Hemicellulose in corn straw:Extracted fromalkali solution and produced 5-hydroxymethyl furfural in HCOOH/HCOONa buffer solution☆
2016-06-12YanLiHongxianFanXueqingYuSongmeiZhangGangLi
Yan Li,Hongxian Fan,Xueqing Yu,Songmei Zhang,Gang Li*
Hebei Provincial Key Lab of Green Chemical Technology and High Ef fi cient Energy Saving,School of Chemical Engineering and Technology,Hebei University of Technology,Tianjin 300130,China
1.Introduction
Lignocellulosic biomass is a renewable and abundant resource which has great potential to be converted to chemical feedstocks in the future.The main components in the lignocellulosic biomass are cellulose(30%–50%),hemicellulose(20%–40%)and lignin(10%–24%),depending on species[1].The three essential components in the biomass are of extensive applications.Cellulose could be used as feedstock for oxygenated materials or even be reduced to give petroleum-like starting materials.Lignin could be used as a source for aromatic compounds if it can be depolymerised in a controlled fashion.In addition,hemicellulose in corn straw is an amorphous heteropolysaccharide consisting of hexose or pentose units,such as mannans,xylans,arabinans and galactans[2].Due to the compositional complexity of hemicellulose,the hydrolysis reaction is constituted of parallel paths that lead to a complex mixture of sugars(xylose,glucose,arabinose,and mannose)and organic acids,such as formic acid,acetic acid and levulinic acid[3].One possible structure for the hemicellulose[4]in corn straw was illustrated in Fig.1.
However,due to the complexity of hemicellulose structure,limited research has been reported on the degradation of hemicellulose.In tradition,considerable efforts had been devoted to the conversion of cellulose or a few hexoses into HMF,which emerged as promising options to replace fossil fuel-based organics for the production of valuable biofuels and chemicals[5],such as N,N-dimethylformamide[6],5-ethoxymethylfurfural[7],and levulinic acid[8].Antal and Mok used H2SO4as a catalyst to degrade fructose in subcritical water at 250°C,and achieved HMF yield of 53%[9].Jianget al.found that more than 69.2%yield of HMF could be achieved at 170°C for 70 min in the dehydration of sugars in the aqueous/butanol media,which was enhanced by using formic acid[10].Pandeyet al.carried out a continuous dehydration of D-glucose into HMF under mild conditions,using SO3H-functionalized acidic ionic liquids as catalysts and H2O-4-methyl-2-pentanone biphasic system as solvent[11].Herein reported a new reaction pathway in polar aprotic solvents(i.e.THF)without the presence of water to produce HMF from cellulose under mild reaction conditions(140–190 °C and 5%H2SO4)and gained the highest HMF yield of 44%[12].Furthermore,Jae-An Chun revealed that using HCl–CrCl3as co-catalysts could efficiently promote the conversion of starch to HMF and the yield was as high as 73%[13].
Lignocellulosic biomass has a very strong resistance to microbial and enzymatic deconstruction due to many factors such as the crystallinity of cellulose,cell wall polymer chemistry and particle size[14].Therefore,pretreating these biomass materials or separating and extracting more pure components by chemical processes might lead to easy ways for subsequent degradation.After the pretreatment,the hemicellulose derived from the biomass such as corn straw would lead to a high potential application.Hence,in this work,hemicellulose which was extracted from corn straw was chosen as our main raw material.Then it was converted into HMF in the acidic buffer solution of formic acid/sodium formate buffer system.The buffer solution of formic acid and sodium formate not only provides a suitable reaction media but also act as an effective catalyst with the advantages as follows:
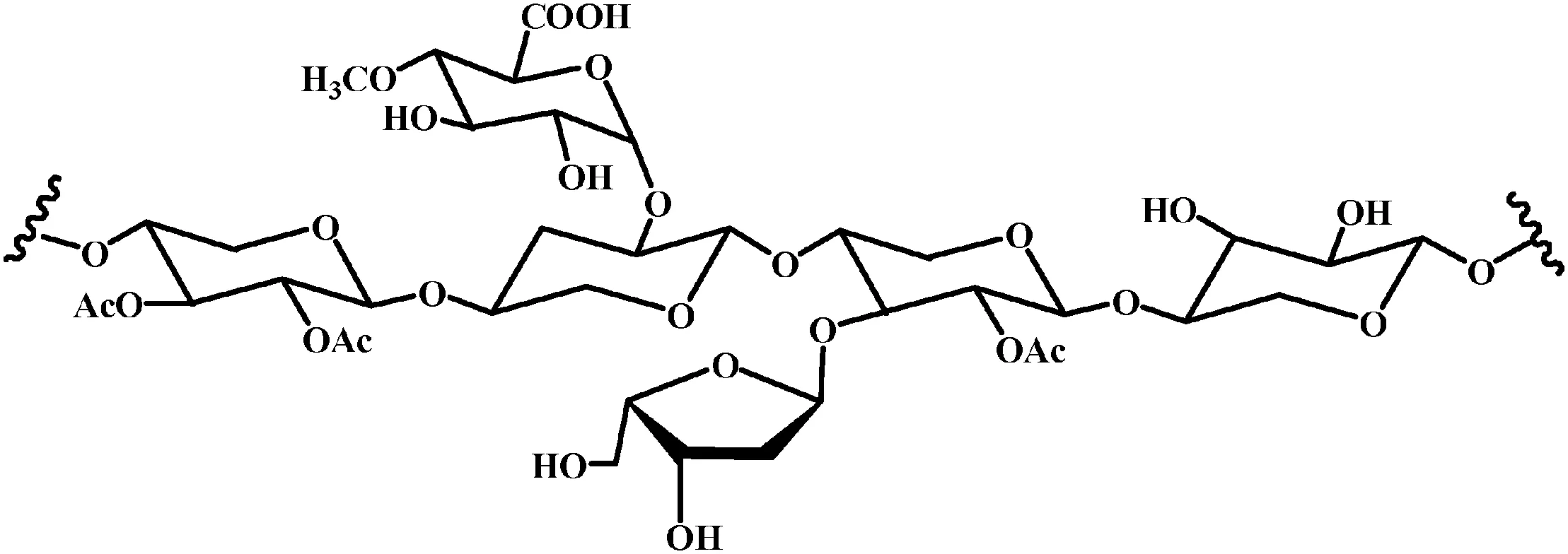
Fig.1.The structure of hemicellulose.
