褐煤基改良剂对石灰性土壤复合体铅赋存形态的影响
2016-06-18赵珂丁满化党领高巍杨秋云王代长刘世亮
赵珂, 丁满, 化党领, 高巍, 杨秋云, 王代长, 刘世亮
(河南农业大学资源与环境学院,450002,郑州)
褐煤基改良剂对石灰性土壤复合体铅赋存形态的影响
赵珂, 丁满, 化党领†, 高巍, 杨秋云, 王代长, 刘世亮
(河南农业大学资源与环境学院,450002,郑州)
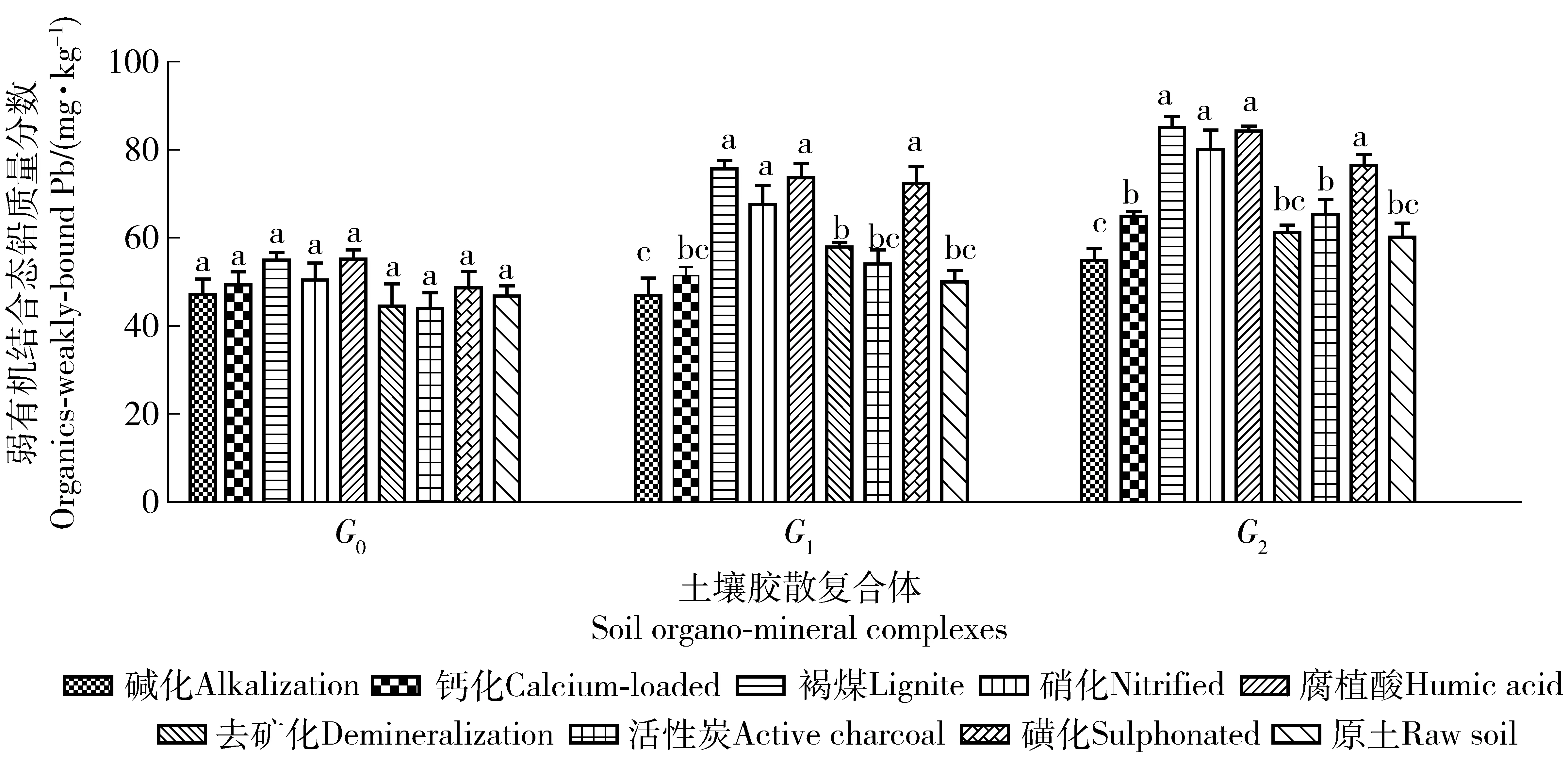
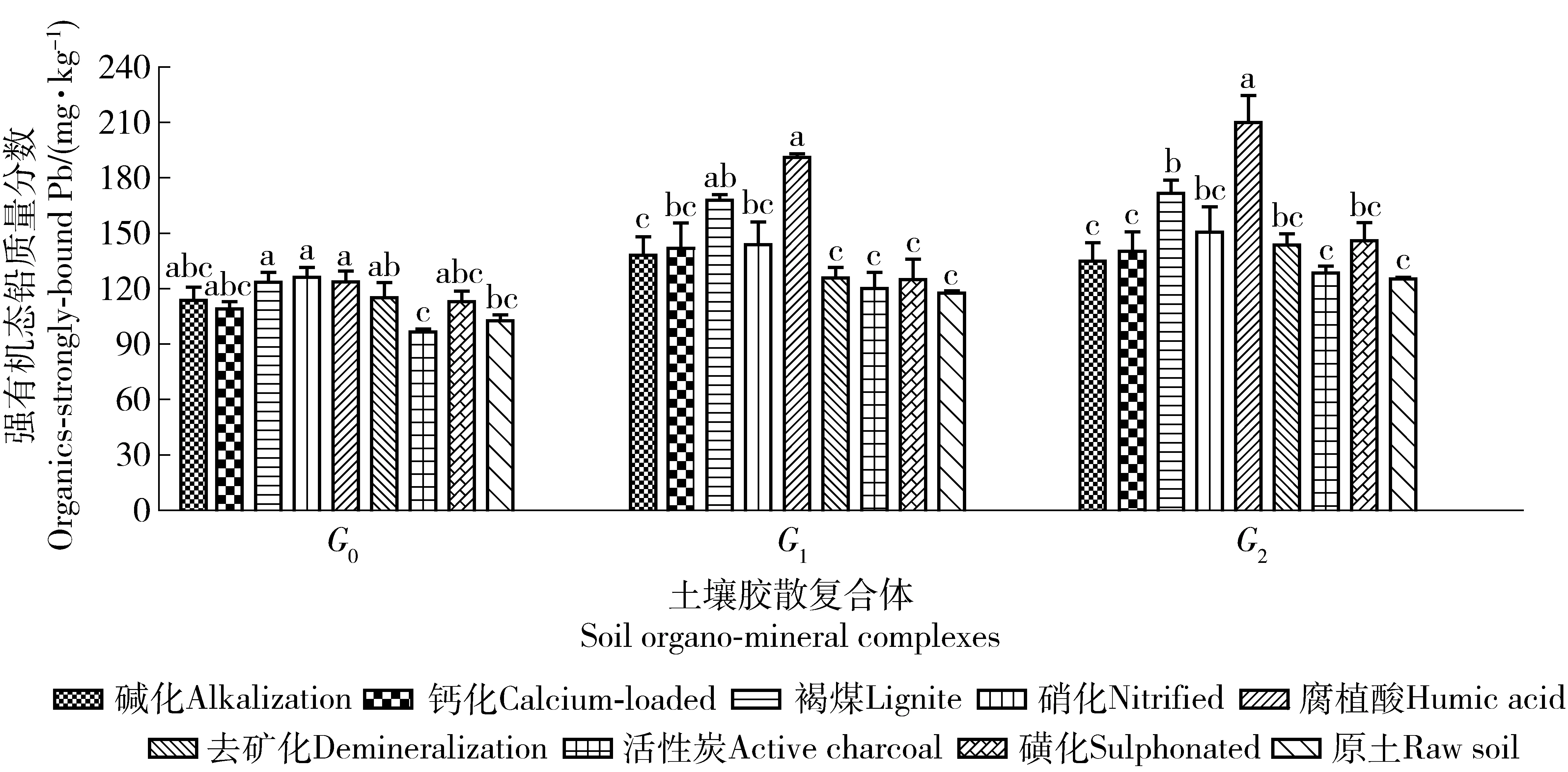
摘要:为了解褐煤基材料对土壤复合体铅形态的影响和污染退化修复机制,将褐煤以及褐煤基改性材料,混入铅污染的土壤中培养4个月,提取其中的土壤复合体,测定各组复合体中的各形态铅。结果表明:施用褐煤基有机材料后,水稳性复合体增加。1)6种铅化学形态在各复合体中分布状况不同。各改良剂处理的离子交换态、铁锰氧化物结合态和碳酸盐结合态铅在复合体中分布的大小顺序均为:G0>G1>G2,各处理从G0到G1,交换态铅质量分数下降了8.74%~32.22%,从G1到G2各处理下降了2.73%~26.74%;弱有机态和强有机态、残渣态铅分布顺序为:G0 关键词:褐煤基改良剂; 土壤修复; 铅形态; 胶散复合体; 石灰性土壤 环境污染导致的土壤退化是目前影响土壤环境质量的重要表现。重金属污染的所有修复方法中,土壤改良剂对于稳定或活化重金属,以便降低土壤污染等级是一种有效手段。土壤有机质积极调控着土壤的物理、化学和生物学过程[1]。褐煤与一般生物性修复材料相比,其有机部分抵抗微生物分解的能力强,其与金属离子形成的络合或螯合物在土壤中也更稳定。褐煤含氧量很高,这些氧存在于羧基和羟基里,是离子交换的活性点[2-3]。褐煤是一种储量非常丰富的富含天然腐植酸类物质,含有羧基、醌基、羰基和甲氧基等活性基团,是一种良好的天然有机离子交换剂,具有优异的吸附性能,用于土壤重金属污染修复潜力巨大。长期以来,腐植酸被用于废水处理移去有毒离子[4-5],氢氧化钙改性褐煤[6]可从水中高效移去重金属Pb2+、Cd2+、Cu2+。磺化褐煤是溶液中吸附Cr6+的高效吸附剂[7],经磺化或碱化处理,对重金属离子的吸附速率、吸附性能和吸附容量均有显著改善[8]。褐煤对多离子复合污染是一种廉价吸附材料[9],可不需进一步化学处理即具有较高的吸附能力和经济性,尤其高效吸附毒性离子铅和镉[10]。褐煤使土壤中可被植物吸收的重金属形态下降,植物体吸收量显著下降[11],能够稳定化酸性沙土中的Pb[12]。国内外目前多是研究了褐煤对污水中重金属的吸附去除效果,而直接用于土壤重金属修复研究的不多;石灰性土壤胶散复合体中重金属形态的研究资料未见报道;根据实验室溶液吸附试验得到的结论,不能原封不动地用于指导土壤重金属修复;直接用褐煤改良土壤,造成其氮质量分数低、C/N比高和酸性大等不良影响。因此,将褐煤进行改性后,施入重金属污染土中,研究褐煤基材料对土壤不同复合体中重金属形态改变的影响,评价褐煤基材料对重金属活化或钝化的效果,以便筛选和生产高效改良剂,为修复污染退化土壤提供理论支持。 1材料与方法 1.1供试土壤 供试土壤为河南省济源市西北郊克井镇青多村某铅冶炼企业周围200 m处、多重金属重度污染的0~20 cm原位土壤,土壤类型为黄土母质发育的褐土,质地为黏壤质,1~0.25 mm粗砂粒占比甚微,0.25~0.05 mm细砂粒质量分数低,0.05~0.001 mm间的粉粒和粗黏粒质量分数为56.50%,小于0.001 mm的细黏粒占39.80%。土壤pH值为8.05,有机质、速效磷、速效钾、碱解氮、全铅和全镉的质量分数分别为27.13、28.45、145.30、190.65、1985.76和29.35 mg/kg。 1.2试验方法 选取云南昭通褐煤进行改性。试验共设钙化褐煤[13]、磺化褐煤[8]、碱化褐煤[8]、硝化褐煤[14]、褐煤制活化碳[15]、去矿化褐煤[16]、褐煤制腐殖酸[17]和碱溶酸析法等8个改良剂处理和1个原污染土为对照。每个处理用塑料盆装土500 g,重复3次。根据先行开展的生菜盆栽试验效果,确定土壤中添加3%的褐煤基改性材料,与土壤混合均匀培养,为与野外温度接近,培养的温度条件为9—12月期间的自然室温,水分条件用称量法控制相对含水量为75%。120 d后,每个处理取土40.00 g风干,过20目筛,按胶散法[18]提取3组复合体(胶散复合体为小于10 μm的胶体颗粒,第1组是水分散复合体G0,第2组是钠质分散复合体G1,第3组是钠质研磨分散复合体G2)。 