A3-3基体石墨的氧化行为
2016-06-20周湘文卢振明李馨楠唐亚平
周湘文, 卢振明, 李馨楠, 张 杰, 刘 兵, 唐亚平
(清华大学 核能与新能源技术研究院,先进核能技术协同创新中心,先进反应堆工程与安全教育部重点实验室,北京100084)
A3-3基体石墨的氧化行为
周湘文,卢振明,李馨楠,张杰,刘兵,唐亚平
(清华大学 核能与新能源技术研究院,先进核能技术协同创新中心,先进反应堆工程与安全教育部重点实验室,北京100084)
摘要:采用自行搭建的热重实验平台对798~973 K温度范围内温度对A3-3基体石墨的氧化行为进行研究,氧化剂为100 mL/min的空气。不同温度下石墨试样均被氧化至失重10%~15%。结果表明,基体石墨的氧化速率(OR)随着温度的升高显著提升,温度为973 K时基体石墨的OR约为798 K时的70倍。虽然973 K时氧气供给速率与平均碳消耗速率的比值仅为4.3,但石墨OR的Arrhenius曲线依然保持了很好的线性关系,表明该温度下基体石墨的氧化机理没有发生改变。在798-973 K温度范围内,A3-3基体石墨在空气中的氧化均处于化学区,其活化能为176 kJ/mol,Arrhenius氧化方程可描述为:OR=2.967 3×108·exp(-21 124.8/T),单位为wt%/min。与堆内的核级结构石墨相比,基体石墨的活化能相对较低,说明基体石墨在空气中更易被氧化,这主要跟基体石墨中含有未完全石墨化的树脂炭有关。
关键词:氧化行为; 基体石墨; 化学控制区; 活化能
English edition available online ScienceDirect ( http:www.sciencedirect.comsciencejournal18725805 ).
1Introduction
High temperature gas-cooled reactors (HTGR), which have passive safety features and usefulness for hydrogen production, are expected to become the nuclear reactors of the next generation. Graphite are widely used in fuel matrix and the structural materials such as the moderator and reflectors in the core of HTGR[1]. Based on the difference of fuel elements, the HTGR can be divided into two different concepts: the block type reactor and the pebble bed reactor. Whereas the core of a block reactor mainly consists of highly graphitized nuclear graphite, and contains only small amounts of fuel element matrix graphite (MG), the active pebble bed core consists of the largest part of fuel element MG[2]. For example, MG in the demonstration plant of HTR-PM under construction in China is composed of about 90% of fully graphitized filler, plus 10% of incompletely graphitized (coked) binder[3]. A graphitization process of the spherical fuel elements in the high temperature range of 2 700-3 000 ℃ cannot be performed due to the following reasons[4]: 1) uranium diffusion out of the fuel particle kernels would become significant at temperature of >2 100 ℃; 2) at temperature of >2 100 ℃, the structure of pyrocarbon layers should change while the anisotropy of the crystallographic orientation of the pyrocarbon should rise sharply.
Graphite oxidation is regarded as one of the most critical safety issues in HTGR for degrading the integrity of graphite components and the retention of fission products inside coated particles and fuel elements during normal and, particularly, under off-normal conditions. In the event of an air-ingress incident, the graphite in the moderator and reflector and MG of the fuel elements are exposed to oxygen at high temperature. Because of its ungraphitized binder content, the fuel pebbles are suspected to behave unfavorably in case of the air-ingress incident compared to the structural nuclear graphite in pebble bed reactor[2]. Qiu et al.[5]studied the oxidation behavior of the fuel element matrix material for HTR-10. The oxidation kinetics of three main components of the MG such as natural flake graphite, artificial graphite and char carbon derived from the phenol resin were studied. Results showed that the artificial graphite had the lowest oxidation rate, followed by the natural graphite, and char carbon had the highest oxidation rate. Although there have been much research work related on the oxidation properties of structural nuclear graphite such as IG-110[6-9]and NBG-18[7-10], only a few researches have been performed on the oxidation behavior of the MG[2, 5].