①Using the acid buffer solution with precise pHvalue could improve the catalytic selectivity of hemicellulose to the preparation of HMF;
②Choosing the buffer solution might avoid excessive using of organic acids so as to reduce the corrosion on equipments;
③This method turned out to be an affordable way to save economy cost,and the development of process conditions was thought to be practical in applications.
Since the buffer system has advantages to maintain a constant and specific pH than other solutions,the degradation process is expected to be stable.The aim of the study was to investigate the degradation of hemicellulose in a buffer solution and to explore the optimal condition including reaction temperature,time and pH to achieve the maximum yield of HMF.
2.Experimental
2.1.Materials
Corn straw used in this study was obtained from the experimental farm of Hebei Academy of Agriculture and Forestry,China.It was oven dried at 60°C for 24 h after deionized water was washed.Then it was ball-milled and screened after it was chopped into small pieces to achieve a size of less than 0.25 mm and larger than 0.18 mm prior to the hydrolysis.Methyl alcohol(HPLC Grade)and acetonitrile(HPLC Grade)were supplied by Concord Technology Co.Ltd.,China.The rest of the chemicals used in the experiments were of analytical grades and purchased from Tianjin Kermel Chemical Reagents Co.Ltd.,China.Ultrapure water was prepared in a lab by Ultrapure Water Polishing System.
2.2.Optimization the extraction of cellulose,hemicellulose in corn straw
The corn straw powder pretreated above was soaked in 1.5 mol·L-1aqueous NaOH solution with a 1:10 powder to extractant ratio(g·ml-1).The dispersions were allowed to stir gently for 4 h at 55 °C in a glass beaker and then filtered to get the filtrate.The residue was filtered off and washed thoroughly with water until the filtrate was neutral,and then dried in the oven at 50°C for 16 h.The filter liquor was neutralized to pH=6–7 with 10 vol%HCl,and the solubilized hemicelluloses were isolated by precipitation of the concentrated filtrates with four volumes of 95 vol%ethanol.After filtration,the isolated hemicellulose was thoroughly washed with 70 vol%ethanol and dried in vacuum for 24 h.The alkali lignin was obtained by precipitation at pH=1–2 adjusted with 10 vol%HCl from the corresponding supernatants after evaporation of ethanol.The isolated lignin preparations were purified by washing with acidified water(pH=2.0),and then freeze-dried.The ashes in corn straw were calcinated in the muffle furnace(Shenyang,RJM-28-10,China)at500°C for200 min.All the contents of cellulose lignin and hemicellulose represented the mean of at least triplicate.
In this process,different conditions including temperature,extraction time,solid-to-liquid ratio,precipitants,and the concentration of NaOH solution were changed to achieve the maximum of hemicellulose.
2.3.Molecular weight distribution and FT-IR characterization of hemicellulose
The molecular weight of soluble hemicellulose was determined by gel permeation chromatography(GPC)of the high performance liquid chromatography system(HPLC)(Shimadzu LC-20AD,Japan)equipped with a Ultrahydrogel™ 250(7.8 mm× 300 mm,Waters)column.The temperature of column compartment was set to 65°C,the mobile phase was ultrapure water at a flow rate of 0.8 ml·min-1,and injecting sample solution of20μlin volume was run for 15 min.The recommended maximum pressure for this column was 20 MPa,and all the experiments were conducted in accordance with this upper limit.All the molecular weight distribution and related polymer-relevant parameters were calculated by Shimdzu Chemstation GPC add-on software.