铅形态分级及测定分析方法:分级采用Tessier连续提取法[19],火焰原子吸收分光光度计测定;土壤其他理化性质测定参考鲁如坤方法[20]。采用Microsoft Excel 2007、SPSS 20.0和GraphPad Prism 5.0对所得数据进行处理分析,方差分析采用Duncan新复极差法。 2结果与分析 2.1不同改良剂处理土壤胶散复合体的质量分数 由表1可知,未加8种有机改良剂前,原土中非水稳性复合体G0组在土壤中质量分数最高,其次是去矿化。添加褐煤基改良剂后,G1组和G2组复合体质量分数以腐植酸、褐煤、硝化褐煤和磺化褐煤的处理较活性炭等其他处理高。土壤中的非水稳性G0组复合体向水稳性G1组和G2组复合体转化,这对于土壤理化性质、土壤稳定团聚体和重金属的赋存状态具有重要影响,有利于提高土壤抗侵蚀能力。 表1 土壤各胶散复合体的质量分数 注:G0为水分散复合体,G1为钠质分散复合体,G2为钠质研磨分散复合体。分别对同组复合体不同处理的铅形态含量作方差分析与多重比较,P<0.05。下同。Note: G0named as water-dispersing complex, G1as NaCl-dispersing complex, G2as NaCl-grinding-dispersing complex. Variance analysis and multiple comparison of content of different lead chemical speciation in the same group of complexes with different treatments atP<0.05. The same as below. 2.2对土壤胶散复合体中铅形态的影响 2.2.1离子交换态铅图1示出,从G0到G1,各处理交换态铅质量分数下降了8.74%~32.22%,G1到G2各处理下降了2.73%~26.74%。即交换态离子主要存在于非水稳性G0组有机无机复合体中,G1和G2作为不易变化的复合体形态,其易交换态铅质量分数也随之下降。各处理的交换态质量分数比较,G0组复合体中,碱化、钙化、腐植酸和活性炭处理的离子交换态铅质量分数显著下降,而褐煤、硝化、去矿化和磺化对交换态铅的影响较小。G1组和G2组复合体中,各处理的离子交换态铅质量分数均有所下降,以褐煤和活性炭下降最多。改性与未改性的褐煤相比,碱化、钙化、腐植酸和活性炭显著降低G0组中交换态铅的质量分数,G1组中,改性材料引起的交换态铅大致有升高趋势,以去矿化和磺化达到了显著性升高。G2组中,改性后导致的交换态质量分数都显著高于改性前的褐煤。 图1 G0、G1和G2复合体的离子交换态铅变化Fig.1 Content of ion-exchangeable Pb in complexes fo G0, G1 and G2 2.2.2碳酸盐结合态铅图2示出,3组复合体的碳酸盐结合态铅质量分数、腐植酸和褐煤处理比原土显著降低。腐植酸处理的G0、G1、G2组复合体的碳酸盐结合态铅质量分数分别比原土低12.25%,12.38%,15.17%。与未改性褐煤比较,除腐植酸使碳酸盐态铅降低外,其他改性均使3组复合体中碳酸盐态铅质量分数升高,但都未达显著差异,碳酸盐态铅在3组复合体中的质量分数差异不大。 图2 G0、G1和G2复合体的碳酸盐结合态铅变化Fig.2 Content of carbonate-bound Pb in complexes of G0, G1 and G2 2.2.3铁锰氧化物结合态铅图3示出,同组复合体各处理与原土比较,3组均未达到显著性差异,但G1和G2组中,腐植酸明显降低了铁锰氧化物态质量分数,其他改性处理多为升高。与褐煤相比,G1组碱化和钙化显著提高了铁锰氧化物质量分数,G0和G2组各改性与褐煤比无显著性差异。3组复合体中铁锰氧化物质量分数比较,各材料处理均表现为G0>G1>G2,均值分别为1 324.89、1 287.58和1 263.58 mg/kg。 图3 G0、G1和G2复合体的铁锰氧化物结合态铅变化Fig.3 Content of Fe-Mn-oxide-bound Pb in complexes fo G0,G1 and G2 图4 G0、G1和G2复合体的弱有机结合态铅变化Fig.4 Content of organics-weakly-bound Pb in complexes of G0,G1 and G2 图5 G0、G1和G2复合体的强有机结合态铅变化Fig.5 Content of organics-strongly-bound Pb in complexes of G0, G1 and G2 2.2.4弱有机结合态铅图4示出,从G0到G1到G2,各处理的弱有机态铅质量分数都明显增加,G0到G1的增加幅度为6.89%~48.48%,G1到G2为5.70%~26.18%。