Interaction Study of Ferrocene Derivatives and Heme by UV-Vis Spectroscopy
2016-07-12HANGuochengFENGXiaozhenLIANGJintaoXIAOWenxiangCHENZhencheng
HAN Guo-cheng, FENG Xiao-zhen, LIANG Jin-tao, XIAO Wen-xiang, CHEN Zhen-cheng
School of Life and Environmental Sciences, Guilin University of Electronic Technology, Guilin 541004, China
Interaction Study of Ferrocene Derivatives and Heme by UV-Vis Spectroscopy
HAN Guo-cheng, FENG Xiao-zhen, LIANG Jin-tao, XIAO Wen-xiang, CHEN Zhen-cheng*
School of Life and Environmental Sciences, Guilin University of Electronic Technology, Guilin 541004, China
The interaction between ferrocene derivatives, such as Fc(COOH)2(λmax=286 nm), Fc(OBt)2(λmax=305 nm), Fc(Cys)(λmax=289 nm) and heme(λmax=386 nm) were studied by UV-Vis spectroscopy, respectively.The results show that, when the concentration of heme is fixed, the absorbance of heme increases with the increase of Fc(COOH)2and Fc(Cys) concentration, the absorbance of heme almost keep the same when Fc(OBt)2concentration increases; when the concentration of ferrocene derivatives are fixed, the absorbance of Fc(COOH)2and Fc(Cys) also increases with the increase of heme concentration, the absorbance of Fc(OBt)2almost keep the same when heme concentration increase.It is demonstrated that the hydrogen bonding interactions happen between Fc(COOH)2, Fc(Cys) and heme, none of Fc(OBt)2, the formation of hydrogen bonding lead to the growth of molecular chain, the bigger molecule can absorb more energy and increase the absorbance.Meanwhile, the stability of molecule is affected by the formation of hydrogen bonding, when the reaction time increases from 0.5 h to 18 h and 48 h, the absorbance atλmax=384 nm change from 2.64 to 2.53 and 2.51 with fixed concentration of Fc(COOH)2, the absorbance atλmax=384 nm change from 1.76 to 1.72 and 1.68 with fixed concentration of heme, the absorbance atλmax=397 nm change from 2.74 to 2.63 and 2.55 with fixed concentration of Fc(Cys), and the absorbance atλmax=397 nm change from 1.82 to 1.58 and 1.49 with fixed concentration of heme, respectively.
UV-Vis spectroscopy; Ferrocene derivatives; Heme; Hydrogen bond
Introduction
Hemoglobin can transport oxygen in higher organisms, this kind of protein is composed of four chains, twoαand twoβchains, each chain has a ring of heme, which is the active center of hemoglobin[1].It is also related with many clinical diseases, such as diabetes, leukemia, anemia and heart diseases[2-4].Therefore, it has great significance to quantitative detect hemoglobin.Several analytical methods for detecting hemoglobin in pharmaceutical or biological systems have been reported.These methods include capillary electrophoresis[5], electrochemistry[6], spectrophotometry[7], spectrofluorimetry[8-9], chemiluminescence[10], and high performance liquid chromatography[11]and so on.
It is well known that ferrocene (Fc) is an organometallic compound consisting of two cyclopentadienyl rings bound on opposite sides of a central metal Fe atom.The rapid growth of organometallic chemistry is attributed to the discovery of ferrocene and its many derivatives.Most importantly, ferrocene and its derivatives have lots of applications, for examples, they can be used in asymmetric catalysis and enantioselective synthesis and are widely used as redox sensors which can be incorporated into large proteins to act as redox relays[12].They are convenient for protein labelling based on amide bond formation between the ferrocene carboxylic acid and amino acids.Ferrocenes exhibit a well-characterized one-electron reversible oxidation wave and are useful as an electrochemical probe in the development of electrochemical sensor technologies that enabled the detection of a wide range of biomolecules[13].Furthermore, ferrocene can be used as a molecular scaffold that supports B-sheet-like interactions between two peptide chains[14].
Herein, we take advantage of ferrocene derivatives as yellow compounds, heme as red compound which show optical property, and they all have some functional groups.Therefore, the UV-Vis spectroscopy can be used for the studies of interaction between ferrocene derivatives and heme, which can provide a new way to explore the quantitative detection of hemoglobin based on the reaction of ferrocene derivatives and heme.