The spectra of hemicellulose before and after degradation were characterized by Fourier transform infrared spectroscopy(FT-IR)(Bruker,Vector 22,Germany).The progress was carried out by a diffuse reflectance accessory with KBr as standard.For sample preparation about 100 mg of KBr and with 1 mg of sample were ground in an agate mortar.The sample mixture was scanned in the middle infrared range from 4000 cm-1to 500 cm-1at a spectral resolution of 4 cm-1.
2.4.Degradation of hemicellulose under formic acid/sodium formate buffer solution and product analysis
The catalytic hydrolysis of hemicellulose was performed in a heavy iron batch autoclave equipped with a liner of polytetra fluoroethylene.As a typical run,0.3 g hemicellulose powder and 30.0 ml formic acid/sodium formate buffer solution of different pH were charged in the reactor,tightened the cover of autoclave and the mixture was then heated from 150 °C to 230 °C(interval of 20)with duration of 150–230 min(interval of 20)and the pH 0.6 to 1.0(interval of 0.1)in the oven.
Monosaccharide analysis was performed on the HPLC of Shimadzu Prominence System equipped with two LC-20AD quaternary gradient pumps,an evaporative light scattering detector(ELSD)(Alltech,LC-2000ES,USA).Pure air was used as the ELSD nebulizer gas(2.0 L·min-1),drift tube temperature was set to 95 °C.Separation was primarily achieved on an XBridge BEH Amide Column(4.6 mm×250 mm,3.5 μm,Waters)and the column oven maintained at 30 °C.The solvent gradient consisted of a linear increase in the amount of water in acetonitrile(H2O,vol%):0–1 min(10%),1–70 min(10%–50%).The flow rate was set to 1.0 ml·min-1,and injections of 5 μl were adopted.
The identification of product was conducted on the NMR Spectrometer(Bruker AV-400,Germany).The degradation liquid was repeatedly extracted with ethyl acetate three times,and then the extract solution was distilled and concentrated under reduced pressure at 28°C.The concentrated solution was purified through column chromatography with GF254 silica gel(eluant:the ratio of ethyl acetate to dichloromethane was 1:10).The purified product was identified by NMR,the main product of hemicellulose degradation was HMF,and its NMR data was as follows:1H NMR(400 MHz,CDCl3):δ9.52(s,1H,CHO),7.32–7.23(d,1H,CH),6.51–6.52(d,1H,CH),4.68(s,2H,CH2),2.0(s,1H,OH).13C NMR(400 MHz,CDCl3):178.1,157.6,153.1,122.0,111.6,57.1.MS(70 eV)m/z(%):126(M+,58),124(12),109(10),97(100),81(8),69(36),53(17).
The content of the aqueous phase from the hemicellulose degradation process was analyzed by the same HPLC equipped with a SPDM20A UV detector and HRC-ODS C18 reversed-phase column(4.6 cm×25 cm,ShimPack)at 40°C constant column temperature.The mobile phase consisted of methanol and pure water with a volume portion of 23:77 with a flow rate of 1.0 ml·min-1.The main product HMF was quantified on UV chromatograms with a detector wavelength of 284 nm,and injections of 10 μl were adopted.
2.5.Determination of yields for products
The total amount of HMF was determined based on the calibration curves obtained from the standard compound by the mean of external standard method.And yields were calculated by the following equation:

3.Results and Discussion
3.1.Chemical composition of corn straw and extraction of hemicellulose
Alkali treatment roughages could dissolve lignin,silica and hemicellulose,while cellulose was not dissolved[15].The optimal extraction of the composition of the raw material(%on a dry basis)was shown in Table 1 which was obtained at 55 °C for 4 h by 1.5 mol·L-1NaOH solution with a solid to liquid ratio of 1:10.And corresponding yields were calculated by the equations below:


Table 1The composition of the raw material(%on a dry basis)
And the different conditions of hemicellulose extraction were presented in Table 2.The highest yield of hemicellulose was 34.16%,with the best condition of the reaction time of 4 h with a solid to liquid ratio of 1:10 at 55 °C in 1.5 mol·L-1NaOH solution.However,the content of hemicellulose in other lignocellulosic materials such as barley straw(27.00%)[9],rice straw(25.10%),and wheat straw(33.30%)[16],which showed differences of percentage varied from different materials.
Four different solvents methanol,ethanol,acetone,and tetrahydrofuran were adopted as precipitant.Table 2 also showed the differences of four reagents on hemicellulose yields and weight-average molecular weightwhich was tested by GPC method.For the little difference of four precipitants on theof corn straw hemicellulose,it could be basically concluded that the same hemicellulose was obtained by the above four solvents.Nevertheless,the yields of these results might determine the choice of solvent that ethanol precipitation showed the optimal hemicellulose yield of 34.16%.