说明弱有机态在相对稳定的G1和G2组复合体中质量分数较多。各处理比较,G0组各处理的弱有机态铅质量分数未达到显著性差异;G1和G2组的褐煤、腐植酸、硝化和磺化显著高于原土,G1组这4种材料分别比原土高了51.23%、47.35%、35.06%和44.59%,G2组分别高了41.35%、40.03%、32.95%和27.11%。改性后碱化、钙化、去矿化和活性炭显著降低了弱有机态铅的质量分数,而腐植酸、硝化和磺化与改性前无显著影响。 2.2.5强有机结合态铅图5示出,从G0到G1到G2,强有机态铅质量分数逐渐增加。增加幅度从G0到G1平均为24%,从G1到G2平均为6.27%。说明在稳定性复合体中,强有机态铅赋存较多。G0组复合体中,褐煤、硝化和腐植酸处理的强有机态铅质量分数显著增加,8种材料的提高幅度为5.44%~38.29%;G1组中提高了1.95%~62.23%,褐煤和腐植酸处理的强有机态铅质量分数分别提高了42.47%和36.95%;G2组中提高了2.51%~67.65%,褐煤和腐植酸处理比原土分别提高62.23%和67.65%。褐煤改性后,多数降低了强有机态铅质量分数。 2.2.6残渣态铅图6示出,3组复合体中,各处理的残渣态铅质量分数均与原土无显著性差异。G0组中,碱化和钙化比褐煤显著降低了残渣态铅质量分数,分别低了15%和17%。G1和G2组中,残渣态铅各处理均未达到显著性差异。G0、G1和G2中的各处理残渣态铅范围分别为318.51~381.82、352.73~451.40和333.15~441.21 mg/kg。说明残渣态铅在稳定性复合体G1和G2中赋存质量分数高于G0中,褐煤基有机材料对残渣态铅的影响不显著或具有不确定性。 图6 G0、G1和G2复合体的残渣态铅变化Fig.6 Content of residual Pb in complexes of G0, G1 and G2 3结论与讨论 1) 施入有机材料后,提高了水稳性复合体在土壤中的质量分数,腐植酸、褐煤、硝化褐煤和磺化褐煤形成更多的水稳性复合体,腐植酸处理形成的水稳性G1和G2组最多,有利于稳定土壤结构和提高抗侵蚀力。交换态、铁锰氧化物态铅和碳酸盐态铅主要赋存于非水稳性复合体中,强、弱有机态铅和残渣态铅主要存在于水稳性复合体中。稳定复合体和团聚体形成后,能够稳定土壤结构,增强对重金属的固定或钝化能力,提高土壤抗侵蚀,并降低重金属溶解、淋溶和环境风险。 2) 有机材料均引起了3组复合体中交换态铅质量分数的下降,普遍提高强有机态铅质量分数,部分材料提高了弱有机态铅质量分数,表现出褐煤基有机材料对铅的显著钝化作用,但对残渣态和铁锰氧化物态铅没有显著影响。其材料可以作为铅污染退化土壤的有效修复剂。可交换态、碳酸盐态、Fe/Mn氧化物态和有机态4种形态存在的重金属合称为“有效态重金属”[20]。残渣态重金属在自然界正常条件下不易释放[21]。几种铅形态受有机材料影响后,总的变化规律是交换态铅显著下降,弱有机态和强有机态主要是显著升高,碳酸盐铅受到部分有机材料影响而改变,而残渣态铅很少受到外源有机物料的影响而改变,铁锰氧化物态也很少改变,反映出可交换态与2种有机态的互动比较明显。残渣态影响较小,与文献[21]的说法一致。然而J.Pavel等[22]认为,施入腐植酸钾后,铁锰氧化物态铅的下降和残留态铅的显著增加,是由于腐植酸的酸性和其对铅的强烈的复合能力所致。这与本文的研究结果“褐煤基有机材料对铁锰氧化物态和残留态铅的影响较小”存在差异。 3) 褐煤改性后比改性前主要提高了交换态铅和碳酸盐态铅质量分数,降低了强、弱有机态铅质量分数。本文中多种改性处理,对降低或提高土壤铅有效性等方面的影响,均可从已有研究结果如腐殖酸的结构和元素特征[23-25]、褐煤活性炭特征[26]、褐煤去矿化后酸性特征[27-28]、硝酸改性褐煤特征[29]、荷钙褐煤特征[9]、磺化褐煤特征[30]中获得一定程度的理论解释,但其准确作用机制还需进一步研究。 4参考文献 [1]李文军,彭保发,杨奇勇.长期施肥对洞庭湖双季稻区水稻土有机碳、氮积累及其活性的影响[J].