微波法制备硫/膨胀石墨复合材料及其锂硫电池性能
2016-06-20朱福良赵金平
朱福良, 杨 志,, 赵金平, 赵 薪
(1.兰州理工大学 材料科学与工程学院,甘肃 兰州730050;2.中国科学院兰州化学物理研究所 清洁能源化学与材料实验室, 甘肃 兰州730050)
微波法制备硫/膨胀石墨复合材料及其锂硫电池性能
朱福良1,杨志1,2,赵金平2,赵薪1
(1.兰州理工大学 材料科学与工程学院,甘肃 兰州730050;2.中国科学院兰州化学物理研究所 清洁能源化学与材料实验室, 甘肃 兰州730050)
摘要:采用简单的微波辅助的方法成功制备了硫/膨胀石墨复合材料。膨胀石墨可以用作锂硫电池中阴极的微型容器及集流体。通过控制硫与膨胀石墨的配比成功控制了复合材料中硫颗粒的大小。当硫与膨胀石墨的比例为10∶1时,可以得到相对较均匀的硫颗粒。同时研究了不同条件下所制样品的锂硫电池性能。结果表明,硫的含量与硫颗粒的大小对锂硫电池的容量非常重要。当硫与膨胀石墨的比例为10∶1时,在0.1 C放电速率下,复合材料具有最高的放电容量1 020 mAh·g-1。
关键词:膨胀石墨; 锂硫电池; 微波; 硫颗粒尺寸
English edition available online ScienceDirect ( http:www.sciencedirect.comsciencejournal18725805 ).
1Introduction
Lithium-sulfur (Li-S) battery is a very attractive battery. Compared with the traditional lithium ion batteries, it has many merits[1]. For example, the battery has a high theoretical specific capacity of 1 672 mAh/g and a high theoretical specific energy of 2 600 Wh/kg based on the reaction between lithium and sulfur[2]. Moreover, sulfur is easily available and environmental friendly[3-4]. However, Li-S battery has obvious two drawbacks. On one hand, the low electronic/ionic conductivity of sulfur would limit the operating current of the battery[5]. On the other hand, sulfur as the active materials would easily dissolve into the liquid electrolyte during the discharge process and then transform into insoluble Li2S and Li2S2[6,7]. Li2S and Li2S2will finally adhere onto the surface of cathode materials, which will occur in every charge/discharge cycle, restrict the reaction of the inner sulfur[1], and lead to the loss of the capacity.
To overcome these problems, several technological contributions are attempted, such as the optimization of the organic electrolyte via introducing room temperature ionic liquid or fabrication of the sulfur composites with the electronic conductors[8]. The electronic conductors include nano metal oxides[9], solid solutions[10], transition metals[11], carbon materials[12]and conductive polymers[13]. Since the carbon materials have high specific surface area, high pore volume, extensive pore structure and good conductivity, they are proved to be effective and facile candidates to improve the sulfur utilization and restrain the solubility of lithium polysulfides[8]. Lots of carbon materials are used to modify the sulfur cathode, such as mesoporous carbon[9], carbon nanotubes[13], carbon nanofibers[14], and graphene[15], all of which have shown improved performance. Among these, graphene sheets, one-atom-thick layers of sp2-hybridized carbon atoms packed in a honeycomb lattice, are advantageous for wrapping sulfur owing to their large lateral size, good conductivity and the flexible structure[16]. And some graphene-based material is more suitable for improving the performance. But the preparation for some carbon-sulfur materials includes complicated multi-steps with strict synthetic conditions[17]. Li et al[18]have prepared a graphene-wrapped carbon/sulfur composite for lithium-sulfur battery by changing the hydrophilicity of graphene oxide during reduction, in which the hydrophobic graphene closely wraps around the hydrophobic carbon surface. And the first discharge capacity is about 1 578.3 mAh·g-1(0.15 A·g-1), higher than the pure carbon/sulfur composite. Li[19]have prepared a graphene/sulfur composite by chemical deposition sulfur on the graphene oxide. And the first discharge capacity is 1 320 mAh·g-1(0.02 C). Tang[20]have prepared a nitrogen-doped carbon nanotube/graphene-sulfur composite, by the chemical vapor deposition for the nitrogen-doped carbon nanotube/graphene followed by a melt-diffusion infiltration strategy. The initial discharge capacity is 1 152 mAh·g-1and nearly 76% capacity is retained after 80 cycles. Above all the composites show excellent performance, but the materials preparation involves complicated multi-steps with strict synthetic conditions.