3.2.Monosaccharides from hemicellulose and conversion to HMF
HPLC–ELSDmethod was selected and used in the present study as it is a simple and accurate way to analysis carbohydrate.In previous studies,lignocellulose biomass had been hydrolyzed under acid conditions to produce monosaccharides[17].The strong response of degraded solution by ELSD showed good retention features and baseline shape.In the present study,the extracted hemicellulose was degraded and five monosaccharides in the degradation solution were identified by comparing the retention time of the reference standards as shown in Fig.2.These mono-saccharides almost came from the cleavage of glycosidic bonds in hemicellulose of corn straw.In this process,the hemicellulose macromolecules were cut into smaller sugar units which were easy to be degraded into monosaccharides or small molecule compounds.
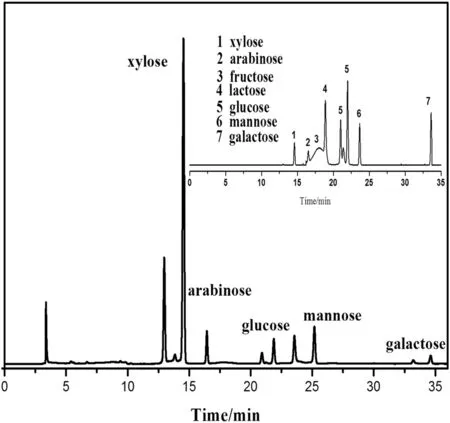
Fig.2.HPCL–ELSD chromatograms of monosaccharide from hemicellulose.

Table 2Different conditions of hemicellulose extraction
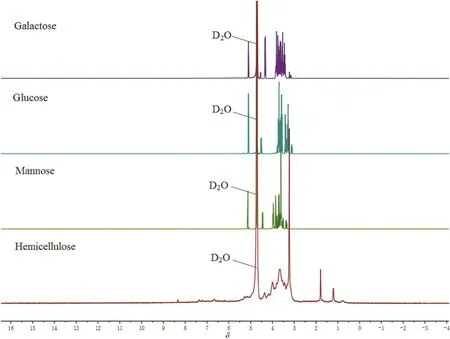
Fig.3.1H NMR comparison spectra of hemicellulose and hexoses.
However,there may be other sugars in this hydrolysis progress which were not detected from the unknown diffraction peaks in Fig.2.In addition,the corresponding disaccharide might also be produced in the degradation of hemicellulose.
The chromatograms of seven standard sugar mixtures are also shown as reference.
Fig.3 presented a group of comparable1HNMR of hemicellulose and hexose spectra in D2Oat25.0°C.The assignments of the1HNMRsignals for glucose proposed by Roslundet al.presented the proton signals for β-glucose at 3–4 and α-glucose at 3–4 and δ =5.21[18].It was obvious that hexoses presented characteristic peaks at 3–4 and the hemicellulose of corn straw also showed superposed peaks at this position,which implied that hemicellulose contained the similar structures with hexoses.
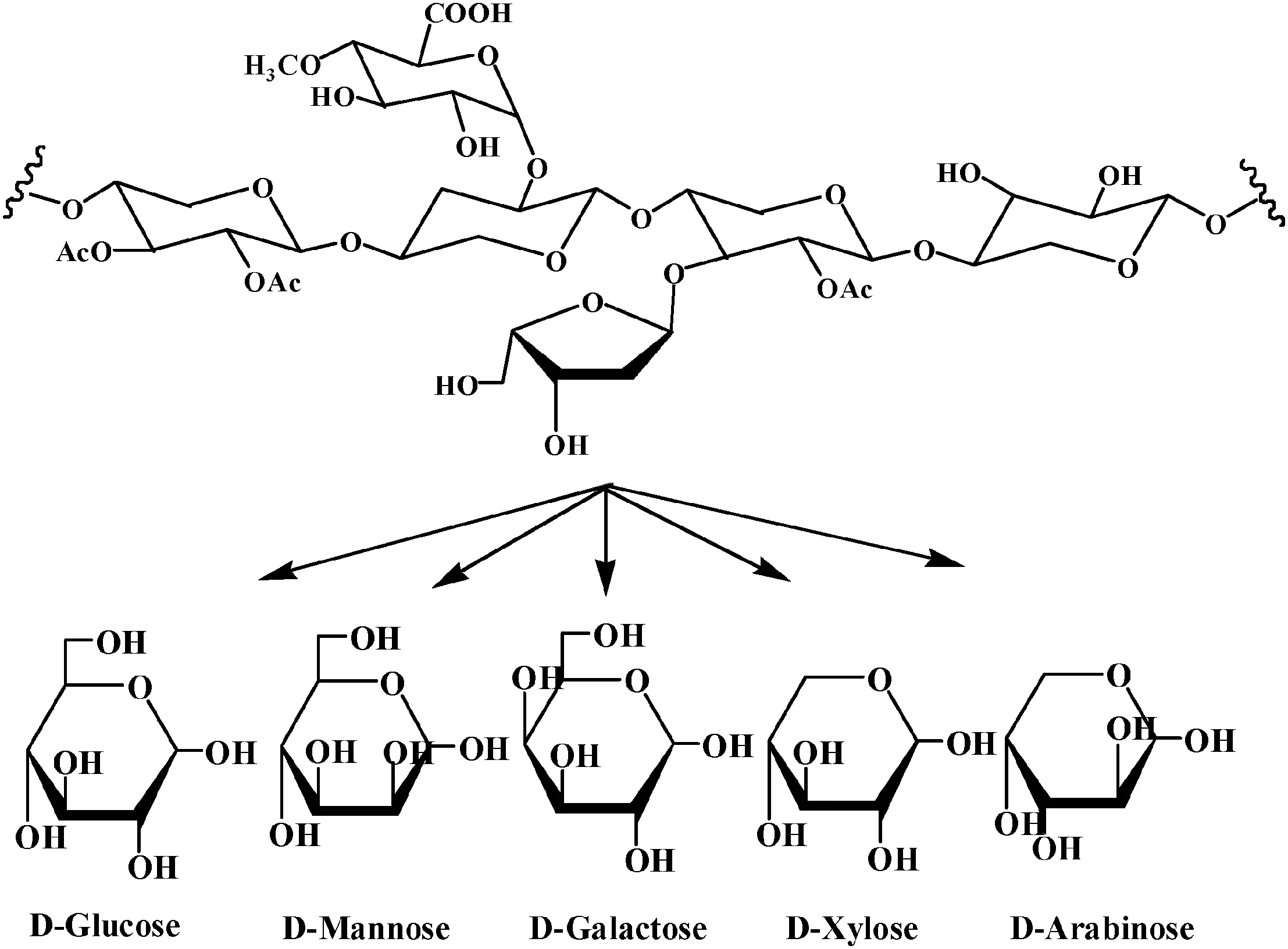
Fig.4.Monosaccharides from hemicellulose after degradation.