1 Experimental
1.1 Apparatus and chemicals
A UV-2550 spectrophotometer was used to perform all spectral experiments.All reagents used were of analytical grade.The ferrocene derivatives, such as Fc(COOH)2, Fc(OBt)2, Fc(Cys) were synthesized according to reported procedures, which structure are shown in Fig.1[15-16].Heme and ethylenediamine (EDA) were purchased from Aladdin Company (Shanghai, China).All aqueous solutions were prepared with Milli-Q water.

Fig.1 The structure of four compounds
1.2 Preparation of solutions
1.0 mmol·L-1stock solution of Fc(COOH)2was prepared by dissolving Fc(COOH)2in 10.0 mmol·L-1EDA solution (pH 8.5).1.0 mmol·L-1stock solution of Fc(OBt)2was prepared by dissolving Fc(OBt)2in 10.0 mmol·L-1EDA solution.0.5 mmol·L-1stock solution of Fc(Cys) was prepared by dissolving Fc(Cys) in 10.0 mmol·L-1EDA solution.0.5 mmol·L-1stock solution of heme was prepared by dissolving heme in 10.0 mmol·L-1EDA solution.
2 Results and discussion
2.1 Interaction of Fc(COOH)2 and heme
2.1.1 UV-Vis spectra of Fc(COOH)2, heme and their interaction
Because most ferrocene derivatives and heme are fat soluble, they do not dissolve in water easily, so it should find suitable promoter to dissolve the above stuffs for experiments.First, we try some organic solvents, such as methanol and acetonitrile, ferrocene derivatives can dissolve these organic solvents easily, but heme does not.Second, we consider that Fc(COOH)2and heme have free —COOH group, they can dissolve alkaline solution, we try triethylamine.Although triethylamine can dissolve Fc(COOH)2and heme, there are no signals when do UV-Vis scan.Then we try ethylenediamine, it works, so ethylenediamine was taken as the promoter for experiments.Fig.2 shows the UV-Vis spectra of 250 μmol·L-1Fc(COOH)2, 25 μmol·L-1heme and their interaction (taken H2O as baseline).
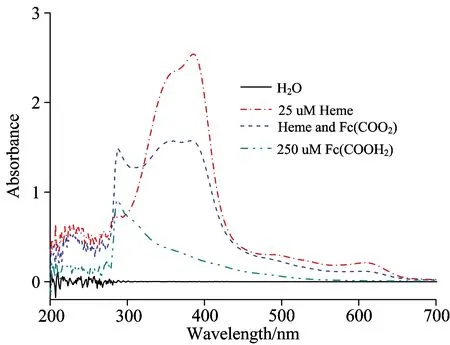
Fig.2 UV-Vis spectra of Fc(COOH)2, heme and their interaction
It can be seen that UV-Vis spectra of Fc(COOH)2, heme are different from their interaction as shown in Fig.2.From the review of maximum absorption wavelength, theλmaxof Fc(COOH)2and heme are 286 and 386 nm, respectively.After the reaction, it shows threeλmaxof 288, 355 and 384 nm, which are corresponding to characteristic peak of Fc(COOH)2and heme, respectively.From the review of absorbance, before the reaction, the absorbance of Fc(COOH)2and heme are 0.92 and 2.55, respectively.After the reaction, the absorbance of three peaks is about 1.53.So it exists interaction between Fc(COOH)2and heme.
2.1.2 Fix concentration of Fc(COOH)2
In order to figure out the mechanism interaction of Fc(COOH)2and heme, a titration experiment was carried out, that is to fix the concentration of Fc(COOH)2as 250 μmol·L-1, react with different concentration of heme (1~50 μmol·L-1), Fig.3 shows UV-Vis spectra of 250 μmol·L-1Fc(COOH)2and different concentration of heme reaction for 0.5 h.