Graphite oxidation in air is controlled by chemical kinetics at low temperature (Regime I), but becomes diffusion-limited at high temperature (regime II)[11]. At even high temperature oxidation is strictly limited to the surface layers (Regime III) and is controlled by the mass transfer of gas species (O2) through the boundary layer at the solid/gas interface. In regime III, oxidation rates are not material-dependent and kinetic measurements are not deemed necessary[12]. The increase in porosity throughout the volume of graphite during oxidation in regime I (and to a lesser extent in regime II) has a high impact on the mechanical and thermal properties of graphite[12,13]. Therefore an in-depth examination of the oxidation kinetics of graphite in regime I is essential. However, the kinetic parameters including particularly the temperature limits for the oxidation of MG in air in regime I, are still not well understood. In order to examine the question, the A3-3 MG, which has been successfully manufactured[3], is selected as the experimental material and its oxidation behaviors in air are studied in this paper.
2Experimental
2.1Material and specimen
The matrix mixture was designated as the “A3-3” MG which contained ~90 wt% graphite and only ~10 wt% of the less stable resin char after carbonization and high temperature purification[3, 12]. The typical properties of the A3-3 MG are listed elsewhere[3]. As shown in Fig. 1, a circular column was drilled from the A3-3 MG pebble and machined symmetrically into two cylindrical specimens with 10 mm in diameter and 25 mm in height. Oxidation tests were performed twice at each temperature by using the two specimens from the same pebble to improve the reliability of test data. All the pebbles were from the same production batch. As the grain sizes of natural flake graphite and artificial graphite in the MG are smaller than 100 μm[3], the size of the cylindrical specimen (φ10×25 mm2) is large enough to be representative of the microstructure of the MG.

Fig. 1 Schematic illustrution of specimens machined
2.2Oxidation experiments
The oxidation experiments were performed in air by using a home-made experiment setup which was consisted of a three-zone vertical tube furnace (500 mm long) and an analytical balance with a weigh-below port feature. A cylindrical tube made of quartz glass (outer diameter of 60 mm, inner diameter of 42 mm, 800 mm long) placed inside the furnace extends on the upper end. The balance (resolution 0.1 mg) is placed on top of the vertical furnace, and is thermally shielded by circulating water so that the balance operates at constant (room) temperature. The MG specimens are placed in a wire basket of oxidation-resisting steel hanging on a thin quartz chain from the weigh-below port of the balance. Care was taken that the MG specimens were placed in the center of the constant temperature zone of the furnace (about 100 mm long). The specimen temperature is measured by a K-type thermocouple placed inside the furnace, not touching it, but no more than 5mm from the MG specimen. Inert gas (oxygen-free nitrogen) or air is introduced from the bottom. The nitrogen and air are analytical pure with a purity of 99.999% in volume. The test specimens are preheated and weight stabilized (no less than 30 min) in the target temperature in a flow of nitrogen, after which the flow through the vertical quartz tube is switched to air with a flow rate of 100 mL/min. The weight variations are recorded while maintaining the specimens in the air flow at the constant oxidation temperature.
2.3Oxidation temperatures
For the oxidation of graphite in air, the temperature limits for each regime were given by Hinssen et al.[14]. At temperatures below 773 K,the oxidation belongs to the regime I (chemical regime). In the temperature range of 773-1 173 K,the oxidation belongs to the regime II (in-pore diffusion controlled regime). When the temperatures are higher than 1 173 K,the oxidation belongs to the regime III (boundary layer regime). Meanwhile, Blanchard et al.[15]gave another classification in which the range of in-pore diffusion controlled regime was 873-1 173 K. Taking the difference of components between the MG and polycrystalline graphite into consideration, temperatures such as 798, 823, 873, 923 and 973 K were chosen to study the oxidation behavior of MG.