Expanded graphite which has a hierarchical pore structure is low in cost and easily to obtain, so it can be used as the cathode material for Li-S batteries. Expanded graphite is prepared by expanding intercalated graphite, which is an exothermic process. So it is beneficial for the preparation sulfur/expanded graphite (S/EG) composites. In this paper, a facile method is successfully used to prepare the S/EG composites assisted with microwave. The sulfur and expanded graphite is mixed and the S/EG composites is easily obtained during the graphite expanding process by microwave irradiation. The S/EG composite as a Li-S battery cathode has a high discharge capacity. This method provides an experimental basis for the subsequent preparation of sulfur/carbon composites.
2Experimental
2.1Preparation of S/EG composite
Firstly, the EG was prepared by expanding the graphite intercalated compound through rapid thermal expansion at 800C in a muffle furnace. In brief, the natural flake graphite was mixed with concentrated sulfuric acid (98%) and concentrated nitric acid (65%) (4∶1, v/v), then the mixture was vigorously stirred for 12 h to ensure the formation of expandable graphite intercalation compound. After that the intercalated graphite was washed with deionized water to neutral and then dried. The dried expandable graphite intercalation compound was rapidly expanded at 800C for 20 s in a muffle furnace. Then the expanded graphite and sulfur was mixed together with different mass ratios (S∶EG=4∶1,6∶1,8∶1,10∶1,12∶1, m/m), and the mixture was put into a microwave oven and irradiated for 2 min at about 400 W. In this process, expanded graphite was used as the heat producer and the heat transfer medium. And the heat can be transferred to the sulfur surface, leading to the sublimation of sulfur that was loaded into the pore of the expanded graphite when the sulfur was cooled.
2.2Characterization
The morphology and microstructure of the S/EG composite was characterized by scanning electron microscopy (FESEM, JSM 6701F). The microstructure of the S/EG material was also analyzed by powder X-ray diffraction (XRD, CuKαradiation, Panalytical X’ Pert Pro). The thermal gravimetry (STA 449F3) was used to analyze the quantity of loaded sulfur from 25 to 500 ℃ at a heating rate of 10 ℃/min under the protection of nitrogen.
2.3Electrochemical measurements
The S/EG composite and acetylene black (electrical conductor) with polyvinylidene fiuoride binder (8∶1∶1, weight ratio) were grinded with a mortar into N-methyl-2-pyrrolidone (NMP) to form a slurry. In this process, the NMP and polyvinylidene fiuoride are used as a diluent and binder, respectively. The S/EG composite could be ground to form a uniform slurry. Then the slurry was coated onto aluminum foil and dried under vacuum to form the working electrode at 60 ℃ for 12 h. 2032-type coin cells were assembled in an argon-filled glove box using lithium foil as the counter electrode. The electrolyte was 1 M bis(trifiuoromethane) sulfonimide lithium salt (LiTFSI) dissolved in a mixture of 1,3-Dioxolane (DOL) and Dimethoxyethane (DME) (1∶1 v/v) containing LiNO3(1 wt%). The cathode, separator, and anode were pressed by a sealing machine to ensure a tight contact. The batteries were cycled between 1.5 and 3.0 V with a battery test instrument Land CT2001A battery test system at 0.1 C (1 C=1 672 mA·g-1) at room temperature. CV tests were performed on a CHI660D electrochemical workstation at a scan rate of 0.2 mV·s-1from 1.5 to 3.0 V. The calculation of specific discharge capacities was based on the mass of elemental sulfur.
3Results and discussion
The microstructure of the S/EG composites after microwave at different mass ratios of sulfur to EG is characterized by SEM. As illustrated in Fig. 1, the SEM images indicate that all the S/EG composites possess an ideal layer-by-layer structure with a hierarchical pore structure. There are some particles embedded into the layers or pores of expanded graphite. When the ratios of sulfur to expanded graphite is 4∶1, 6∶1, 8∶1 and 10∶1, no large sulfur particles are found and the particles are about tens to hundreds of nanometers as shown in the red rectangle. Lots of large sulfur particles about 1 μm are found when the ratio is 12∶1.
The temperature of EG is increased by absorbing microwave energy when it is irradiated by microwave. The sulfur is sublimed when it is heated by EG. When the mixture is cooled down the sulfur vapor enter into the pores of the particles and deposited on EG. The higher is the content of sulfur, the more are the sulfur particles formed, but when the sulfur content increases to a limit, the sulfur can not sublime completely, so the particle size increases.So the size of sulfur particles is controlled by controlling the amount of sulfur.