Fig.3 UV-Vis spectra of 250 μmol·L-1Fc(COOH)2and different concentration of heme reaction for 0.5 h
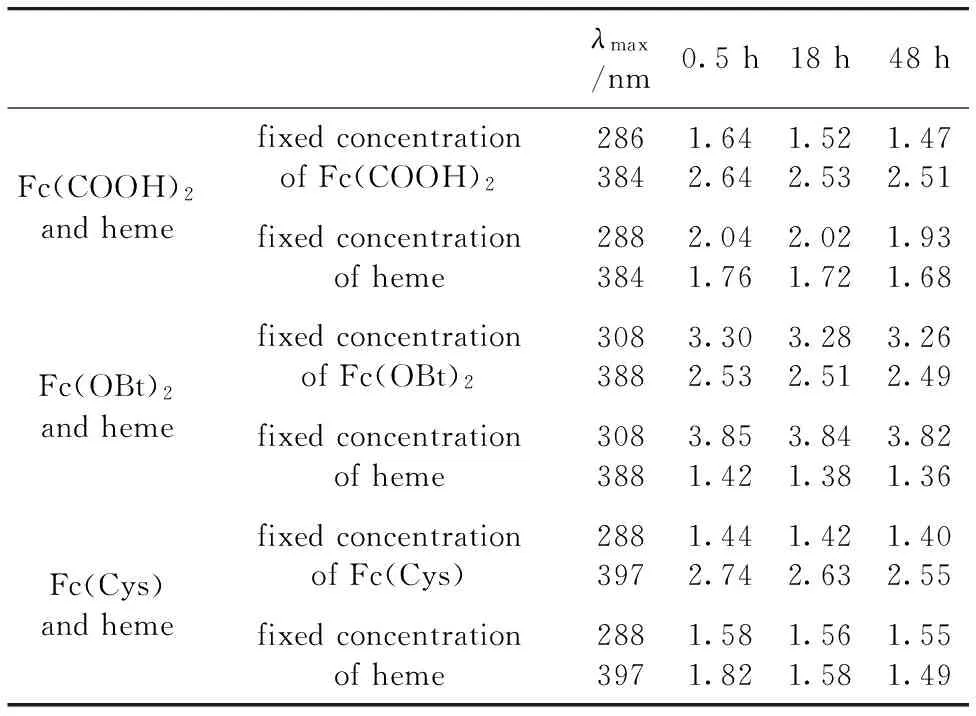
Table 1 Absorbance of ferrocence derivatives and heme reaction for different time
From Fig.3, it can be seen that when the concentration of Fc(COOH)2is fixed, not only the absorbance of heme increases with the increase of hemeconcentration, but also the absorbance of Fc(COOH)2increases with the increase of hemeconcentration.When the reaction time increases from 0.5 h to 48 h, the absorbance at maximum absorption wavelength (λmax=384 nm) has a little change, taken 50 μmol·L-1heme as example, the absorbance change from 2.64 to 2.53 and 2.51 as shown in Table 1, after 18 h and 48 h reaction, respectively.So it can conclude that the whole solution is unstable to show it exists interaction between Fc(COOH)2and heme.
2.1.3 Fix concentration of heme
Now the concentration of heme is fixed as 25 μmol·L-1, react with different concentration of Fc(COOH)2(10~450 μmol·L-1), Fig.4 shows UV-Vis spectra of 25 μmol·L-1heme and different concentration of Fc(COOH)2reaction for 0.5 h.
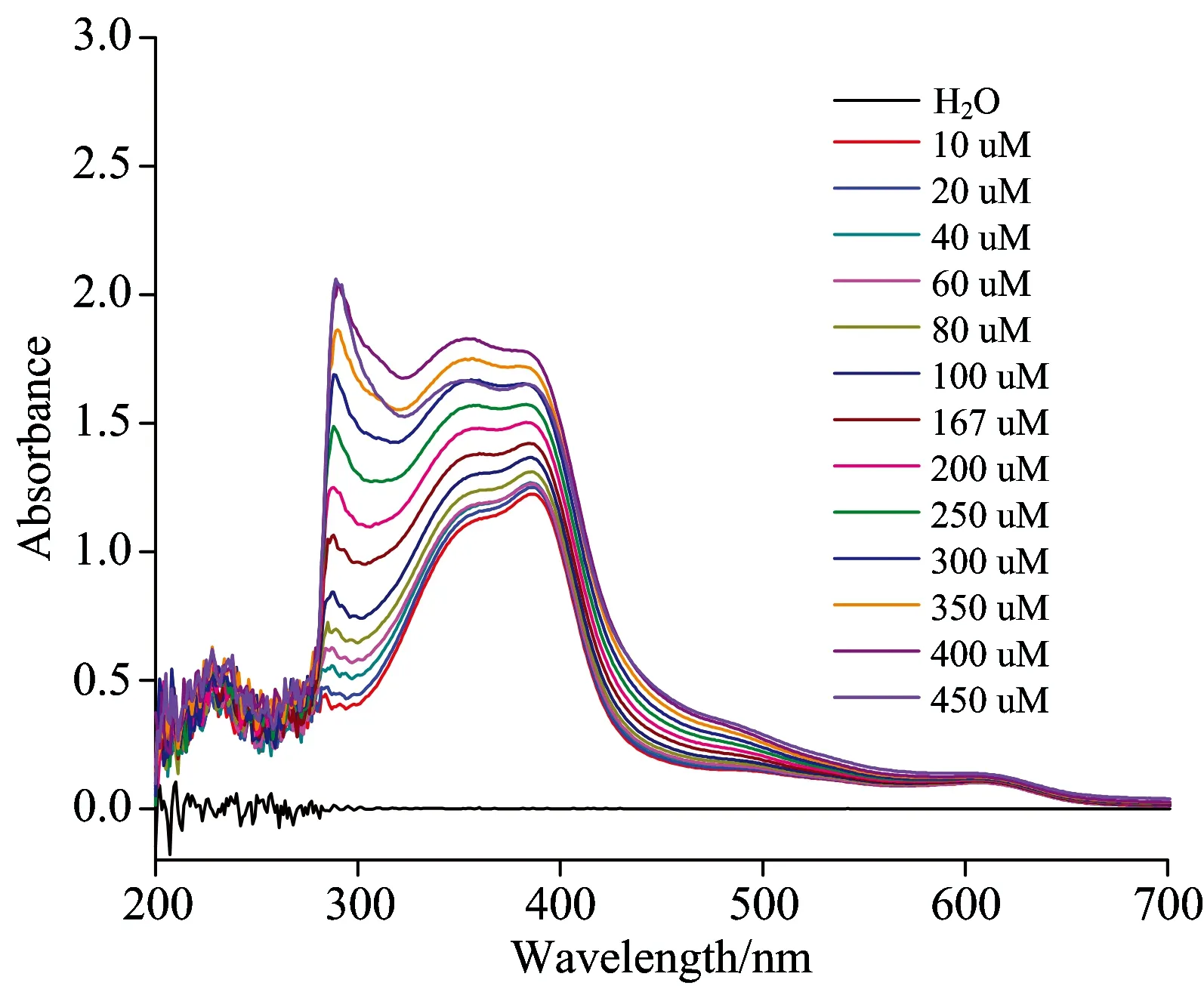
Fig.4 UV-Vis spectra of different concentration of Fc(COOH)2and 25 μmol·L-1heme reaction for 0.5 h
From Fig.4, it can be seen that when the concentration of heme is fixed, not only the absorbance of Fc(COOH)2increases with the increase of Fc(COOH)2concentration, but also the absorbance of heme increases with the increase of