Bioactive constituents from the leaves of Quercus phillyraeoides A.Gray for α-glucosidase inhibitor activity with concurrent antioxidant activity
2016-06-15AnstsiWheniIndriningsihSnroThin
Anstsi Wheni IndriningsihSnro Thin
a The United Graduate School of Agricultural Sciences,Ehime University,3-5-7 Tarumi,Matsuyama,Ehime 790-8566,Japan
b Research Unit for Development of Chemical Engineering Process,Indonesian Institute of Sciences,Gading,Playen,Gunungkidul,Yogyakarta 55581,Indonesia
c Department of Applied Biosciences,Faculty of Agriculture,Ehime University,3-5-7 Tarumi,Matsuyama,Ehime 790-8566,Japan
Received 6 October 2015;received in revised form 18 February 2016;accepted 28 February 2016
Available online 5 March 2016
Abstract Several α-glucosidase inhibitory constituents were isolated from the methanolic extract of the leaves of Quercus phillyraeoides A. Gray (Q.phillyraeoides) using a bioassay-guided fractionation technique. Further separation and purificatio of the methanol-soluble fraction led to the isolation of constituents with moderate and strong inhibitory activities against α-glucosidase:β-sitosterol-D-glucoside(1)and condensed tannin fractions(2,3,4,5,and 6).Compound 1 and fractions 2–6 had inhibitory concentration(IC50)values against α-glucosidase from Saccharomyces cerevisiae of 118.8, 2.79, 2.78, 3.10, 2.60, and 3.14 μg/mL, respectively, while quercetin as the standard had an IC50 value of 4.80 μg/mL.Furthermore,the significan antioxidant activities were evaluated using several assays,such as the DPPH radical scavenging,hydrogen peroxide radical scavenging, reducing power, and β-carotene-linoleate bleaching assays, and the results suggested that the isolated constituents showed their possible application for treating the hyperglycemia-induced oxidative stress. The results of the present study showed the potential of Q.phillyraeoides as a rich source of natural antidiabetic medicine.
Keywords: Quercus phillyraeoides A.Gray;α-Glucosidase inhibitor;Antioxidative activity;Lineweaver–Burk plot
1. Introduction
Diabetes mellitus (DM) is a common chronic disease that has become a serious health issue due to its associated complications.There are two types of DM:type 1 DM,caused by the destruction of pancreatic beta cells,resulting in an insulin defi ciency,and type 2 DM results from defects in insulin secretion or insulin resistance[1,2].DM is characterized by high blood glucose levels,which lead to complications such as hypertension,neuropathy, nephropathy, retinopathy, and diabetic foot ulcers[3].Type 2 DM is the most frequently encountered form of DM,accounting for more than 80%of all cases[4].The number of DM patients has markedly increased in the past few years and is estimated to rise to 366 million by 2030[5].
Current DM treatments are based on the use of synthetic drugs, which are often associated with a number of serious adverseeffects[6].Therefore,thedevelopmentofbetterpharmaceuticals as alternatives for the treatment of DM without any side effects is urgently needed.Natural compounds from plants have attracted much attention because they become alternatives to the currently used synthetic DM drugs.Ethnobotanical studies have identifie approximately 1200 plants in the world with antidiabetic potential [7]. Several studies also suggested the application of medicinal plant extracts to antidiabetic treatments due to fewer side effects than those of synthetic medicines[8,9].Another advantage is that natural compounds may be safely consumed in the daily diet,thereby reducing the risk of DM[10].
However, free radicals have also been suggested to cause DM [11]. Although free radicals typically originate from the surrounding environment,several physiological and biochemical processes in the human body also produce reactive oxygen species, such as the superoxide radical, hydroxyl radicals, and peroxyl radicals,as by-products[12].Therefore,the search for antidiabetic and antioxidant drug constituents from plants has been attracting increasing attention in recent years.
Quercus phillyraeoidesA. Gray (Q. phillyraeoides) of the familyFagaceaeis an evergreen tree that is distributed in the limestone mountains and acid bed rocks of East Asia (Korea,China and Japan). The leaves ofQuercusspecies have been used in Korean folk medicine for dysentery, diarrhea, hemorrhage, dermatitis, and the exclusion of extravasated blood[13]. Previous phytochemical studies onQ. phillyraeoidesled to the identificatio of several tannins from the leaves ofQ. phillyraeoides[14]. However, the bioactivities ofQ.phillyraeoideshave not yet been examined.
The objective of the present study was to identify the active constituents from the leaves ofQ. phillyraeoidesthat are responsible for inhibitory activity against α-glucosidase and subsequently evaluate the kinetics responsible for this enzyme inhibitory activity.Anin vitroassay of α-glucosidase inhibitory activity was conducted using an α-glucosidase enzyme obtained fromSaccharomycescerevisiae(S.cerevisiae)yeast.Theantioxidant properties of isolated constituents were also evaluated using several assays as an assessment to alleviate oxidative stress related to DM.These assays may be used for preliminary observationsintheevaluationofpharmacologicalactivitiesandalsoto verify the medicinal effects of these active constituents isolated from plants.
2. Materials and methods
2.1. General instrumentation and reagents
An analysis using gas chromatography(GC)was conducted on a GC-FID 2014 model (Shimadzu, Japan). The electron ionization mass spectra (EI-MS) of isolated constituents were recorded on a GC Mass Spectrometer(GC-MS QP 2010 Plus,Shimadzu, Japan) and Fast Atomic Bombardment Mass Spectrometer(FAB-MS,Shimadzu,Japan).TLC was run on silica gel 60 F254pre-coated plates(Merck 5554)and spots were detected using UV light.