As far as the role ofhydrogen ion was concerned,the firststep tended to promote the fragmentation of the polymer chain in hemicellulose,in which produced glucan,xylan,arabinan,mannan,and galactosan.Secondly,the acid continued to release protons that broke the glycosidic bonds between the sugar monomers in the polymeric chains.The breaking of these bonds releases several compounds,mainly sugars such as glucose,mannose,galactose,xylose and arabinose[19].The result of degradation was illustrated in Fig.4.
The literature recorded a hypothesis for the mechanism of dehydration of hexoses to HMF.This acid catalyzed sequence proceeding through a 2,5-anhydride intermediate.Antalet al.[20]deduced that the acid catalyzed the reaction of hexoses to 2,5-anhydride intermediate,then HMF was formed by losing three water molecules as shown in Fig.5.
3.3.Structural characterization of hemicellulose and degradation liquid
FTIR spectroscopy was used to investigate the changes of glucosidic bond in hemicellulose before and after the decomposition process which were shown in Fig.6.The absorption band at 3439 cm-1was associated with the intermolecular and intramolecular O–H stretching vibration,mainly those on the monosaccharide residues[11].Enhancement in the intensity of this peak indicated that hydrogen bonds and glucosidic bond in hemicellulose were disrupted or the O–H stretching vibration in HMF.The peak at 2910 cm-1corresponded to the C–H stretching vibration of alkyl groups in aliphatic bonds of hemicellulose[12],and the relative absorption decreased noticeably meant that the methyl and methylene portions of hemicellulose were ruptured.The distinct appearance peak at 1735 cm-1was responsible for C–O stretching vibration ofO-acetyl groups which might belong to HMF.Band at 1249 cm-1corresponded to carboxyl groups and vibration of C–O[21].The absorption band at 1057 cm-1,which was due to C–O stretching at C-3 and C–O–C stretching at the β-(1,4)-glycosidic linkages originated from typical of xylans.Decrease of the peak at 890 cm-1which referred to the domain of β-glycosidic bonds between sugars[22]indicated the generation of smaller molecule.

Fig.6.FTIR spectra of hemicellulose before and after degradation.
3.4.The optimization of HMF yield from hemicellulose using HCOOH/HCOONa buffer solution
The dehydration of hemicellulose to HMF in HCOOH/HCOONa buffer solution was influenced by several parameters,including temperature,pH,reaction time and the result was illustrated in Fig.7.