Fc(COOH)2concentration.When the reaction time increases from 0.5 h to 48 h, the absorbance at maximum absorption wavelength (λmax=384 nm) has a little change, taken 25 μmol·L-1heme as example, the absorbance change from 1.76 to 1.72 and 1.68 as shown in Table 1, after 18 h and 48 h reaction, respectively.So it can conclude that the whole solution is unstable to show it exists interaction between Fc(COOH)2and heme as shown as results of fixed Fc(COOH)2concentration.
2.1.4 Mechanism study
From Fig.2, after the reaction of Fc(COOH)2and heme, the UV-Vis spectra is different from UV-Vis spectra of Fc(COOH)2, and UV-Vis spectra of heme.Fc(COOH)2and heme show theirλmaxat 286 and 386 nm, respectively.After the reaction, it shows threeλmaxat 288, 355, 384 nm.From the review of absorbance, before the reaction, the absorbance of Fc(COOH)2and heme are 0.92 and 2.55, respectively.After the reaction, the absorbance of three peaks is about 1.53.From results of fixed concentration of Fc(COOH)2and heme, although one stuff concentration is fixed, its absorbance increases with increase of another stuff concentration, when consider that Fc(COOH)2and heme have free —COOH group, so the hydrogen bonding interactions happen between Fc(COOH)2and heme to form intermolecular hydrogen bond[17], lead to the growth of molecular chain, the bigger molecular can absorb more energy and increase the absorbance.Meanwhile, bigger molecules are more unstable, so the absorbance decreases with the increase of reaction time.