3Results and discussion
3.1Density profile of the A3-3 MG pebbles
In the common isostatic molding process, there are density gradients which should change gradually from the edge to the center in the forming product due to the loss of the pressure. In order to avoid the density gradients in the A3-3 MG, the pre-molding of fuel zone at a low pressure of 5 MPa was applied before the final molding of the fuel element at a pressure of ~300 MPa in the cold quasi-isostatic molding of pebble fuel elements. The same process was used to prepare the A3-3 MG pebbles. Furthermore, the density of the A3-3 MG which is directly related to its porosity has a significant influence on its oxidation behavior. However, the density profiles of the prepared A3-3 MG pebbles have never been reported. In order to verify the density distribution throughout the pebbles, the A3-3 MG pebble with a diameter of 60 mm was used in this study. First of all, the average density of the pebble was calculated by its weight and volume. Then, the density profile of the pebble was measured by machining a thin layer (1 mm) from the outer surface of the pebble, and accurately weighing the residual core after each machine step. The density of the layer removed was calculated by comparing with the previous measurements. This operation was repeated until the diameter of the residual core pebble reached 32 mm which was too small to be machined. The average density of the residual pebble was also acquired by dividing its weight by its volume. Fig. 2 shows the density profile of the A3-3 MG pebble. The average densities of the original pebble and residual core pebble are 1.721 and 1.723 g/cm3, respectively. The density distributions throughout the pebble layer of the diameter between 32 mm to 60 mm almost totally lie in the range of 1.70~1.77 g/cm3. The density profile shows a good uniformity throughout the pebble, which is necessary to guarantee the representativeness of the oxidation property of the cylindrical samples for the whole A3-3 MG pebbles.

Fig. 2 Density profile of the A3-3 MG pebble.
3.2Oxidation behavior of the A3-3 MG
Fig. 3 shows the mass losses as a function of oxidation time for the A3-3 MG in flowing air in the temperature range from 798 to 973 K. All the samples (except for the samples oxidized to a mass loss of ~11 wt% at 823 K) were oxidized to a weight loss of ~15 wt%. It can be seen that the oxidation temperatures have remarkable influences on the oxidation time. The oxidation time, when a weight loss of ~15 wt% is reached, decreases evidently with the increasing temperature. When the oxidation temperature is 798 K, it takes around two weeks (more than 18 000 minutes) to reach the weight loss of 15 wt%. Meanwhile, when the oxidation temperature increases to 973 K, the time is no more than 200 minutes to approach the same weight loss. The oxidation time at 798 K is more than 70 times as much as that at 973 K.
The variations of oxidation rate with oxidation burn-off are shown in Fig. 4. The oxidation rate can be obtained by taking the derivative of the mass changes with respect to oxidation time in Fig. 3. For all the temperatures in the chemical regime, the temperature plays a critical role in determining the oxidation rate of the A3-3 MG, which increases significantly with temperatures. At low temperatures of 798 and 823 K, the oxidation rate is very low and reaches its maximum at low burn-off, and then decreases slightly. At relatively high temperatures, the oxidation rate increases evidently with the increasing burn-off and approaches the maximum, and then remains stable. The higher are the oxidation temperatures, the more burn-off is when the oxidation rates reach their maxima and remain almost constant.
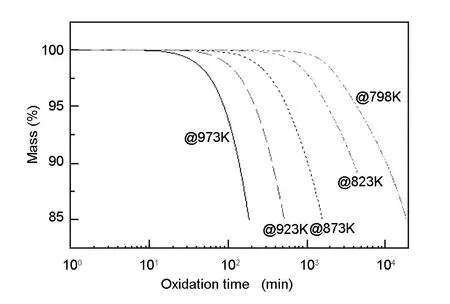
Fig. 3 Mass loss with time for the A3-3 MG at 798-973 K.
The differences in oxidation rates related to the burn-off are related to the changes of the total reaction surface area. As is known, A3-3 MG is a porous media. The reaction between carbon atoms and oxidizing gas occurs at the wall of the pores. At low temperatures such as 798 and 823 K, the oxidation rate is very low and there is no essential change in graphite microstructure. Therefore, the total reaction surface and the concentration of oxidizing gas are almost constant throughout the process of oxidation. As the temperature increases, the oxidation rates increase remarkably at relatively high temperatures, changing the microstructure of the MG. The closed pores are opened to increase the reaction surface. The fresh additional surface leads to an increase in the oxidation rate. On the other hand, as the oxidation proceeds, some of the micro-pores are interconnected into meso-pores and macro-pores, which should decrease the reaction surface. The reaction surface will reach a maximum and remain stable with increasing burn-off to 15 wt% where there is a balance between the two aspects.