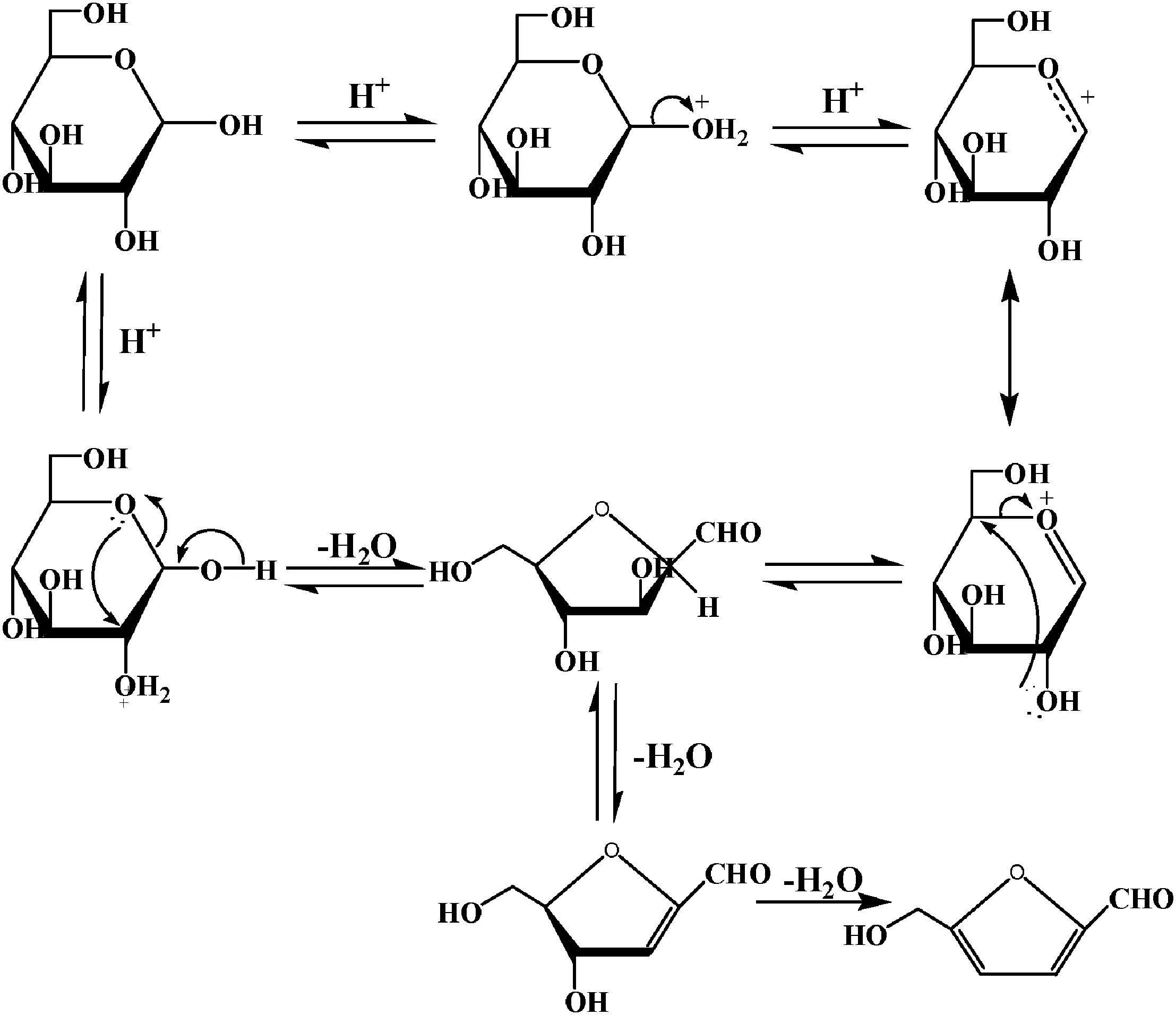
Fig.5.Mechanism of producing HMF.

Fig.7.Different conditions on hemicellulose degradation.
The effect of reaction temperature on the hemicellulose dehydration into HMF was studied,and the experiments were conducted at150,170,190,210,and 230°C in the condition of 190 min and pH=0.8.The increase in temperature was favorable for improving the initial yield of HMF to some extent and the optimal condition was obtained at 190°C for 190 min.However,when the temperature was higher than 210°C,the production yield decreased,this ought to be explained by the decomposition and polymerization reactions of both reactant and HMF just as what had been reported under normal conditions[23].On the basis of other researches,even though the rise in temperature would shorten the reaction time and improve HMF production efficiently,the yield decreased when the temperature exceeded at a certain value[24].Moreover,the color of the reaction mixture turned to deep brown from pale yellow at high temperature for long reaction period,which was attributed to the fact that long reaction time with high temperature might provide sufficient energy to bring about the side reactions,such as the condensation of HMF with intermediates of the pentose-to-HMF conversion,resinification reactions of HMF with itself[25],and fragmentation or decomposition reaction of pentose[26].
Next,the hemicellulose dehydration reaction time was varied from 150 to 230 min(interval of 20)to analyze the rate of HMF formation as a function of time under 190°C,pH=0.8.It was obvious that the yield of HMF showed quite low with short reaction time,only about 40%.Nevertheless,a conspicuous increase in reaction time after 170 min gave a beneficial effect on yields,giving about53%HMF.With the extended response time,further constant increase in reaction time up to 190 min under similar condition with the maximum HMF yield of 82%was possibly due to the preferential formation of soluble polymers[27].Owing to the existence of incompletely dehydrated intermediates in the dehydration during shorter time,the HMF yield was relatively poor.When the reaction time increased to 170 min,the result showed the favorable yield of HMF.The yield decreased with the extending reaction time which demonstrated that HMF was no longer the favored product[28].Thus,190°C and 190 min were selected as the appropriate reaction temperature and the reaction time for the dehydration.