All chemicals used were purchased from commercial available sources and were used without further purification α-Glucosidase [(EC 3.2.1.20)] type I fromS. cerevisiae,p-nitrophenyl α-D-glucopyranoside (p-NPG), 1,1-diphenyl-2-picrylhydrazyl (DPPH), β-carotene, potassium ferricyanide[K3Fe(CN)6], trichloroacetic acid, ferric chloride (FeCl3),hydrogen peroxide, and bis(trimethylsilyl) acetamide (BSA)were purchased from Wako Pure Chemical Industries, Ltd.(Osaka, Japan). Quercetin, Tween 40, and gallic acid were purchased from Sigma–Aldrich Co., Ltd. (Tokyo, Japan).All solvents used in this study (methanol, ethanol, toluene,pyridine, ethyl acetate, chloroform,n-hexane, and acetone)were purchased from Wako Pure Chemical Industries,Ltd.
2.2. Plant material
The leaves ofQ. phillyraeoideswere collected from a site in Ehime University, Matsuyama, Japan, in September 2014.Voucher specimens have been deposited in the Department of Plant Chemistry, Faculty of Agriculture, Ehime University,Japan.The leaves were dried naturally.
2.3. Extraction and isolation procedures
The dried leaves ofQ. phillyraeoideswere powdered and extracted twice with methanol(1:8,w/w)at room temperature for 3 days.The methanol filtrat was concentrated using rotary evaporator under reduced pressure.The methanolic extract was partitioned successively using solvents with increasing polarity fromn-hexane,chloroform,ethylacetate(EtOAc),andmethanol(MeOH) to obtainn-hexane-, chloroform-, ethyl acetate-, and methanol-soluble fractions. All extracts were screened for α-glucosidase inhibitory activity, with the methanol-soluble fraction exhibiting the highest activity. The active methanol soluble fraction (90.3 g) was separated by column chromatography over a silica gel(100 mesh).The column was eluted with solvents of increasing polarities and a stepwise elution fromnhexane(100%),ethyl acetate(EtOAc,50%)inn-hexane,EtOAc(100%), and EtOAc with variable concentrations in methanol(MeOH)to MeOH 100%to obtain six fractions(F1–F6).Fraction F4, which exhibited the highest α-glucosidase inhibitory activity among the fractions examined,was further separated by silica gel column chromatography using solvents with increasing polarities and a stepwise elution fromn-hexane (100%),ethyl acetate (EtOAc, 50%) inn-hexane, EtOAc (100%), and EtOAc with variable concentrations in methanol (MeOH) to MeOH 100% to obtain fi e fractions (F41-F45). Compound 1(15.2 mg)was isolated as a white buff powder from fraction F42 by silica column chromatography followed by the recrystallization of compound 1 from methanol.Fractions 2 to 6(81.7 mg,15.1 mg,54.9 mg,553 mg,and 313 mg,respectively)were isolated from F44 using Sephadex LH-20 column chromatography by eluting with methanol and water in gradients.
Acid hydrolysis of 1: Compound 1 (1.5 mg) was reflu ed with 2 N HCl in aq. MeOH for 4 h to give β-sitosterol and Dglucose,which were confirme with an available standard using thin layer chromatography. Furthermore, the β-sitosterol was further confirme using GC analysis.
Thiolysis of fractions 2–6: A solution of the fraction in methanol (4000 ppm, 50 μL) was reacted with 2 N HCl in methanol(50 μL),followed by the addition of benzyl mercaptan(BM)in methanol(100 μL;5:95,v/v).The solution was heated at 90◦C for 5 min [15]. The reaction was stopped by placing the solution in an ice bath. Prior to the GC–MS analysis, the trimethylsilane (TMS) derivation of the result of the thiolysis product was conducted by reacting the sample with pyridine(10 μL)and bis(trimethylsilyl)acetamide(BSA,20 μL).
2.4. HPLC analysis
The condensed tannin fractions (2–6) were analyzed using gel permeation chromatography(GPC)on Waters HPLC,using a Shodex Asahipak GF-310 HQ column (Japan). The elution conditions were as follows: solvent, CHCl3:MeOH (50:50);isocratic eluent;fl w rate 0.5 mL/min;photodiode array(PDA)detector. The molecular weights of fractions 2–6 were estimated using polystyrene (PS, Polymer Laboratories) as the standard.
2.5. GC/GC–MS analysis
The GC analysis of β-sitosterol as a result of the hydrolysis of compound 1 was conducted using the GC-FID 2014 model (Shimadzu) with a TC-5 capillary column (crossbond 5%diphenyl-95%dimethyl polysiloxane,GL Sciences,Tokyo,Japan)with a fil thickness of 30 m×0.25 mm×0.25 μm.The carrier gas helium was delivered at a constant fl w rate of 1.01 mL/min,column pressure of 117 kPa,and interface temperature of 320◦C.The column oven temperature program started at 150◦C(held for 1 min at 150◦C),and was then programmed at 10◦C/min to 320◦C(held for 10 min at 320◦C).The total analytical time was 28 min. The detector temperature was 320◦C with an injection volume of 2 μL.
The GC–MS analysis of the trimethylsilylated samples of fractions 2–6 was conducted on a GC–MS QP 2010 Plus with a TC-1 capillary column (100% dimethyl polysiloxane, GL Sciences, Tokyo, Japan) with a fil thickness of 30 m×0.25 mm×0.25 μm. The carrier gas helium was delivered at a constant fl w rate of 1.46 mL/min, column pressure of 100 kPa, and interface temperature of 260◦C. The column oven temperature program started at 80◦C, and was then programmed at 10◦C/min to 300◦C (held for 10 min at 300◦C).The total analytical time was 32 min with an injection volume of 2 μL.