2.2 Interaction of Fc(OBt)2 and heme
2.2.1 UV-Vis spectra of Fc(OBt)2, heme and their interaction
Fig.5 shows the UV-Vis spectra of 250 μmol·L-1Fc(OBt)2, 25 μmol·L-1heme and their interaction.

Fig.5 UV-Vis spectra of Fc(OBt)2, heme and their interaction
It can be seen that UV-Vis spectra of Fc(OBt)2and heme are similar to their interaction except for absorbance as shown in Fig.5.For the difference of heme absorbance at 397 nm, it shows one half of heme absorbance duo to the concentration of heme is diluted double when it mixture with Fc(OBt)2solution.For the difference of heme absorbance at 304 nm, it shows about two-thirds of Fc(OBt)2absorbance when it mixture with heme solution.So there is no reaction happen between Fc(OBt)2and heme.
2.2.2 Fix concentration of Fc(OBt)2
In order to figure out whether there exists reaction between Fc(OBt)2and heme, we also carried out titration experiments, to fix the concentration of Fc(OBt)2as 250 μmol·L-1, react with different concentration of heme (1~50 μmol·L-1), Fig.6 shows UV-Vis spectra of 250 μmol·L-1Fc(OBt)2and different concentration of heme reaction for 0.5 h.
From Fig.6, it can be seen that when the concentration of Fc(OBt)2is fixed, the absorbance of heme increases with the increase of hemeconcentration, but the absorbance of Fc(OBt)2almost keep the same when concentration of heme increases.When the reaction time increases from 0.5 h to 48 h, the absorbance at maximum absorption wavelength do not change obviously (Table 1), so it can conclude that the whole solution is stable and there is no interaction between Fc(OBt)2and heme.
2.2.3 Fix concentration of heme
In order to verify there is no interaction between Fc(OBt)2and heme, now the concentration of heme is fixed as 25 μmol·L-1, react with different concentration of Fc(OBt)2(10~500 μmol·L-1), Fig.7 shows UV-Vis spectra of 25 μmol·L-1heme and different concentration of Fc(OBt)2reaction for 0.5 h.

Fig.6 UV-Vis spectra of 250 μmol·L-1Fc(OBt)2and different concentration of heme reaction for 0.5 h

Fig.7 UV-Vis spectra of different concentration of Fc(OBt)2and 25 μmol·L-1heme reaction for 0.5 h
From Fig.7, it can be seen that when the concentration of heme is fixed, only the absorbance of Fc(OBt)2increases with the increase of Fc(OBt)2concentration, the absorbance of heme increases almost keep the same.When the reaction time increases from 0.5 h to 48 h, the absorbance at maximum absorption wavelength do not change obviously (Table 1), so it can conclude that the whole solution is stable and there is no interaction between Fc(OBt)2and heme.
2.2.4 Mechanism study
From above discussion of interaction of Fc(OBt)2and heme, and combine the structure of Fc(OBt)2, this compound has no free —H, so it can’t form intermolecular hydrogen bond between two molecules and there is no interaction between Fc(OBt)2and heme.
2.3 Interaction of Fc(Cys) and heme
2.3.1 UV-Vis spectra of Fc(Cys), heme and their interaction
Fig.8 shows the UV-Vis spectra of 100 μmol·L-1Fc(Cys), 25 μmol·L-1heme and their interaction.

Fig.8 UV-Vis spectra of Fc(Cys), heme and their interaction
It can be seen that UV-Vis spectra of Fc(Cys), heme are different from their interaction as shown in Fig.16.From the review of maximum absorption wavelength, theλmaxof Fc(Cys) and heme are 289 and 386 nm, respectively.After the reaction, it shows threeλmaxat 288, 350 and 397 nm, which are corresponding to characteristic peak of Fc(Cys) and heme.From the review of absorbance, before the reaction, the absorbance of Fc(Cys) and heme are 0.71 and 2.55, respectively.After the reaction, the absorbance of two peaks is about 1.02 and 1.46, respectively.So, there exist interaction between Fc(Cys) and heme.
2.3.2 Fix concentration of Fc(Cys)
In order to figure out the mechanism interaction of Fc(Cys) and heme, a titration experiment was carried out.First, fix the concentration of Fc(Cys) as 100 μmol·L-1, react with different concentration of heme (1~50 μmol·L-1), Fig.9 shows UV-Vis spectra of 100 μmol·L-1Fc(Cys) and different concentration of heme reaction for 0.5 h.