Furthermore,we evaluated the influence of solution pH at 0.6,0.7,0.8,0.9,and 1.0 respectively on the conversion of hemicellulose into HMF at 190°C,190 min.The column chart showed that variations in pH strongly affected the HMF yields which obtained from hemicellulose.When the pH value of the reaction medium was 0.6,the HMF yield reached to the maximum of 87%.While,it resulted in a significant decline in HMF yield when the pH was lower than 0.9.It was significant for the effect of pH on HMF formation,and more HMF was gained at lower pH values[29].HMF was possibly oligomerized or polymerized to gain soluble polymeric byproducts at high pH values,which might be similar to what had been discussed under normal conditions[23].
3.5.Reusability of the HCOOH/HCOONa buffer solution catalyst
Table 3 summarized the catalytic performance of reused HCOOH/HCOONa buffer solution catalyst.When the hemicellulose washydrothermally degraded over the fresh catalyst,the HMF was 82%.Meanwhile the pH of buffer solution was 0.8 to 0.86.Then adding enough buffer solution to maintain the pH=0.80 and 30 ml in volume.In the second run,the pH was significantly decreased to 1.12 and the yield was 76%.The catalytic efficiency and yields were decreased with the increase of reaction times but the catalytic effect keeps in a good stand when supplementing excess buffer solution to guarantee the pH value.Hence,it was necessary to add appropriate dosage of fresh catalyst on the conversion of hemicellulose with the reused catalyst in order to reach almost the same catalytic performance.

Table 3Reusability of the HCOOH/HCOONa buffer solution catalyst
4.Conclusions
This study investigated the catalytic degradation of corn straw hemicellulose in HCOOH/HCOONa buffer solution to produce 5-hydroxymethyl furfural.The effect of operation conditions was examined and optimized for achieving the maximum yield of hemicellulose in the extraction and the maximum yield of HMF in the hemicellulose degradation.
In the extraction of hemicellulose from corn straw,alkaline solution was used and optimal conditions were found to be:reaction time of 4 h,solid to liquid of 1:10,extraction temperature of 55°C and sodium hydrate concentration of 1.5 mol·L-1.Under these conditions,hemicellulose extraction was 34.16%using ethanol as precipitant.
The optimized conditions for the hemicellulose degradation were found to be:the buffer solution pH of 0.8,operation temperature of 190°C and reaction time of 190 min.Under these conditions,the HMF yield was 82%.Although the yield was slightly lower than that of chemical synthesis and fermentation process,this degradation has greater potential for developing renewable chemicals in the future,due to the advantages of simpler process and easier operation procedure.
Acknowledgments
The authors would like to thank Professor Shusheng Pang,Department of Chemical and Process Engineering,University of Canterbury,for valuable discussions and linguistic revision.
[1]M.Anggraini,A.Kurniawan,K.O.Lu,et al.,Antibiotic detoxification from synthetic and real effluents using a novel MTAB surfactant-montmorillonite(organoclay)sorbent,RSC Adv.4(31)(2014)16298–16311.
[2]M.A.Paivi,S.Tapio,H.Bjarne,et al.,Synthesis of sugars by hydrolysis of hemicelluloses—A review.Chem.Rev.111(9)(2011)5638–5666.
[3]Y.Lu,Kinetic and mechanistic studies of a biomimetic catalyst for hemicellulosic biomass hydrolysis,ProQuest,2008.
[4]T.D.Matson,B.Katalin,A.V.Iretskii,et al.,One-pot catalytic conversion of cellulose and of woody biomass solids to liquid fuels,J.Am.Chem.Soc.133(35)(2011)14090–14097.
[5]W.Yang,A.Sen,One-step catalytic transformation of carbohydrates and cellulosic biomass to 2,5-dimethyltetrahydrofuran for liquid fuels,Chem Sus Chem3(5)(2010)597–603.
[6]M.Chatterjee,Hydrogenation of 5-hydroxymethylfurfural in supercritical carbon dioxide–water:A tunable approach to dimethylfuran selectivity,Green Chem.16(3)(2014)1543–1551.
[7]Y.Zu,P.Yang,J.Wang,et al.,Efficient production of the liquid fuel2,5-dimethylfuran from 5-hydroxymethylfurfural over Ru/Co3O4catalyst,Appl.Catal.B Environ.146(3)(2014)244–248.
[8]J.F.White,Top value-added chemicals from biomass volume II—Results of screening for potential candidates from biorefinery lignin,Biomass Fuels2(2007)263–275.
[9]M.J.Antal,W.S.L.Mok,G.N.Richards,Mechanism of formation of 5-(hydroxymethyl)-2-furaldehyde from d-fructose and sucrose,Carbohydr.Res.199(1)(1990)91–109.
[10]N.Jiang,R.Huang,W.Qi,et al.,Effect of formic acid on conversion of fructose to 5-hydroxymethylfurfural in aqueous/butanol media,Bioenergy Res.5(2)(2012)380–386.