2.6. α-Glucosidase inhibitory activity
The inhibitory activity of α-glucosidase was evaluated as reported by Kim et al.[16].Samples were dissolved in dimethyl sulfoxide at various concentrations (10 μL) and then treated withp-NPG (250 μL, 3 mmol/L) in phosphate buffer solution(490 μL, 100 mmol/L, pH 7). The solution was pre-incubated at 37◦C for 5 min. Two hundred and fift microliters of αglucosidase (0.065 U/mL) was then added and the reaction continued for 15 min. The reaction was stopped by the addition of 1 mL of 0.2 M Na2CO3. The mixtures were measured at 400 nm using a UV–Vis spectrophotometer. The percentage inhibition of α-glucosidase inhibitory activity was calculated using Eq.(1).whereA0is the absorbance of the control andA1is absorbance in the presence of the sample.The inhibitory concentration(IC50)of the samples was calculated using a regression analysis from the graph plotting scavenging activity against concentration.All experiments were carried out in triplicate and the results are expressed as the mean±SD of three determinations.
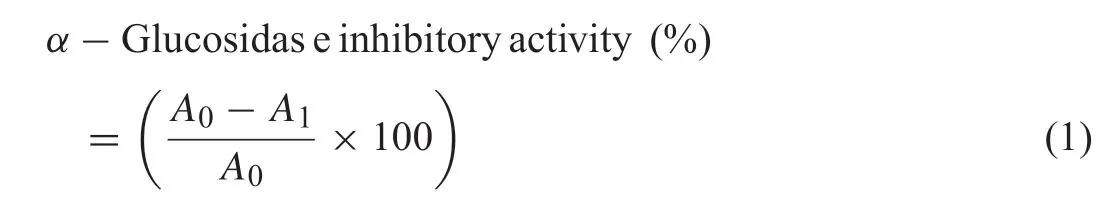
2.7. Enzyme kinetics
All isolated constituents were evaluated for their kinetics in inhibiting α-glucosidase activity. The type of inhibition of the active constituents against α-glucosidase was determined using increasing concentrations ofp-NPG as a substrate in the absence or presence of active constituents as inhibitors at different concentrations.The type of inhibition was determined using a Lineweaver–Burk plot analysis.
2.8. DPPH free radical scavenging activity
The antioxidant activities of constituents 1–6 were determined by a DPPH radical scavenging assay as conducted according to Yen and Chen,1995[17]with slight modifications Samples were dissolved in methanol at various concentrations,treated with DPPH(1 mmol/L in methanol),and left to stand for 30 min at room temperature in the dark.Absorbance was measured at 517 nm using a UV–Vis spectrophotometer.The ability of the samples to scavenge the DPPH radical was calculated using Eq.(1).
2.9. Hydrogen peroxide radical scavenging activity
The abilities of constituents 1–6 to scavenge hydrogen peroxide were determined according to the method of Wang et al.[18].Asolutionofhydrogenperoxide(40 mmol/L)wasprepared in phosphate buffer saline (PBS, pH 7.4). Samples at various concentrations in 4 mL distilled water were added to hydrogen peroxide solution (0.6 mL). The solution was left to stand for 10 min and absorbance was measured at 230 nm.The ability of the samples to scavenge the H2O2radical was calculated using Eq. (1). All experiments were carried out in triplicate and the results are expressed as the mean±SD of three determinations.
2.10. Reducing power assay
The reducing power assay was performed according to a previously described method by Oyaizu, 1986 [19] with minor modifications A test sample solution (1 mL, 20 g/mL) was mixed with phosphate buffer (2.5 mL) and potassium ferricyanide (2.5 mL). The mixture was incubated at 50◦C for 20 min.Trichloroacetic acid(2.5 mL)was added to the mixture,which was then centrifuged at 3000 rpm for 10 min.The upper layer of the solution (2.5 mL) was mixed with distilled water(2.5 mL)andafreshlypreparedferricchloridesolution(0.5 mL).Absorbance was measured at 700 nm.Antioxidant activity was calculated using Eq.(1).All experiments were carried out in triplicate and the results are expressed as the mean±SD of three determinations.
2.11. β-Carotene-linoleate model assay
The antioxidant activities of constituents 1–6 in the βcarotene-linoleate model system were assessed as reported by Jayaprakasha et al. [20]. A solution of β-carotene was prepared by dissolving 2 mg of β-carotene in 10 mL of chloroform.Two milliliters of the solution was then transferred into a boiling flas containing 20 mg linoleic acid and 200 mg Tween 40.Chloroform was removed using a rotary evaporator and 50 mL of distilled water was slowly added. Aliquots of the emulsion(4.8 mL) were transferred into different test tubes containing 0.2 mL of samples in methanol.These tubes were incubated at 50◦C in a water bath.As soon as the emulsion was added to each tube,the zero time absorbance was measured at 470 nm using a spectrophotometer.Absorbance readings were then recorded at 20-min intervals.Antioxidant activity was calculated using Eq.(1).All experiments were carried out in triplicate and the results are expressed as the mean±SD of three determinations.
2.12. Statistical analysis
All assays were conducted in triplicate. Statistical analyses were performed with SPSS 16.0 for an analysis of variance(ANOVA) followed by Duncan’s test. Differences atp<0.05 were considered significant
3. Results
3.1. Isolation and structure identification
The methanol-soluble fraction in the methanolic extract ofQ.phillyraeoideswas fractionated using silica gel column chromatography and followed by recrystallization to give compound 1, while Sephadex LH-20 column chromatography led to the isolation of condensed tannin fractions 2–6(Fig.1).