中国农业科学,2015,48 (3):488. Li Wenjun,Peng Baofa,Yang Qiyong. Effects of long-term fertilization of organic carbon and nitrogen accumulation and activity in a paddy soil in double cropping rice area in Dongting lake of China[J].Scientia Agricultura Sinica,2015,48(3):488.(in Chinese) [2]Lafferty C, Hobday M. The use of low rank brown coal as an exchange material[J]. Fuel, 1990, 69:78. [3]Murakami K, Yamada T, Fuda K, et al.Selectivity in cation exchange property of heat-treated brown coals[J]. Fuel, 2001, 80:599. [4]Ucurum M. A study of removal of Pb heavy metal ions from aqueous solution using lignite and a new cheap adsorbent (lignite washing plant tailings) [J].Fuel,2009, 88(8):1460. [5]Pehlivan E, Arslan G.Removal of metal ions using lignite in aqueous solution-low cost biosorbents[J]. Fuel Processing Technology,2007,88:99. [6]Miluše J, Miroslav P,Jan H,et al. Removal of heavy metals from water by lignite-based sorbents[J]. Fuel,2004,83:1197. [7]Zhang Runhu, Wang Bo, Ma Hongzhu.Studies on Chromium (Ⅵ) adsorption on sulfonated lignite[J]. Desalination,2010,255:61. [8]张怀成,王在峰,李建义,等.褐煤经磺化及碱化处理对重金属离子吸附性能研究[J].环境化学,1999,18(5):482. Zhang Huaicheng,Wang Zaifeng, LiJianyi, et al.Studies on the adsorption characteristics of lignite activated by sulphonation or alkalization for heavy-metal ions[J].Environmental Chemistry,1999,18(5):482. (in Chinese) [9]Leoš D,Miloslav P. Removal of metal ions from multi-component mixture using natural lignite[J].Fuel Processing Technology, 2012,101:29. [10]Martina H, Jiri M, Ivana S, et al. Sorption of metal ions on lignite and the derived humic substances[J]. Journal of Hazardous Materials, 2009,161 :559. [11]Pusz A. Influence ofbrown coal on limit of phytotoxicity of soils contaminated with heavy metals[J]. Journal of Hazardous Materials, 2007,149:590. [12]Nikolett U, Márk R, Eszter D,et al.Stabilization of Cr, Pb, and Zn in soil using lignite[J].Soil and Sediment Contamination: An International Journal,2014,23(3):270. [14]王鲁敏,邓昌亮,殷军港,等.硝化褐煤对铬离子溶液的吸附研究[J].