[11]K.K.Pandey,A study of chemical structure of soft and hardwood and wood polymers by FTIR spectroscopy,J.Appl.Polym.Sci.71(12)(1999)1969–1975.
[12]R.Weingarten,A.Rodriguez-Beuerman,F.Cao,et al.,Selective conversion of cellulose to hydroxymethylfurfural in polar aprotic solvents,Chem Cat Chem6(8)(2014)2229–2234.
[13]J.A.Chun,J.W.Lee,Y.B.Yi,etal.,Direct conversion of starch to hydroxymethylfurfural in the presence of an ionic liquid with metal chloride,Tex.Dent.J.62(6)(2010)326–330.
[14]L.Yang,C.Jie,J.Mao,et al.,Sodium carbonate–sodium sulfite pretreatment for improving the enzymatic hydrolysis of rice straw,Ind.Crop.Prod.43(1)(2013)711–717.
[15]M.G.Jackson,Review article:The alkali treatment of straws,Anim.Feed Sci.Technol.2(2)(1977)105–130.
[16]F.R.Tao,C.Zhuang,Y.Z.Cui,etal.,Dehydration of glucose into 5-hydroxymethylfurfural in SO3H-functionalized ionic liquids,Chin.Chem.Lett.25(5)(2014)757–761.
[17]X.Liu,N.Ai,H.Zhang,et al.,Quantification of glucose,xylose,arabinose,furfural,and HMF in corncob hydrolysate by HPLC–PDA–ELSD,Carbohydr.Res.353(9)(2012)111–114.
[18]M.Roslund,P.Tahtinen,M.Niemitz,et al.,Complete assignments of the1H and13C chemical shifts and J(H,H)coupling constants in NMR spectra of D-glucopyranose and all D-glucopyranosyl–D-glucopyranosides,Carbohydr.Res.343(1)(2008)101–112.
[19]Q.Q.Wu,Y.L.Ma,X.Chang,et al.,Optimization and kinetic analysis on the sulfuric acid-catalyzed depolymerization of wheat straw,Carbohydr.Polym.129(2015)79–86.
[20]M.J.Antal,T.Leesomboon,W.S.Mok,et al.,Mechanism of formation of 2-furaldehyde from d-xylose,Carbohydr.Res.91(1991)71–85.
[21]D.Ferreira,A.Barros,M.A.Coimbra,et al.,Use of FT-IR spectroscopy to follow the effect of processing in cell wall polysaccharide extracts of a sun-dried pear,Carbohydr.Polym.45(2)(2001)175–182.
[22]T.Guo,X.Tong,C.Yi,et al.,Tin-catalyzed efficient conversion of carbohydrates for the production of 5-hydroxymethylfurfural in the presence of quaternary ammonium salts,Carbohydr.Res.370(14)(2013)33–37.
[23]F.Benvenuti,C.Carlini,P.Patrono,et al.,Heterogeneous zirconium and titanium catalysts for the selective synthesis of 5-hydroxymethyl-2-furaldehyde from carbohydrates,Appl.Catal.A Gen.193(1–2)(2000)147–153.
[24]A.Avci,B.C.Saha,G.J.Kennedy,et al.,High temperature dilute phosphoric acid pretreatment of corn stover for furfural and ethanol production,Ind.Crop.Prod.50(10)(2013)478–484.
[25]J.Q.Li,The chemistry and technology of furfural and its many by-products,Chem.Eng.J.81(1)(2001)338–339.
[26]R.Karinen,K.Vilonen,P.Niemelä,Biore fining:Heterogeneously catalyzed reactions of carbohydrates for the production of furfural and hydroxymethylfurfural,Chem Sus Chem4(8)(2011)1002–1016.
[27]I.Jiménez-Morales,M.Moreno-Recio,J.Santamaría-González,et al.,Mesoporous tantalum oxide as catalyst for dehydration of glucose to 5-hydroxymethylfurfural,Appl.Catal.B Environ.154–155(7)(2014)190–196.
[28]H.Liu,C.Hua,C.Song,et al.,Commercially available ammonium salt-catalyzed efficient dehydration of fructose to 5-hydroxymethylfurfural in ionic liquid,Inorg.Chim.Acta428(2015)32–36.
[29]P.Kavousi,H.Mirhosseini,H.Ghazali,et al.,Formation and reduction of 5-hydroxymethylfurfural at frying temperature in model system as a function of amino acid and sugar composition,Food Chem.182(2015)164–170.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- A comprehensive fractal char combustion model☆
- Kinetic effects of nanosecond discharge on ignition delay time☆
- Modification and sequential treatment of EU-1 zeolite in mild alkali and alkaline-acid conditions
- Catalytic kinetics of dimethyl ether one-step synthesis over CeO2–CaO–Pd/HZSM-5 catalyst in sulfur-containing syngas process☆
- Development of a bifurcation analysis approach based on gPROMS platform☆
- Investigation on molar heat capacity,standard molar enthalpy of combustion for guaiacol and acetyl guaiacol ester