Compound 1: A white buff powder; melting point:283–285◦C; HRFAB-MS [M+H]+:m/z577.8562 for C35H60O6; EI-MS,m/z414 ([M-C6H10O5]+). Its FABMS and EI-MS properties were consistent with those of β-sitosterol-D-glucoside [21,22]. Thus, compound 1 was identifie as β-sitosterol-D-glucoside.The acid hydrolysis of 1 resulted in β-sitosterol and D-glucose.The results of hydrolysis were confirme using thin layer chromatography with an available standard while β-sitosterol was further confirme using gas chromatography analysis.The GC chromatogram of β-sitosterol is shown in Fig.2.
Fractions 2–6:light brown powders.The acid-butanol assay of fractions 2–6 resulted in red colored anthocyanidin, which indicated that the fractions were condensed tannins [23]. The retention time of fractions 2–6 on GPC were 6.81, 12.78,15.36,10.55,and 15.87 min,respectively,therefore the molecular weights of fractions 2–6 were 5700, 4200, 3500, 4700 and 3400 g/mol, respectively, which were estimated using polystyrene as the standard. The TMS derivatives of the thiolysis products of fractions 2–6 were analyzed using GC–MS and resulted in catechin (TMS derivatives, EI-MS,m/z: 650)and epicatechin (TMS derivatives,m/z: 650) as the terminal
Fig.1. Structures of isolated constituents:(a)β-Sitosterol-D-glucoside(1);(b)Schematic representation of condensed tannin fractions with n=17(2),n=12(3),n=10(4),n=14(5),and n=9(6).
units. Benzylthiocatechin and benzylthioepicatechin were also detected and consistent with previous finding [24]. The GC chromatogram of the typical thiolysis products of fractions 2–6 is shown in Fig.3.
3.2. α-Glucosidase inhibitor activities and their kinetic inhibition of α-glucosidase activity
The IC50values for the α-glucosidase inhibitory activities of the isolated constituents and quercetin as the standard are summarized in Table 1. The condensed tannin fractions (2–6) had highα-glucosidaseIC50valuesintherangeof2.60–3.14 μg/mL,and no significan difference was observed from that of quercetin as the standard (P<0.05) with an IC50value of 4.81 μg/mL.Compound 1 showed moderate inhibitory activity against αglucosidase with an IC50value of 118.8 μg/mL, which was significantl lower than those of quercetin and the other isolated fractions.
The inhibitory mechanisms of the isolated constituents and quercetin using Lineweaver–Burk plots are shown in Fig.4.The values for the inhibition constants(Ki)are given in Table 1.
3.3. Antioxidant activity
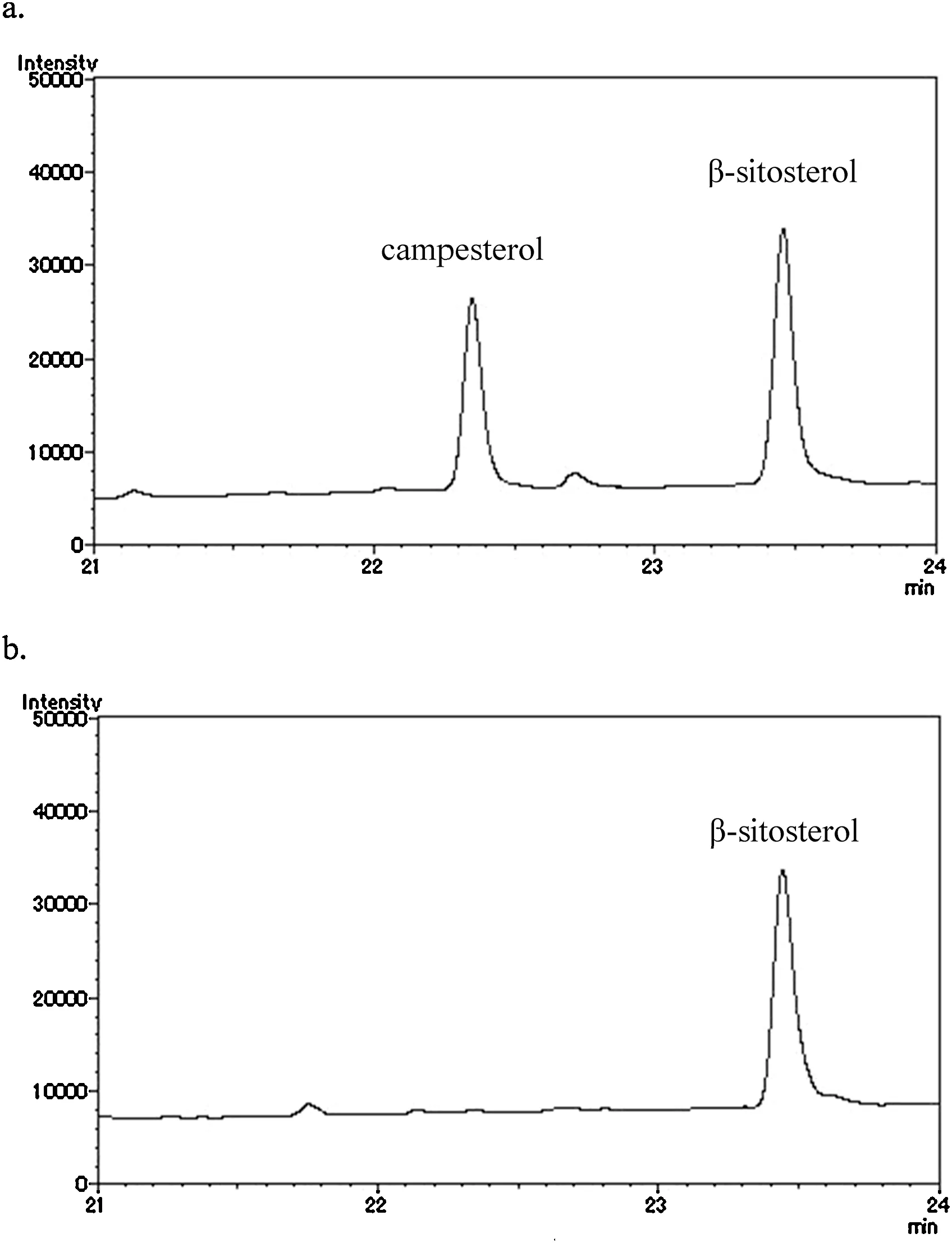
Fig. 2. GC chromatograms of (a) the standard: β-sitosterol, rt: 23.4 min and campesterol, rt: 22.3 min; (b) β-sitosterol resulting from the acid hydrolysis of 1.