3.3Arrhenius plot and activation energy of the A3-3 MG[16]
The kinetic parameters of the oxidation reaction, namely the activation energy and the logarithm of pre-exponential factor have been calculated using the Arrhenius Equation as shown in Equation (1):
OR=A·exp[-Ea/(RT)]
(1)
WhereORis the oxidation rate of the reaction ,Ais the pre-exponential factor with the same unit asOR,Ris the ideal or universal gas constant that has the value of 8.314 J·K-1·mol-1,Tis the absolute temperature andEa is the activation energy of the reaction in J·mol-1. The kinetic parameters could be obtained from the temperature dependence of oxidation rates measured over the temperature range where Arrhenius plots are linear, which is defined as the “kinetic” or “chemical control” oxidation regime. The activation energy and pre-exponential factor are calculated from linearized form of Arrhenius equation, that is, from the slope and intercept of the linear plot of the logarithm of oxidation rate versus the inverse of absolute temperature (1/T):
ln(OR)=ln(A)-Ea/(RT)
(2)
For typical nuclear grade graphite materials, it was found that the practical range of testing temperatures was from about 500-550 ℃ up to about 700-750 ℃.
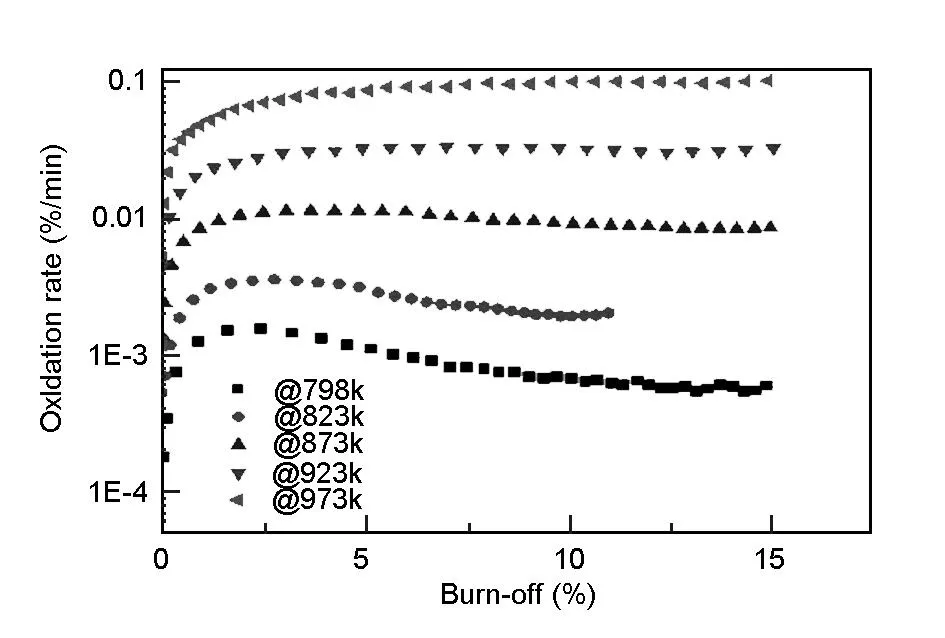
Fig. 4 Variation of oxidation rate
The oxidation rate (OR) is determined by a linear fit of the weight loss plotted against time (Fig. 3) in the range from 5% to 10% loss of original specimen weight. Experience has shown that this is the most linear part of the weight loss versus time curve because there is an introduction period where reactive surface is created when weight loss is below 5% of the starting weight of specimen. For weight loss above 10% of the starting weight of specimen, the sample dimensions become significantly distorted. The Arrhenius plot of the A3-3 MG at different temperatures is shown in Fig. 5. The slope of the plot is -21.124 8, from which the activation energy (Ea) can be calculated to be 176 kJ/mol. In previous studies the activation energy measured for most graphite materials was in the range of 190-210 kJ/mol[9]. The relatively low activation energy of the MG indicates that the MG is easier to be oxidized in air than the structural nuclear graphite materials. Also from the value of the intercept the pre-exponential factor (A) can be obtained to be 2.967 3×108which has the same unit as the oxidation rate. Therefore, the Arrhenius equation for the A3-3 MG in the range of temperatures up to 973 K which determines the oxidation rate can be described as:
OR=2.967 3×108·exp(-21 124.8/T)