环境化学,2001,20(1):54. Wang Lumin, Deng Changliang, Yin Jungang, et al. Study on adsorption of nitrify lignite for chromium-ion solution[J].Environmental Chemistry,2001,20(1):54.(in Chinese) [15]罗道成,郑李辉.用褐煤活化一步法制备活性炭的研究[J].煤化工,2009,37(5):25. Luo Daocheng, ZhengLihui. Study on the preparation of activated carbon by one-step method of carbonization-activation with lignite coal[J]. Coal Chemistry Industry,2009,37(5):25.(in Chinese) [17]黄金凤,赵义龙,赵金香,等.腐植酸的提取及其成分含量测定[J].四川畜牧兽医,2007,34(5):27. Huang Jinfeng, Zhao Yilong, Zhao Jinxang, et al. The extraction and determination of composition of humic acid[J]. Sichuan Animal & Veterinary Science,2007,34(5):27. (in Chinese) [18]化党领,张一平.塿土不同施肥条件下土壤胶散复合体研究[J].土壤肥料,1999(1):9. Hua Dangling, Zhang Yipeng. Study on soil organo-mineral complex on Lou-soil[J].Soil and Fertilizer, 1999,(1):9.(in Chinese) [19]Tessier A, Campbell P G, Bisson M. Sequential extraction procedure for the speciation of particulate trace metals[J]. Analytical Chemistry,1979,51(7):844. [20]周康民,汤志云,黄光明,等.土壤中重金属形态分析方法研究[J].江苏地质,2007,31(3):165. Zhou Kangmin, Tang Zhiyun, Huang Guangming, et al. Methodology study on heavy metal morphology analysis[J]. Jiangsu Geology, 2007,31(3):165. (in Chinese) [21]李宇庆,陈玲,仇雁翎,等.上海化学工业区土壤重属元素形态分析[J].生态环境,2004,13(2):154. Li Yuqing, Chen Ling, Qiu Yanling, et al. Speciation of heavy metals in soil from Shanghai Chemical Industry Park[J]. Ecology and Environment, 2004, 13(2):154. (in Chinese) [22]Pavel J, Jana V, Lucie H,et al.Effects of inorganic and organic amendments on the mobility (leachability) of heavy metals in contaminated soil: A sequential extraction study[J].Geoderma, 2010,159:335. [23]喻伟.伊敏褐煤腐殖酸结构特征及热解动力学分析[J].科技创新与生产力,2011(6):94. Yu Wei. Analysis on features of humus acid texture of lignite coal and pyrolysis dynamics[J]. Sci-Tech Innovation & Productivity, 2011(6):94. (in Chinese) [24]Martyniuk H, Wieckowska J. Adsorption of metal ions on humic acids extracted from brown coals[J]. Fuel Process Technology, 2003,84(1/2/3):23. [25]Clemente R, Bernal M P. Fractionation of heavy metals and distribution of organic carbon in two contaminated soils amended with humic acid[J]. Chemosphere, 2006,64(8):1264. [26]陈雯,刘中华,范艳青,等.