The antioxidant activities of the six isolated constituents were determined using several assays:DPPH free radical scavenging activity,hydrogen peroxide radical scavenging activity,reducing power,and β-carotene-linoleate model assays.The results of the DPPH and hydrogen peroxide radical scavenging activity assays of the isolated constituents are presented in Table 2.
The scavenging capacities of the isolated constituents in the DPPH free radical scavenging activity assay ranged between 9.34 and>500 μg/mL.Of the isolated constituents,the highest DPPH scavenging capacity was exhibited by fraction 5,followed by fractions 3,2,6,and 4 with IC50values of 9.34,10.53, 10.84, 12.98, and 13.12 μg/mL, respectively. Quercetin and gallic acid were used as positive controls in this experiment and had IC50values of 8.45 and 3.41 μg/mL, respectively.The IC50values of fractions 5, 2, and 3 were not statistically significan different from that of quercetin as the standard,but were statistically significan lower than that of gallic acid(Table 1) atP<0.05 in a statistical analysis with ANOVA followed by Duncan’s test.The weakest activity was observed for compound 1 with an IC50value of>500 μg/mL in the DPPH free radical scavenging activity assay, which was significantl lower than those of the standard and other isolated fractions.The present results suggested that the isolated constituents reduced the DPPH radical to corresponding hydrazine and,thus, showed the potential of constituents isolated fromQ.phillyraeoidesto be better sources of antioxidants.

Table 1 α-Glucosidase inhibitory activities,inhibition constants(Ki value),and modes of compound 1 and fractions 2–6 from Q.phillyraeoides leaves againstS.cerevisiae α-glucosidase.
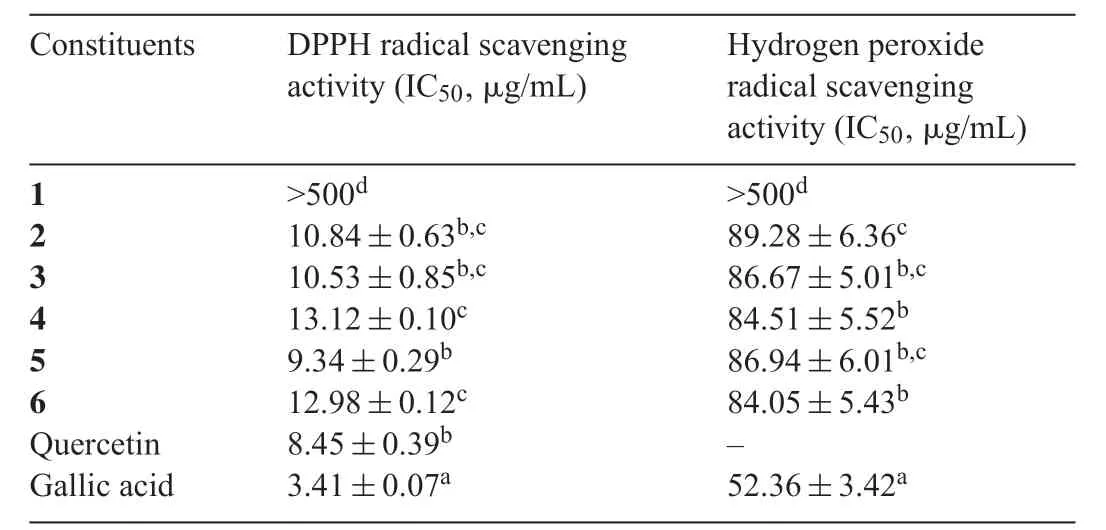
Table 2 Antioxidant activities of isolated compound 1 and fractions 2–6 using DPPH and hydrogen peroxide radical scavenging activities.