From Fig.9, it can be seen that when the concentration of Fc(Cys) is fixed, not only the absorbance of heme increases with the increase of heme concentration, but also the absorbance of Fc(Cys) increases with the increase of heme concentration.When the reaction time increases from 0.5 h to 48 h, the absorbance at maximum absorption wavelength (λmax=397 nm) change obviously, taken 50 μmol·L-1heme as example, the absorbance change from 2.74 to 2.63 and 2.55 as shown in Table 1, after 18 h and 48 h reaction, respectively.So it can conclude that the whole solution is unstable to show there exist interaction between Fc(Cys) and heme.
2.3.3 Fix concentration of heme
Now the concentration of heme is fixed as 25 μmol·L-1, react with different concentration of Fc(Cys) (1~200 μmol·L-1), Fig.10 shows UV-Vis spectra of 25 μmol·L-1heme and different concentration of Fc(Cys) reaction for 0.5 h.

Fig.9 UV-Vis spectra of 100 μmol·L-1Fc(Cys) and different concentration of heme reaction for 0.5 h

Fig.10 UV-Vis spectra of different concentration of Fc(Cys) and 25 μmol·L-1heme reaction for 0.5 h
From Fig.10, it can be seen that when the concentration of heme is fixed, not only the absorbance of Fc(Cys) increases with the increase of Fc(Cys) concentration, but also the absorbance of heme increases with the increase of Fc(Cys) concentration.When the reaction time increases from 0.5 h to 48 h, the absorbance at maximum absorption wavelength (λmax=397 nm) also change obviously, taken 25 μmol·L-1heme as example, the absorbance change from 1.82 to 1.58 and 1.49 as shown in Table 1, after 18 h and 48 h reaction, respectively.So it can conclude that the whole solution is unstable to show there exists interaction between Fc(Cys) and heme as shown as results of fixed Fc(Cys) concentration.
2.3.4 Mechanism study
From Fig.8, after the reaction of Fc(Cys) and heme, the UV-Vis spectra is different from UV-Vis spectra of Fc(Cys), and UV-Vis spectra of heme.Fc(Cys) and heme show theirλmaxat 289 and 386 nm, respectively.After the reaction, it shows threeλmaxat 288, 350, 397 nm.From the review of absorbance, before the reaction, the absorbance of Fc(Cys) and heme are 0.71 and 2.55, respectively.After the reaction, the absorbance of three peaks is about 1.02 and 1.46, respectively.From results of fixed concentration of Fc(Cys) and heme, although one stuff concentration is fixed, its absorbance increases with increase of another stuffconcentration, when consider that Fc(Cys) has free —NH group and heme has —COOH group, so the hydrogen bonding interactions happen between Fc(Cys) and heme to form intermolecular hydrogen bond, lead to the growth of molecular chain, the bigger molecular can absorb more energy and increase the absorbance.Meanwhile, bigger molecules are more unstable, so the absorbance decreases with the increase of reaction time as results of interaction between Fc(COOH)2and heme.
3 Conclusions
In this work, the interaction between Fc(COOH)2, Fc(OBt)2, Fc(Cys) and heme were studied by UV-Vis spectroscopy, respectively.The results show that the interactions happen between Fc(COOH)2, Fc(Cys) and heme to form intermolecular hydrogen bond based on —COOH/—NH— and —COOH group, none of Fc(OBt)2.It can provide a new way to explore the quantitative detection of hemoglobin based on the reaction of ferrocene derivatives and heme.
[1] Bluher S, Markert J, Herget S, et al.Current Diabetes Reports, 2012, 12(2): 147.
[2] Khera P K, Joiner, C H, Carruthers A, et al.Diabetes, 2008, 57(9): 2445.
[3] Sun W, Jiang H, Jiao K.Journal of Chemical Sciences, 2005, 117(4): 317.
[4] Bodei L, Kidd M, Paganelli G, et al.European Journal of Nuclear Medicine and Molecular Imaging, 2015, 42: 5.
[5] Tunc S, Duman O, Soylu I, et al.Journal of Hazardous Materials, 2014, 273: 36.
[6] Matysiak E, Donten M, Kowalczyk A, et al.Biosensors and Bioelectronics, 2015, 64: 554.