To further obtain the particle size distribution, we randomly select one hundred sulfur particles in the red rectangle in Fig. 1. The result is shown in Fig. 2. It is found that the percentage of sulfur particles between 20 and 50 nm reaches a maximum of 79% at the S/EG mass ratio of 10∶1. While the particles between 50-100 nm and 100-150 nm are found in all the samples, but their percentages in the sample at 10∶1 is the lowest.

Fig. 1 SEM images of the S/EG composites with different mass ratios of

Fig. 2 The particle size distribution of
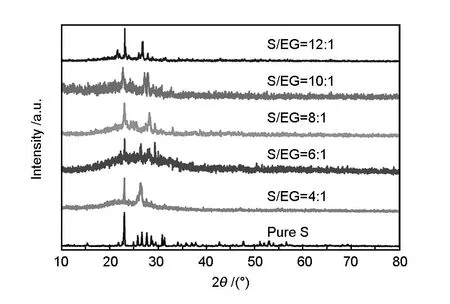
Fig. 3 XRD patterns of the S/EG composites
To investigate the state of the sulfur in the S/EG composite after microwave irradiation XRD patterns are recorded. Firstly, the pure sulfur displays sharp diffraction peaks that agree well with the characteristic pattern of S8[21]. Compared with the pure sulfur sample, all of the S/EG composites at different ratios of S/EG after microwave irradiation show the diffraction peaks of bulk crystal sulfur in the form of S8. This is also consistent with the SEM images.
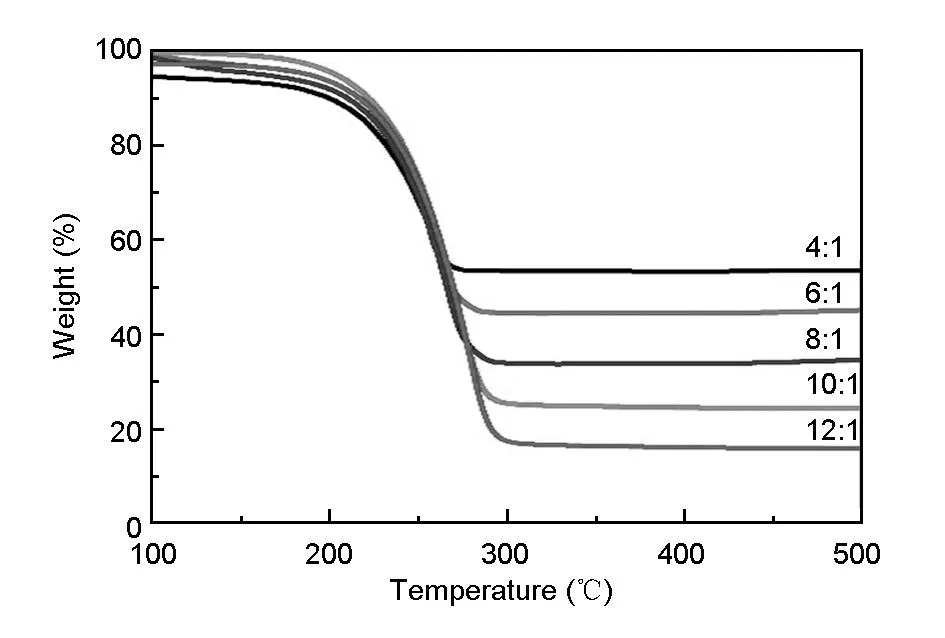
Fig. 4 Thermo gravimetric analysis (TGA) curves of the S/EG
The thermal decomposition characteristic of the S/EG composites at different ratios of S/EG after microwave irradiation under nitrogen atmosphere is investigated by TGA. The expanded graphite is prepared at 800C, so here, the weight loss of the composites is caused by sulfur. The TGA curves in Fig. 4 show that the weight loss of the S/EG composites after microwave irradiation is about 50, 55, 64, 71 and 76% at the S/EG ratios of 4∶1, 6∶1, 8∶1, 10∶1 and 12∶1, respectively. This result indicates that in the composites, the quantity of sulfur increase with the S/EG ratio. For testing the electrochemical properties of the S/EG composites, the batteries assembled were investigated by galvanostatic charge-discharge measurement. Fig. 5a shows the cyclic performance of the S/EG cathodes with different S/EG ratios. The initial discharge capacity increases from 347 to 950 mAh·g-1with the S/EG ratios from 4∶1 to 10∶1. With a further increase of the S/EG ratio to 12∶1, the capacity is decreased to 556 mAh·g-1.