(3)
The activation energy in the chemical control regime measured in the past studies for most graphite materials was in the range of 190-210 kJ/mol[9]. Otherwise, the reported activation energies for fuel matrix[9]and filler of the A3-27 MG[2]were 160 and 166 kJ/mol, respectively. Furthermore, the activation energies of natural graphite and synthetic graphite which were the main components of MG were 188 and 170 kJ/mol, respectively[17]. The activation energy of the A3-3 MG in this study is 176 kJ/mol, which is in good coincidence with the values reported before. From Fig. 5, the value of Adj. R-Square (0.995 13) is very close to 1.000 00, indicating a good linearity of the plot. It is largely assumed that the linearity of Arrhenius plots indicates that oxidation has a unique mechanism[18]; for graphite materials, oxidation in the range of temperatures up to 973 K where the Arrhenius plot is linear is commonly assumed to occur in the chemical control regime (Regime I).
Based on the previous literature results, it is concluded that, at least for the graphite samples of 1~2 cm diameter, the oxidation is in the chemical control regime (Regime I) as long as the oxygen supply rate is roughly at least 10 times higher than the rate of carbon oxidation. If the oxygen flow rate is less than about 10 times of what would be needed to sustain the rate of carbon oxidation at the highest temperature, the Arrhenius plot bends and the oxidation mechanism moves into the in-pore diffusion control regime (Regime II)[9]. In this study the ratio of oxygen supply rate (mol/min) to average carbon loss rate (mol/min) at several temperatures between 798 and 973 K is shown with arrows for the A3-3 MG in Fig. 5. As the oxidation accelerates with the increment of temperature, the ratio of oxygen supply rate to carbon loss rate declines, but it is always higher than 10 when the temperature is not higher than 923 K. When the temperature is 973 K, the ratio drops to 4.3 which is below 10. However, the Arrhenius plot does not bend and show a good linearity, which might be considered as an indication that the oxidation mechanism did not change in the temperature range investigated. The contradiction of the ratio of oxygen supply rate to average carbon loss rate between in this study and in previous studies probably due to the triple different elements in the MG indicates that further comprehensive researches on the oxidation behavior of the A3-3 MG are needed.
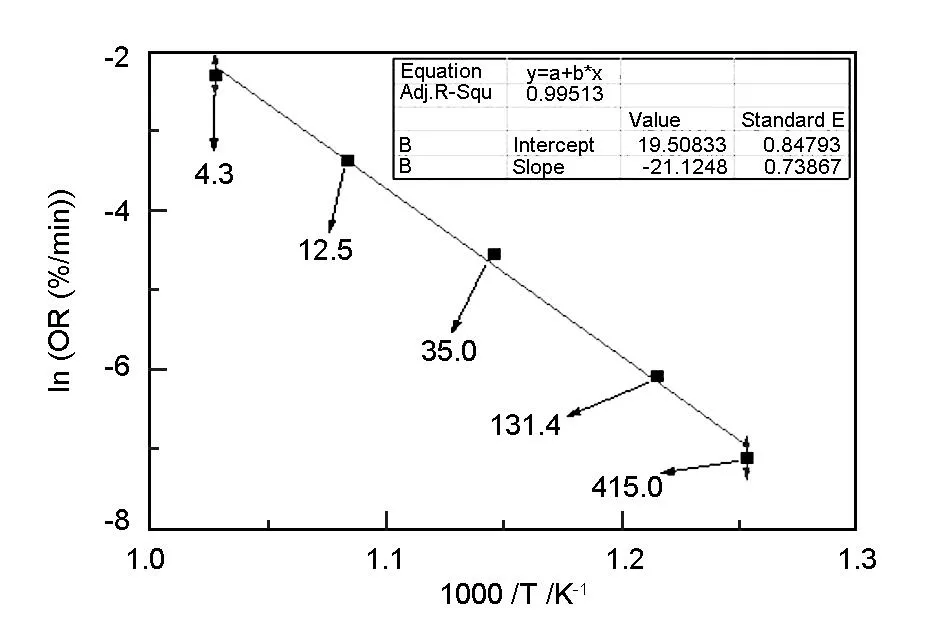
Fig. 5 The Arrhenius plot of the A3-3 MG at several temperatures.