[7] Zhan T R, Yang Q, Zhang Y M, et al.Journal of Colloid and Interface Science, 2014, 433: 49.
[8] Madrakian T, Bagheri H, Afichami A, et al.Journal of Luminescence, 2014, 155: 218.
[9] Liu B S, Yang C, Yan X N, et al.Spectroscopy Letters, 2012, 45(3): 175.
[10] Cao Juntao, Wang Hui, Chen Yonghong, et al.Spectroscopy and Spectral Analysis, 2014, 34(1): 241.
[11] Lan H Z, Gan N, Pan D D, et al.Journal of Chromatography A, 2014, 1365: 34.
[12] Lin L L, Berces A, Kraatz H B.Journal of Organometallic Chemistry, 1998, 556: 11.
[13] Shipman P O, Lafreniere M A, Colquhoun C D S, et al.Inorganica Chimica Acta, 2012, 391: 195.
[14] Chowdhury S, Schatte G, Kraatz H B.Angewandte Chemie International Edition, 2008, 47: 7056.
[15] Zhang D, Zhang Q, Sua J H, et al.Chemical Communication, 2009, 1700.
[16] Han G C, Ferranco A, Feng X Z, et al.European Journal of Inorganic Chemistry, 2014, 31: 5337.
[17] Beheshti S, Lataifeh A, Kraatz H B.Journal of Organometallic Chemistry, 2011, 696(5): 1117.
*通讯联系人
O657.3
A
紫外-可见光谱法研究二茂铁衍生物与血红素的相互作用
韩国成,冯小珍,梁晋涛,肖文香,陈真诚*
桂林电子科技大学生命与环境科学学院,广西 桂林 541004
采用紫外-可见光谱法(UV-Vis)研究三种二茂铁衍生物[Fc(COOH)2(λmax=286 nm), Fc(OBt)2(λmax=305 nm), Fc(Cys)(λmax=289 nm)]与血红素(heme,λmax=386 nm)的相互作用。实验结果表明:当固定heme浓度时,heme的吸光度随着Fc(COOH)2和Fc(Cys)浓度的增加而增大,而heme的吸光度随着Fc(OBt)2的浓度的增加几乎没有增大; 当分别固定Fc(COOH)2, Fc(Cys)和Fc(OBt)2的浓度时,Fc(COOH)2和Fc(Cys)的吸光度随着heme浓度的增加而增大,而Fc(OBt)2的吸光度随着heme浓度的增加没有变化,说明Fc(COOH)2和Fc(Cys)与heme存在分子间的相互作用,主要是由于Fc(COOH)2和 Fc(Cys)与heme能形成氢键,分子链增长,吸收的能量增加,导致吸光度增大; 而Fc(OBt)2与heme没有分子间的相互作用,是由于Fc(OBt)2没有自由的氢,不能与heme形成分子间的氢键。同时考察了三种二茂铁衍生物与heme 的吸光度随时间的变化,Fc(COOH)2和 Fc(Cys)与heme的吸光度随着时间的增加而减少,而Fc(OBt)2与heme的吸光度随时间的变化几乎没有变化。Fc(COOH)2与Fc(Cys)和heme的反应时间为0.5,18和48 h,当固定Fc(COOH)2浓度时,在λmax=384 nm处的吸光度由2.64分别变为2.53和2.51; 当固定heme的浓度时,在λmax=384 nm处的吸光度由1.76分别变为1.72和1.68; 当固定Fc(Cys)浓度时,在λmax=397 nm处的吸光度由2.74分别变为2.63和2.55; 当固定heme的浓度时,在λmax=397 nm处的吸光度由1.82分别变为1.58和1.49。
紫外-可见光谱法; 二茂铁衍生物; 血红素; 氢键
2015-01-15,
2015-04-21)
Foundation item:The National Natural Science Foundation of China (61301038), Natural Science Foundation of Guangxi Province (2015GXNSFBA139041), National Undergraduate Training Programs for Innovation and Entrepreneurship (201510595044)
10.3964/j.issn.1000-0593(2016)05-1585-07
Received:2015-01-15; accepted:2015-04-21
Biography:HAN Guo-cheng, (1981—), assoicate professor, School of Life and Environmental Sciences, Guilin University of Electronic Technology e-mail: hangc81@guet.edu.cn *Corresponding author e-mail: chenzhcheng@163.com