From the results of Fig. 2 and Fig. 3, it is found that both the sulfur content and the sulfur particle sizes are the main factors for capacity. The S/EG composite with a S/EG ratio of 10∶1 is the best, so we choose this ratio for the next discussion. To identify all the electrochemical reactions in the S/EG composite, the CV and galvanostatic discharge-charge curves are shown in Fig. 5b and 5c, respectively. As shown in Fig. 5b, one oxidation peak and two reduction peaks are observed, which are attributed to the multistep reaction of sulfur with lithium[3]. The transformation of sulfur to lithium polysulfide (Li2Sn, 2
Yang[23]prepared composites using a conductive polymer as coating to improve the performance of lithium-sulfur batteries, and the results show that the performance of the composites with the conductive polymer coating is better than the composites without the coating. Based on this observation, we are going to coat conductive coating on the material to restrain the solubility of lithium polysulfides to improve the performance.
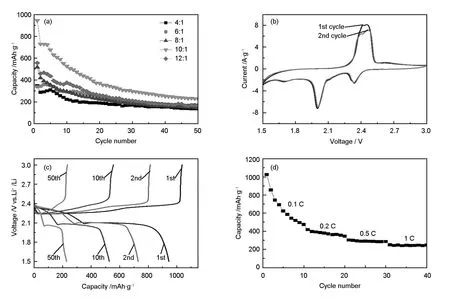
Fig. 5 (a) The cyclic performance of the S/EG composites at different S/EG ratios; (b) Cyclic voltammogram of the S/EG cathode
4Conclusions
We successfully prepared the S/EG composites by microwave irradiation method. The size of sulfur particles loaded in the composites can be easily controlled by the S/EG mass ratio. When the ratio is 10∶1, relatively uniform sulfur particles are obtained. Both the S/EG ratio and the sulfur particle size play an important role for capacity in Li-S battery. It has been confirmed that the cathode with the ratio of 10∶1 shows the highest capacity (1 020 mAh·g-1) at 0.1 C rate. Such facile method is a promising means for preparing the cathode of Li-S batteries.
References
[1]R Elazari G, Salitra A, Garsuch A, et al. Sulfur-impregnated activated carbon fiber cloth as a binder-free cathode for rechargeable Li-S batteries[J]. Advanced Materials, 2011, 23: 5641-5644.
[2]Li S, Xie M, Liu J B, et al. Layer structured sulfur/expanded graphite composite as cathode for lithium battery[J]. Electrochemical and Solid-State Letters, 2011, 14: 105-107.
[3]Yamin H, Gorenshtein A, Penciner J, et al. Lithium sulfur battery oxidation/reduction mechanisms of polysulfides in THF solutions[J]. Journal of The Electrochemical Society, 1988, 135: 1045-1048.
[4]Wang J L, Yang J, Xie J Y, et al. Sulfur-carbon nano-composite as cathode for rechargeable lithium battery based on gel electrolyte[J]. Electrochemistry Communications, 2002, 4: 499-502.
[5]Liang C, Dudney N J, Howe J Y. Hierarchically structured sulfur/carbon nanocomposite material for high-energy lithium battery[J]. Chemistry of Materials, 2009, 21: 4724-4730.
[6]Lai C, Gao X P, Zhang B, et al. Synthesis and electrochemical performance of sulfur/highly porous carbon composites[J]. Journal of Physical Chemistry C, 113 (2009): 4712-4716.
[7]Wang Y, Huang Y, Wang W, et al. Structural change of the porous sulfur cathode using gelatin as a binder during discharge and charge[J]. Electrochimica Acta, 2009, 54: 4062-4066.
[8]Zhang B, Qin X, Lia G R, et al. Enhancement of long stability of sulfur cathode by encapsulating sulfur into micropores of carbon spheres[J]. Energy & Environmental Science, 2010, 3: 1531- 1537.
[9]Choi Y J, Jung B S, Lee D J, et al. Electrochemical properties of sulfur electrode containing nano Al2O3for lithium/sulfur cell[J]. Physica Scripta, 2007, T129: 62-65.
[10]Songa M S, Hana S C, Kima H S, et al. Effects of nanosized adsorbing material on electrochemical properties of sulfur cathodes for Li/S secondary batteries[J]. Journal of The Electrochemical Society, 2004, 151: A791-A795.
[11]Wu F, Wu S X, Chen R J, et al. Electrochemical performance of sulfur composite cathode materials for rechargeable lithium batteries[J]. Chinese Chemical Letters, 2009, 20: 1255-1258.
[12]Wang J L, Yang Y, Xie J Y. A novel conductive Polymer-Sulfur composite cathode material for rechargeable Lithium batteries[J]. Advanced Materials, 2002, 14: 963-965.
[13]Ryu H S, Guo Z, Ahn H J, et al. Investigation of discharge reaction mechanism of lithium-liquid electrolyte-sulfur battery[J]. Journal of Power Sources, 2009, 189: 1179-1183.