4Conclusions
The temperature dependence of oxidation behaviors of the A3-3 MG was investigated in the temperature range of 798-973 K. The oxidation temperatures have remarkable influences on the average oxidation rate of the A3-3 MG at several temperatures. The average oxidation rate at 973 K is more than 70 times as much as that at 798 K. The Arrhenius equation was used to describe the oxidation behavior of the A3-3 MG in the temperature range of 798-973 K. The activation energy of MG is 176 kJ/mol, which is lower than that of structural graphite materials. This indicates that MG is easier to be oxidized in air. The oxidation kinetics of MG in air in the temperature range is in the chemical control regime (Regime I) and the Arrhenius equation could be described as:OR=2.967 3×108·exp(-21 124.8/T). Although the ratio of oxygen supply rate to average carbon loss rate drops to 4.3 at 973 K, the Arrhenius plot maintains a good linearity, which indicates that the oxidation mechanism does not change in the temperature range investigated. The contradiction of the ratio between in this study and in previous studies indicates that further researches related to the oxidation behavior of the A3-3 MG should be done.
Acknowledgments
Thanks for the help of Liu Jiaxiang, Wang Chen and Hu Jiuda from INET, Tsinghua University for the machining of the specimens.
References
[1]ZHOU Xiang-wen, YI Zi-long, LU Zhen-ming, et al. Graphite materials in pebble-bed high temperature gas-cooled reactors [J]. Carbon Techniques, 2012, 6: B9-B13.
[2]Moormann R, Hinssen H-K, Kuhn K. Oxidation behavior of an HTR fuel element matrix graphite in oxygen compared to a standard nuclear graphite[J]. Nuclear Engineering and Design, 2004, 227: 281-284.
[3]Zhou Xiangwen, Lu Zhenming, Zhang Jie, et al. Preparation of spherical fuel elements for HTR-PM in INET[J]. Nuclear Engineering and Design, 2013, 263: 456-461.
[4]Hrovat M, Grosse K H. Manufacture of high corrosion resistant fuel spheres (FS) for high temperature pebble bed modular reactors (PBMR) [C]. Proceedings of the HTR2006, B00000281, Johannesburg, South Africa, 2006.
[5]Xueliang Qiu, Shichao Zhang, Jun He, et al. Oxidation behavior of the matrix materials. IAEA-TECDOC-901: graphite moderate lifecycle behavior [C]. Specialist Meeting on the Graphite Moderator Lifecycle Behavior, Bath, United Kingdom, 1996: 351-362.
[6]Fuller E L, Okoh J M. Kinetics and mechanisms of the reaction of air with nuclear grade graphites: IG-11. Journal of Nuclear Materials, 1997, 240: 241-250.
[7]Kim E S, Lee W W, No H C. Analysis of geometrical effects on graphite oxidation through measurement of internal surface area[J]. Journal of Nuclear Materials, 2006, 348: 174-180.
[8]Choi W K, Kim B J, Kim E S, et al. Oxidation Behavior of IG and NBG nuclear graphites[J]. Nuclear Engineering and Design, 2011, 241: 82-87.