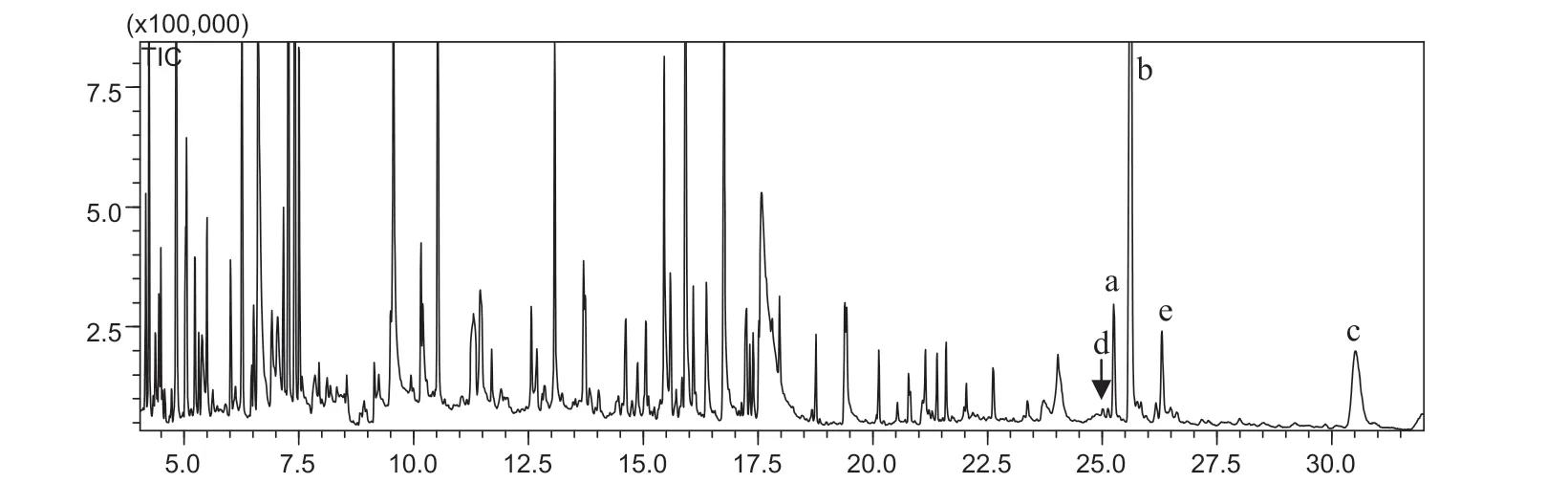
Fig.3. GC chromatogram of the typical thiolysis products of condensed tannin fractions 2–6:(a)catechin,(b)epicatechin,(c)benzylthioepicatechin,(d)benzylthiocatechin isomer,and(e)benzylthioepicatechin isomer.
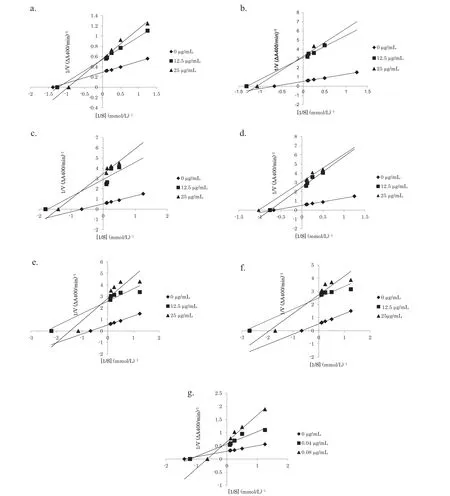
Fig. 4. Lineweaver–Burk plots of compound 1 (a), and fractions 2 (b), 3 (c), 4 (d), 5 (e), 6 (f), and quercetin (g) against S. cerevisiae α-glucosidase at different concentrations of p-NPG.
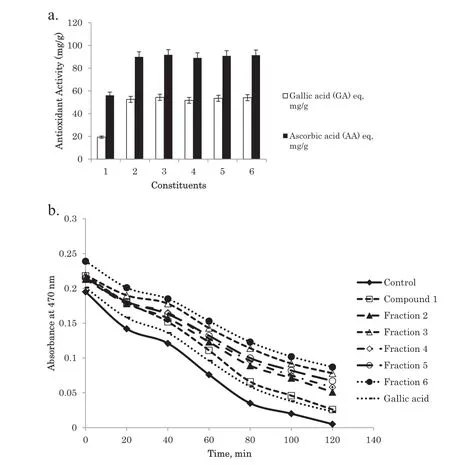
Fig. 5. Antioxidant activities of compound 1 and fractions 2–6 from Q.phillyraeoides:(a)in the reducing power assay which was equivalent to gallic acid(GA)and ascorbic acid(AA).(b)In the β-carotene-linoleate model system at 40 μg/mL with gallic acid as the standard.Control was β-carotene-linoleate solution without addition of antioxidant constituents.
The scavenging abilities of the isolated constituents on hydrogenperoxideareshowninTable2andwerecomparedwithgallic acid as the standard.Fraction 6 exhibited the highest scavenging activity(IC50)of 84.05 μg/mL,followed by fractions 4,3,5,2,and compound 1 with IC50values of 84.51,86.67,86.94,89.28, and >500 μg/mL, while gallic acid as the standard had scavenging activity of 52.36 μg/mL.Based on a statistical analysis,no significan differences were observed in the IC50values of fractions 2–6.This result is in accordance with those obtained for the DPPH scavenging test of fractions 2–6,which had high antioxidant activities.
The results obtained for the isolated constituents in the reducing power assay are depicted in Fig. 5a. The reducing power assaywas usedtodetermine thereducingpotential(Fe3+toFe2+)of the isolated constituents.The results indicated that fractions 2–6 actively reduced Fe3+to Fe2+with values of 52.59,54.35,51.72,53.47,and 54.05 mg/g(gallic acid equivalent)and 89.94,91.72,89.05,90.83,and 91.42 mg/g(ascorbic acid equivalent),respectively, while compound 1 showed the lowest activity at 19.34 mg/g (gallic acid equivalent) and 56.16 mg/g (ascorbic acid equivalent). This result is in accordance with the results of the DPPH and hydrogen peroxide radical scavenging assays.
The abilities of the isolated constituents to prevent the oxidation of the β-carotene-linoleate system was found to be higher than that of gallic acid as the standard and the results are summarized in Fig.5b,which showed that fraction 6 exhibited the strongest ability to prevent β-carotene bleaching, followed by fractions 3,5,4,and 2.Overall,the abilities of fractions 2–6 were stronger than that of gallic acid as the standard while compound 1 was found to have the weakest ability to prevent β-carotene bleaching.
