Sean M H, Aylward L L.Regulatory Toxicology and Pharmacology, 2003,(37): 202.
[6] Buekens A, Huang H.Journal of Hazardous Materials, 1998, 62(1): 1.
[7] Khaleel A, Al-Nayli A.Applied Catalysis B: Environmental, 2008, 80(1-2): 176.
[8] Hsu S H, Huang C S, Chung T W, et al.Journal of the Taiwan Institute of Chemical Engineers, 2014, 45(5): 2526.
[9] Luo M F, He M, Xie Y L, et al.Applied Catalysis B: Environmental, 2007, 69(3-4): 213.
[10] Chi Sheng Wu J, Chang T Y.Catalysis Today, 1998, 44(1-4): 111.
[11] Yang Y, Xu X, Sun K.Journal of Hazardous Materials, 2007, 139(1): 140.
[12] Blasin-Aub V, Belkouch J, Monceaux L.Applied Catalysis B: Environmental, 2003, 43(2): 175.
[13] Alifanti M, Florea M, Somacescu S, et al.Applied Catalysis B: Environmental, 2005, 60(1-2): 33.
[14] Tang W, Wu X, Liu G, et al.Journal of Rare Earths, 2015, 33(1): 62.
[15] Dong Won Lee B R Y.Journal of Industrial and Engineering Chemistry, 2014, 20: 3947.
[16] Scir S, Minic S, Crisafulli C.Applied Catalysis B: Environmental, 2003, 45(2): 117.
[17] van den Brink R W, Krzan M, Feijen-Jeurissen M M R, et al.Applied Catalysis B: Environmental, 2000, 24(3-4): 255.
[18] Oliveira L C A, Lago R M, Fabris J D, et al.Applied Clay Science, 2008, 39(3-4): 218.
[19] Liu Y, Wei Z, Feng Z, et al.Journal of Catalysis, 2001, 202(1): 200.
[20] Li W B, Zhuang M, Wang J X.Catalysis Today, 2008, 137(2-4): 340.
[21] Vu V H, Belkouch J, Ould-Dris A, et al.Journal of Hazardous Materials, 2009, 169(1-3): 758.
[22] Chen Z, Li X, Gao X, et al.Chinese Journal of Catalysis, 2009, 30(1): 4.
[23] Chen Z, Yang Q, Li H, et al.Journal of Catalysis, 2010, 276(1): 56.
[24] Fuji M, Shiroki Y, Menchavez R L, et al.Powder Technology, 2007, 172(1): 57.
[25] Yamuna A, Johnson R, Mahajan Y R, et al.Journal of the European Ceramic Society, 2004, 24(1): 65.
[26] Zhou T, Li L, Cheng J, et al.Ceramics International, 2010, 36(2): 529.
[27] Kobayashi Y, Sumi K, Kato E.Ceramics International, 2000, 26(7): 739.
[28] José Luis Contreras G A F.
[29] Grzybowska B, Sloczynski J, Grabowski R, et al.Journal of Catalysis, 1998, 178(2): 687.
[30] Hakuli A, Harlin M E, Backman L B, et al.Journal of Catalysis, 1999, 184(2): 349.
X132
A
Cr-MnOx/堇青石催化剂制备及催化降解邻二氯苯性能研究
张文睿1,唐爱东2,薛建良1
1.山东科技大学化学与环境工程学院,山东 青岛 266590 2.中南大学化学化工学院,湖南 长沙 410083
采用溶胶凝胶法、浸渍法、共沉淀法以及流变相法制备了新型Cr-MnOx/堇青石催化剂,同时采用X射线衍射(XRD)、扫描电镜(SEM)、热重和差热分析(TG-DTA)、H2-程序升温还原(H2-TPR)以及元素能谱(EDS)技术对催化剂进行表征。经筛选发现,以共沉淀法制备的Cr-MnOx/堇青石催化剂(Cr/Mn=2∶5)催化活性最高。通过表征结果可知,以共沉淀方法制备的催化剂主要活性成分为Mn2O3和Cr2O3,并且具有特殊的球形和较好的氧化还原性能,协同作用的存在有助于催化降解目标污染物邻二氯苯(o-DCB)性能的提高;在60 h之内,o-DCB降解率仍保持在80%以上,具有较好的催化稳定性。
邻二氯苯;Cr-MnOx/堇青石催化剂;共沉淀法;催化氧化
2015-09-23,
2016-01-20)
Foundation item:Scientific Research Foundation of Shandong University of Science and Technology for Recruited Talents (2016RCJJ017) and National Natural Science Foundation of China (51408347)
10.3964/j.issn.1000-0593(2016)09-3075-08
Received:2015-09-23; accepted:2016-01-20
Biography:ZHANG Wen-rui, (1986—), female, PhD, lecturer in College of Chemical and Environmental Engineering, Shandong University of Science and Technology e-mail:wenrui.mao@163.com