褐煤活性炭的制备与性能研究[J].煤炭转化,2004,27(2):61. Chen Wen, Liu Zhonghua, Fan Yanqing, et al. Study on preparation and properties of activated carbon from lignite[J]. Coal Conversion, 2004,27(2):61. (in Chinese) [27]Hadi Z, Nursen O, Mehtap G, et al. Images of demineralized coal surfaces by scanning tunnelling microscopy[J]. Fuel,1996,75(7):855. [28]Julien S, Philippe B, Sebastien M, et al. The influence of demineralisation and ammoxidationon: the adsorption properties of an activated carbon prepared from a Polish lignite[J]. Carbon,2006,44:2549. [29]上官炬,杨直,苗茂谦. 硝酸改性褐煤半焦制备烟气脱硫剂[J].太原理工大学学报,2007,38(3):229. Shangguan Ju, Yang Zhi, Miao Maoqian. The SO2adsorbent prepared by the oxidation with HNO3from lignite semi coke[J]. Journal of Taiyuan University of Technology,2007,38(3):229. (in Chinese) [30]Halina M, Danuta A.Sorption of metal cations on sulphonated brown coals[J].Fuel,1991,70(4):551. (责任编辑:程云郭雪芳) Effects of lignite-based amendments on lead chemical speciation in calcareous soil organo-mineral complexes Zhao Ke, Ding Man, Hua Dangling, Gao Wei, Yang Qiuyun, Wang Daichang, Liu Shiliang (College of Resources and Environment, Henan Agricultural University, 450002, Zhengzhou, China) Abstract:[Background] In order to remediate heavy metal-contaminated calcareous soil, a comparative research on the effect of lignite-based materials on heavy metals speciation of different organo-mineral complexes were conducted for screening out highly effective amendments and understanding the restoration mechanism of pollution degradation. [Methods] All these materials were mixed with lead-contaminated soiland incubated for 120 days at 25 ℃ and 65% relative humidity, thereafter organo-mineral complexes were extracted, and mass fraction of different Pb chemical