4. Discussion
Compound 1 was isolated as a minor component in the methanol soluble fraction using gradientn-hexane,ethyl acetate,and methanol as solvents.The further acid hydrolysis of compound 1 resulted in β-sitosterol and D-glucose, as confirme by TLC and compared with standard samples. The resulted β-sitosterol was also further confirme using gas chromatography. Based on these results, compound 1 was identifie as β-sitosterol-D-glucoside.
Fractions 2–6 were major components in the methanol soluble fraction and were identifie as condensed tannins with estimated molecular weights in the range of 3400–5700 g/mol.Using a thiolysis reaction, the terminal units of fractions 2–6 were released as catechin and epicatechin,which was confirme by a GC–MS analysis.
The present study was conducted in order to evaluate the antidiabetic and antioxidant potentials of constituents isolated fromQ.phillyraeoides.Antidiabetic properties were assessed in termsoftheabilitytoinhibitanintestinalcarbohydrate-digesting enzyme,namely α-glucosidase.α-Glucosidase inhibitors delay theactivityofthisenzymeandalsotheformationofglucosefrom the hydrolysis of carbohydrates, thereby lowering the amount of glucose available for absorption in the intestine; therefore,they play a significan role as chemotherapeutic agents for noninsulin-dependent DM. Acarbose, miglitol, and voglibose are some examples of α-glucosidase inhibitors presently being used in the primary treatment of type 2 DM[25].However,since they lack specificit in their action and cause several side effects,safer anti-hyperglycemic agents are needed.Effective and safe α-glucosidase inhibitors from nature have been sought in the development of physiological functional food or constituents for antidiabetic therapy[26].We investigated the inhibitory activities of constituents isolated from the leaves ofQ.phillyraeoidesagainst α-glucosidase fromS. cerevisiae.p-NPG was used as the substrate and the yellow color of the enzyme’s degradation product,p-nitrophenol,was produced and measured using a spectrophotometer. Quercetin was used as a positive control based on a previous study in which phenolic compounds exhibited stronger inhibitory effects on α-glucosidase than acarbose[27].
The α-glucosidase inhibitory activities of fractions 2–6 were higher than that of compound 1, and this may have been because they consisted of more hydroxyl groups.The removal of hydroxyls from fl vonoids has been shown to decrease αglucosidase inhibitory activity[28].In this study,compound 1 showed moderate activity as an α-glucosidase inhibitor in this study using an enzyme fromS.cerevisiae.A previous study by Ivorra et al.,1988[29],on normoglycemic and hyperglycemic rats revealed that oral treatments with β-sitosterol glucoside isolated from the aerial part ofCentaurea seridisL. var. maritime Lge increased fasting plasma insulin levels. β-Sitosterol glucoside is considered to act by increasing the insulin circulation levels, which was attributable due to their aglycone,β-sitosterol. The higher α-glucosidase inhibitory activities of fractions 2–6 were also in accordance with condensed tannins extracted from the amaranth grain, finge millet, fiel bean,sunfl wer seeds,drumstick,and amaranth leaves[30].Previous studies reported the ability of tannins to inhibit the activity of α-glucosidase. Tannins isolated from tea extracts were found to possess insulin-enhancing properties and regulate hepatic glucose output [31]. Another study revealed that tannins were effective as inhibitors of α-glucosidase activity,similar to synthetic inhibitors such as acarbose and voglibose, which are already being used in the treatment of non-insulin-dependent DM[32].The results of the present study showed the potential ofQ.phillyraeoidesA.Gray as a rich source of natural antidiabetic medicine. These constituents may be employed as lead constituents for potentially new antidiabetic medicines derived from plants.
The inhibitory mechanisms of the isolated constituents were analyzed further using Lineweaver–Burk plots(Fig.4).A substrate (p-NPG) with increasingly higher concentrations was treated with an α-glucosidase enzyme with and without the isolated constituents as inhibitors.The results obtained showed that all of the isolated constituents and quercetin as the standard exhibited a mixed type of inhibition(Table 1).Mixed inhibition is a mode of enzyme inhibition in which the inhibitor binds to the enzyme even if it has already bound to the substrate.Furthermore,in mixed inhibition enzyme kinetics,inhibition occurs as a result of the inhibitor binding to a site other than the active site on the enzyme,known as an allosteric site[33].However,further studies such as a molecular docking approach are required to confir the interaction between the enzyme and substrate.
DPPH is a relatively stable organic radical and it has been widely used to determine the antioxidant activities of natural compounds in an easy, rapid, and sensitive manner [34]. The DPPH alcohol solution is deep purple with an absorption peak at 517 nm, and becomes yellow in the presence of a radical scavenger in the system and when the odd electron of nitrogen in DPPH is paired. In the DPPH scavenging assay, antioxidant activity was indicated by decreasing in absorbance as the DPPH radical received an electron or hydrogen radical from an antioxidant compound making a stable molecule[35].Fractions 2–6 exhibited significan scavenging activities against DPPH free radicals (Table 2). Some previous studies demonstrated the potentially significan biological effects of tannins such as their antioxidant or radical scavenging activitiesin vitro[36]and antidiabetic properties[30,31].The antioxidant activities of tannins have been attributed to their free radical and reactive oxygen species-scavenging properties,as well as the chelation of transition metal ions that initialize the oxidation process[37].Previous finding by Kunyanga et al.,2011[30]showed that the highest condensed tannin content present in the acetonic extract ofvariousfoodsgrowninKenyaexhibitedthehighestDPPHfree radical scavenging activity.Another study reported that tannins extracted from plants exhibited strong DPPH scavenging activity[38,39].Tannins are considered to be superior antioxidants because their oxidation may lead to oligomerizationviaphenolic coupling and increases in the number of reactive sites[40].On the other hand, compound 1 exhibited weaker activity against DPPH radicals. The evaluation of antioxidant activity using a free-radical scavenging assay may provide information on the ability of an antioxidant to prevent radical species from attacking proteins,fatty acids,DNA,amino acids,and sugar in biological or food systems.