[14]Ji X L, Lee K T, Linda F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries[J]. Nature Materials, 2009, 8: 500-506.
[15]Wang H L, Yang Y, Liang Y Y, et al. Graphene-wrapped sulfur particles as a rechargeable Lithium-Sulfur battery cathode material with high capacity and cycling stability[J]. Nano Letters, 2011, 11: 2644-2647.
[16]Wang D W, Zeng Q C, Zhou G M, et al. Carbon-sulfur composites for Li-S batteries: status and prospects[J]. Journal of Materials Chemistry A, 2013, 1: 9382-9394.
[17]Wang D W, Li F, Liu M, et al. 3D aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage[J]. Angewandte Chemie, 2008, 120: 379-382.
[18]李芳菲, 吕伟, 牛树章, 等. 石墨烯包覆炭硫复合物正极材料的制备及其电化学性能[J]. 新型炭材料, 2014, 29: 309-315.
(LI Fang-fei, LU Wei, NIU Shu-zhang, et al. Preparation and electrochemical performance of a graphene-wrapped carbon/sulphur composite cathode[J]. New Carbon Materials, 2014, 29: 309-315.)
[19]Li W J, Rao M M, Zhang Y G, et al, Graphene oxide as a sulfur immobiliaer in high performance lithium/sulfur cells[J]. Journal of the American Chemical Society, 2011, 133: 18522-18525.
[20]Tang C, Zhang Q, Zhao M Q, et al. Nitrogen-doped aligned carbon nanotube/graphene sandwiches: Facile catalytic growth on bifunctional natural catalysts and their applications as scaffolds for high-rate Lithium-Sulfur batteries[J]. Advanced Materials, 2014, 26: 6100-6105.
[21]Zheng W, Wong S C. Electrical conductivity and dielectric properties of PMMA/expanded graphite composites[J]. Composites Science and Technology, 2003, 63: 225-235.
[22]Yamin H, Penciner J, Gorenshtain A, et al. The electrochemical behavior of polysulfides in tetrahydrofuran[J]. Journal of Power Sources, 1985, 14: 129-134.
[23]Yang Y, Yu G H, Wu H, et al. Improving the performance of lithium-sulfur batteries by conductive polymer coating[J]. ACS Nano, 2011, 5: 9187-9193.
作者介绍:杨志,硕士研究生. E-mail: yzqms_127@163.com
Receiveddate: 2016-01-31;Reviseddate: 2016-04-02
Foundationitem: National Natural Science Foundation of China (21303234,51364024); China Postdoctoral Science Foundation(2013M530437).
Authorintroduction: YANG Zhi, Master Student. E-mail: yzqms_127@163.com
Microwave assisted preparation of expanded graphite/sulfur composites as cathodes for Li-S batteries
ZHU Fu-liang1,YANG Zhi1,2,ZHAO Jin-ping2,ZHAO Xin1
(1.SchoolofMaterialsScienceandEngineering,LanzhouUniversityofTechnology,Lanzhou730050,China;2.LaboratoryofCleanEnergyChemistryandMaterials,LanzhouInstituteofChemicalPhysics,ChineseAcademyofScience,Lanzhou730000,China)
Abstract:Expanded graphite/sulfur(EG/S) composites were prepared by microwave irradiation of mixtures of sulfur and expanded graphite with S/EG ratios from 4∶1 to 12∶1. Sulfur was sublimed and entered the EG pores when the mixtures were heated and was condensed to form particles within the pores during cooling. The pores of the EG acted as microcontainers to host the sulfur, and the material, with its interconnected and conductive pore walls, acted as a current collector for the cathode of the Li-S battery. The size of sulfur particles in the EG pores could be controlled by the S/EG mass ratio. When the ratio is 10∶1, relatively uniform size sulfur particles could be obtained. Both the S/EG ratio and the sulfur particle size have an important effect on the capacity increase of the Li-S battery. Using a composite with a S/EG ratio of 10∶1 as the cathode gives the highest capacity of 1 020 mAhg-1at a rate of 0.1 C.
Key words:Expanded graphite; Lithium-sulfur batteries; Microwave; Sulfur particles size
文章编号:1007-8827(2016)02-0199-06
中图分类号:TB333
文献标识码:A
基金项目:国家自然科学基金青年基金(21303234,51364024);中国博士后科学基金(2013M530437).
通讯作者:朱福良,教授. E-mail: chzfl@126.com
Corresponding author:ZHU Fu-liang, Professor. E-mail: chzfl@126.com
DOI:10.1016/S1872-5805(16)60011-2