speciation of soil complexes were assessed. [Results] The results indicated that water-stable complexes were increased with the application of lignite organic materials,the treatments with humic acid,lignite,nitrified lignite and sulphonated lignite transformed more of G1 and G2 complexes from G0, and other results were as follows:1) The distributions of 6 Pb chemical speciation (ion-exchangeable Pb, Fe-Mn-oxide-bound Pb, carbonate-bound Pb, organics-weakly-bound Pb, organics-strongly-bound Pb, and residual Pb) varied in the different complexes G0 (water-dispersing complex), G1(NaCl-dispersing complex),and G2(NaCl-grinding-dispersing complex). For all amendments, the order of the abundance for ion-exchangeable Pb, Fe-Mn-oxide-bound Pb, and carbonate-bound Pbwas G0>G1>G2; the ion-exchangeable Pb decreased 8.74%-32.22% from G0 to G1, and 2.73%-26.74% from G1 to G2; organics-weakly-bound Pb, organics-strongly-bound Pb, and residual Pb in G1 and G2 complexes were more than that in G0. 2) Applying organic materials decreased the content of ion-exchangeable Pb averagely 2.73%-32.22%in 3 complexes, while increased the organics-weakly-bound Pb at a maximum of 51.23% and organics-strongly-bound Pb at a maximum of 67.65%, no significant effect on residual Pb. Organics-weakly-bound Pb in G0 was less significantly affected than that in G1 and G2 by these organic materials, and the content of organics-weakly-bound Pb in G1 complexes treated with lignite, humic acid, nitrified lignite and sulphonated lignite were higher than original soil in the range of 35.06%-51.23%, and in G2 higher in the range of 27.11%-41.35%. All lignite-based materials increased the content of organics-strongly-bound Pb in the range of 5.44%-38.29%, but had no significant effect on residual Pb of G0, G1 and G2. 