The scavenging abilities of compound 1 and fractions 2–6 on hydrogen peroxide need to be evaluated(Table 2)because even though it is not very reactive in human cells,hydrogen peroxide sometimes is toxic because it gives rise to the hydroxyl radical in cells[41].Thus,antioxidants that remove hydrogen peroxide are important in biological and food systems.
The reducing power assay measures the ability of antioxidants to reduce the ferric(Fe3+)ion to ferrous(Fe2+)ion through the donation of an electron,that can be monitored at 700 nm.The ability of an antioxidant to reduce the ferric ion to ferrous ion is an indication of its ability to act as a pro-oxidant in a biological or food system.In the present study,we used gallic acid and ascorbic acid equivalents to determine reducing power abilities.The higher the reducing power value,the greater the reducing power of the tested constituents and, thus, the higher the antioxidant activity.The reducing powers of the isolated constituents ranged between 19.34 and 54.35 μg/mL in gallic acid equivalents and between 56.16 and 91.72 μg/mL in ascorbic acid equivalents(Fig.5a).These potential reducing powers may have been due to the presence of the dihydroxy type of benzene derivatives,catechin and epicatechin,which are integral parts of condensed tannins.The reducing powers of bioactive constituents including the high molecular weight tannins extracted from the peanut hulls and stem bark of Indian laburnum have been closely linked to strong antioxidant activities[39].
The β-carotene-linoleate bleaching assay measures the ability of an antioxidant to inhibit lipid peroxidation. The β-carotene-linoleate bleaching assay was conducted because food generally consists of a lipid and water system with some emulsifie. Therefore, the aqueous emulsion system of β-carotene and linoleic acid was used to evaluate the antioxidant activities of the isolated constituents. The free peroxy radical in this system was formed when oxidized linoleic acid attacked β-carotene molecules, which consequently underwent rapid decolorization.The rate of bleaching of the β-carotene solution was measured by the difference between the initial reading in spectral absorbance at 470 nm at time 0 min and after 120 min.Antioxidant activity was expressed as percent inhibition relative to the control. The results obtained showed that most of the investigated constituents efficientl inhibited the oxidation of emulsifie linoleic acid and, as a result, inhibited β-carotene bleaching. The antioxidant activities of compound 1 and fractions 2–6 fromQ.phillyraeoidesat 40 μg/mL in the β-carotene linoleate model system resulted in fraction 6 having the strongest ability to protect against β-carotene bleaching, followed by fractions 3, 5, 4, 2, and compound 1, which still retained antioxidant activities of 20.01%, 25.78%, 22.10%, 18.42%,14.73%,and 1.05%,respectively,after 120 min of the assay.The results obtained for fractions 2–6 were higher than that of gallic acid as the standard,which had an antioxidant ability of 6.32%.A comparison of antioxidant activities measured with the four methods,i.e., DPPH radical scavenging, hydrogen peroxide radical scavenging, reducing power, and β-carotene-linoleate bleaching assays,demonstrated that all the isolated constituents showed similar results among the four methods.Therefore,we concluded that all these methods were consistent with each other for evaluating the antioxidant activities of isolated constituents from the leaves ofQ. phillyraeoides. Antioxidants have been reported to provide synergistic benefit in the treatment of DM because of their insulin-enhancing potentials [42]. To the best of our knowledge, this study is the firs reported work on the bioassay-guided isolation of active constituents from the leaves ofQ. phillyraeoidesthrough evaluation of both their α-glucosidase inhibitor and antioxidant activities.
5. Conclusions
β-Sitosterol-D-glucoside(1)and fi e condensed tannin fractions (2, 3, 4, 5, and 6) were isolated from the leaves ofQ. phillyraeoides. The α-glucosidase inhibitory and antioxidant activities of the isolated constituents were investigated.Four antioxidant assays were successfully conducted in order to evaluate the antioxidant activities of the plant extracts,with similar results being obtained. Of the isolated constituents, condensed tannin fractions (2–6) showed potent α-glucosidase inhibitory activities and antioxidant activities,while β-sitosterol-D-glucoside(1)showed moderate inhibitory activity against α-glucosidase. These constituents may be employed as lead constituents for potentially new antidiabetic medicine and antioxidants derived from plants. The results of the present study showed the potential ofQ.phillyraeoidesas a rich source of natural antioxidants and antidiabetic medicine.
Conflict of interest
We declare that there is no conflic of interest.
杂志排行
食品科学与人类健康(英文)的其它文章
- Moringa oleifera:A review on nutritive importance and its medicinal application
- Antioxidant,antimicrobial and cytotoxic potential of condensed tannins from Leucaena leucocephala hybrid-Rendang
- Protective effect of rhizome extracts of the herb,vacha(Acorus calamus)against oxidative damage:An in vivo and in vitro study
- In vitro neuroprotective potentials of aqueous and methanol extracts from Heinsia crinita leaves
- The protective effect of dietary fl vonoid fraction from Acanthophora spicifera on streptozotocin induced oxidative stress in diabetic rats