[9]Contescu C I, Azad S, Miller D, et al. Practical aspects for characterizing air oxidation of graphite[J]. Journal of Nuclear Materials, 2008, 381: 15-24.
[10]Lee J J, Ghosh T K, Loyalka S K. Oxidation rate of nuclear-grade graphite NBG-18 in the kinetic regime for VHTR air ingress accident scenarios[J]. Journal of Nuclear Materials, 2013, 438: 77-87.
[11]Clark T J, Woodley R E, deHalas D R. Gas-graphite systems [C]. R. E. Nightingale (Ed.), Nuclear Graphite, Academic Press, New York, 1962: 387.
[12]Moormann R, Hinssen H K, Krussenberg A K, et al. Investigation of oxidation resistance of carbon based first-wall liner materials of fusion reactors[J]. Journal of Nuclear Materials, 1994, 212-215: 1178-1182.
[13]Pappano P J, Burchell T D, Hunn J D, et al. A novel approach to fabricating fuel compacts for the next generation nuclear plant (NGNP) [J]. Journal of Nuclear Materials, 2008, 381: 25-38.
[14]Hinssen H K, Katcher W, Moormann R. Kinetics Der Graphite/sauerstoff Reaction [M]. Juel-1875, 1983.
[15]Blanchard A. Appendix 2. The thermal oxidation of graphite, irradiation damage in graphite due to fast neutrons in fission and fusion systems [R]. IAEA-TECDOC-1154, 2003: 207-213.
[16]Standard test method for air oxidation of carbon and graphite in the kinetic regime [S]. An American National Standard, D7542-09, 2009.
[17]Zaghib K, Song X, Kinoshita K. Thermal analysis of the oxidation of natural graphite: isothermal kinetic studies[J]. Thermochim. Acta, 2001, 371: 57-64.
[18]Hawtin P, Gibson J A, Murdoch R, et al. The effect of diffusion and bulk gas flow on the thermal oxidation of nuclear graphite-I. Temperatures below 500 ℃[J]. Carbon, 1964, 2: 299-309.
Receiveddate: 2016-02-26;Reviseddate: 2016-04-02
Foundationitem: State Scholarship Foundation of China (201406215002); Chinese National S&T Major Project (ZX06901); Tsinghua University Initiative Scientific Research Program (20121088038).
The oxidation behavior of A3-3 matrix graphite
ZHOU Xiang-wen,LU Zhen-ming,LI Xin-nan,ZHANG Jie,LIU Bing,TANG Ya-ping
(InstituteofNuclearandNewEnergyTechnologyofTsinghuaUniversity,CollaborativeInnovationCenterofAdvancedNuclearEnergyTechnology,thekeylaboratoryofadvancedreactorengineeringandsafety,MinistryofEducation,Beijing100084,China)
Abstract:The effects of temperature on the oxidation behavior of the A3-3 matrix graphite (MG) in the temperature range 798-973 K in air with a flow rate of 100 mL/min to burn-offs of 10-15 wt%, were investigated by a home-made thermo-gravimetric experimental setup. The oxidation rate (OR) increases significantly with the temperature. The OR at 973 K is over 70 times faster than at 798 K. The oxidation kinetics of A3-3 MG in air at temperatures up to 973 K is in the reaction control regime, where the activation energy is 176 kJ/mol and the Arrhenius equation could be described as: OR=2.967 3×108·exp(21 124.8/T) wt%/min. The relatively lower activation energy of MG than that of structural nuclear graphite indicates that MG is more easily oxidized.
Key words:Oxidation behavior; Matrix graphite; Chemical control regime; Activation energy
文章编号:1007-8827(2016)02-0182-06
中图分类号:TQ165
文献标识码:A
基金项目:国家公派留学基金(201406215002);国家科技重大专项(ZX06901);清华大学自主科研项目(20121088038).
通讯作者:周湘文,博士,副研究员. E-mail: xiangwen@tsinghua.edu.cn
Corresponding author:ZHOU Xiang-wen, Ph. D, Associate Professor. E-mail: xiangwen@tsinghua.edu.cn
DOI:10.1016/S1872-5805(16)60010-0