3) Compared with raw lignite, the treatments with humic acid, active charcoal, alkalization and calcium-loaded lignite decreased markedly the content of ion-exchangeable Pb in G0 group, but all modified lignite increased ion-exchangeable Pb content in G2 group, as well as sulphonation and demineralization increased ion-exchangeable Pb in G1 remarkably. Except humic acid,many modified lignite-based materials resulted in lower content of organics-strongly-bound Pb in all three groups of complex.There were no significant differences on content of carbonate-bound Pb in all complexes between original and modified lignite.Alkalization and calcium-loaded lignite markedly decreased residual Pb in G0, and the same changes in G1 and G2. Simultaneously, alkalization and calcium-loaded lignite increased significantly Fe-Mn-oxide-bound Pb in G1. [Conclusions] In conclusion, applying lignite-based amendments is effective in increasing water-stable complexes and prompting more organically-bound Pb speciation in contaminated soil, decreasing the fraction of ion-exchangeable Pb, thus it helps establish stable soil structure, enhance the capacity of resisting soil erosion, and decrease the environmental exposure risk by surface runoff and deep percolation. Organic lignite-based amendments improve remarkably content of organically-bound Pb, nevertheless, organic matter has little effect on Fe-Mn-oxide-bound, residual and carbonate-bound Pb. Taking immobilization and economy efficiency into account, raw lignite, humic acid, active charaoal, nitrified lignite and sulphonated lignite are recommended for remediating Pb-contaminated soil. Keywords:lignite-based amendments; soil remediation; lead chemical speciation; organo-mineral complex; calcareous soil 收稿日期:2015-07-23修回日期: 2016-01-04 第一作者简介:赵珂(1988—),女,硕士研究生。主要研究方向:土壤重金属污染修复。E-mail: 1060048917@qq.com †通信 化党领(1964—),男,博士,教授。主要研究方向:土壤学与植物营养学。E-mail: collegehua@163.ocm 中图分类号:S156.2 文献标志码:A 文章编号:1672-3007(2016)01-0123-08 DOI:10.16843/j.sswc.2016.01.015 项目名称: 国家自然科学基金“褐煤基改性材料转化石灰性土壤重金属形态的机理和其对重金属时空变异的影响”(41371311),“硫素对稻根表面铁锰胶膜形成及水稻吸收Cd和As有效性的影响”(41271471)