MCM-36 zeolites tailored with acidic ionic liquid to regulate adsorption properties of isobutane and 1-butene☆
2016-06-12HongxiaLiTaoZhangShaojunYuanShengweiTang
Hongxia Li,Tao Zhang,Shaojun Yuan,Shengwei Tang*
Multiphase Mass Transfer and Reaction Engineering Lab,College of Chemical Engineering,Sichuan University,Chengdu 610065,China
1.Introduction
Catalyst is very important to the chemical industry for the production of a wide variety of products,such as heavy commodity and fine chemicals.Acid catalyst is one of the most important areas in the catalysis field.A wide range of liquid phase industrial reactions use inorganic or mineral acids as catalysts.For example,H2SO4,HF,AlCl3and BF3are usually used as catalysts for Friedel–Crafts alkylations,acylations and sulfonylations,aromatic halogenations,nitrations,isomerizations and oligomerizations.However,these reagents are hazardous and corrosive in handling.They are also difficult and costly to separate and regenerate,and thus leading to a large amount of toxic and corrosive wastes[1].To circumvent the problems of the mineral acid catalysts,solid acid catalysts have been widely used in many reactions owing to their unique advantages,such as easy separation and recycle from the reaction medium[2].
For a heterogeneous catalytic reaction,the adsorption capacity of reactant on the catalyst active sites is a crucial factor to achieve high reaction efficiency.Furthermore,an ideal stoichiometric adsorption ratio of reactants is a prerequisite to obtain good selectivity.However,in the isoparaffin/olefin alkylation process,a preferential adsorption of olefin over isoparaffin on the solid acid catalyst usually takes place[3].The different adsorption capacities may lead to more side reactions,rapid deactivation of the catalyst and a low selectivity of objective product.Generally,the adsorption capacities of reactants on the catalyst active sites mainly depend on the physicochemical properties and surface features of the catalysts.For instance,polar reactants are readily adsorbed on the solid acid catalysts due to their intrinsic surface polarity[4,5].On the other hand,the pore size also has been found to affect the adsorption capacity through steric hindrances[6].Therefore,it is of great significance to seek for an effective way to modulate the relative adsorption capacities of reactants on the solid acid catalysts.
In recent years,more attentions have been paid on the functionalization of solid acid with specific surface features and microstructures.Shenet al.[7]modulated the hydrophobic/hydrophilic balance on SBA-15 catalyst by coupling the surface hydroxyl groups with ethoxytrimethylsilane.This led to a higher and steady paraffin concentration on the surface of catalyst,while restricting the adsorption of olefin.Oginoet al.[8]tuned the pore structures of carbon-based acid catalysts for the improvement of several liquid-phase reactions.Gücüyener et al.[9]reported a selective ethane adsorption on the metal–organic framework ZIF-7 through a gate-opening mechanism.The interactions between the adsorbate and the benzene rings in the narrow ZIF-7 windows dominate the adsorption process.Molecules with very similar sizes but different shapes can be discriminated by the gate-opening effect.
Grafting ionic liquids(ILs)on the support materials is a feasible way to regulate the surface features,microstructures,physicochemical and adsorption properties of catalysts[10–13].The structure of ILs has a significant effect on the adsorption properties of the materials supported by ionic liquid[14].For example,ionic liquids were loaded on the activated carbon[15]and silica gel particles[16]to adsorb SO2in air.Amino acid-based IL was immobilized onto activated carbons to separate CO2[17].Ionic liquids were also covalently conjugated on silica gels or Merrifield resins to remove thiophenic sulfur compounds from fuel[12,18].In addition,the delaminated zeolite ITQ-6 was also functionalized by grafting 3-aminopropyl,3-(methylamino)propyl,or 3-(phenylamino)propyl ligands for improved adsorption of CO2[19].
Our previous work demonstrated that MCM-22 zeolite immobilized with a dual acidic ionic liquid exhibited a significant modulation effect on the adsorption ratio of paraffin/olefin[20].However,the specific surface area and average pore volume of MCM-22 underwent an evident decrease thus resulting in a significant reduction of adsorptive capacity of adsorbates.To further address this problem,MCM-36 is selected as support instead of MCM-22 to provide a higher surface area and a larger pore size in this study.As schematically illustrated in Scheme 1,a novel acidic ionic liquid,1-butyl-3-(triethoxysilylpropyl)imidazolium hydrogen sulfate(i.e.,[BTPIm][HSO4]),was synthesized and further covalently conjugated on the MCM-36 zeolites.The successful immobilization of[BTPIm][HSO4]on the MCM-36 zeolite was confirmed by the Fourier Transform Infrared Spectroscopy(FT-IR),X-ray Photoelectron Spectroscopy(XPS)and Scanning Electron Microscopy(SEM),X-ray Diffraction(XRD),Thermal Gravity Analysis-Differential Thermal Gravity Analysis(TG–DTG)and N2adsorption–desorption measurement.The adsorption performance of[BTPIm][HSO4]-immobilizied MCM-36(defined as MCM-36-IL)was investigated by determining the adsorption capacity of isobutane and 1-butene on a home-made static adsorption equipment.
2.Experimental
2.1.Materials
The 3-chloropropyltriethoxysilane(99%)and 1-butylimidazole(99%)were purchased from Nuotai Chem.Co.(Shanghai,China).Hexadecyltrimethylammonium chloride(CTAC,99%)was obtained commercially from Kelong Chem.Co.(Chengdu,China).Tetrapropylammonium hydroxide(TPAOH,25%aqueous solution)was purchased from Tianjin Guangfu Fine Chemical Research Institute(Tianjin,China).Isobutane and 1-butene were obtained from Chengdu Haoyun Gas Co.LTD(Chengdu,China)with a purity of higher than 99.5%.Anhydrous ethanol and dichloromethane used in the synthesis of([BTPIm][HSO4])were obtained by a reduced-pressure distillation and stored over 5 Å molecular sieves.
2.2.Preparation of MCM-36
The MCM-22 precursor MCM-22(P)was synthesized according to the procedures described elsewhere[21].The wet cake of MCM-22(P)(25–30%solid),hexadecyltrimethylammonium chloride and tetrapropylammonium hydroxide(25%)mixed together with a relative mass ratio of 1:5.64:2.44:21.47(dried MCM-22(P):CTAC:pure TPAOH:H2O)in a flask,then the reaction was conducted under stirring(~500 r·min-1)at room temperature for 16 h.After filtration and washing to neutral state by deionized water,the swollen MCM-22 precursor(it was named as MCM-22-SW)was obtained.Then MCM-22-SW reacted with pillaring agent tetraethylorthosilicate with a relative mass ratio of1:10 at363 Kfor25 h under nitrogen atmosphere.After filtration and drying,the resulting solid were hydrolyzed in water with a mass ratio of 1:10 at 313 K for 6 h,and the solution pH value was controlled at about 8 by NaOH solution.After filtration,the solid was dried at room temperature overnight and then was calcined at 823 K for 8 h to obtain the product of Na-MCM-36.Na-MCM-36 was reacted with excess NH4NO3aqueous solution(1 mol/L)under continuously stirring at 353 K for 8 h.After filtration,drying and calcined in air atmosphere at 773 K for 6 h,the product of acidic H-MCM-36 was obtained.
2.3.Synthesis of ionic liquid[BTPIm][HSO4]
As showed in Fig.1 the synthesis of acidic ionic liquid[BTPIm][HSO4]was performed using a similar procedure described previously[22].Briefly,1-butylimidazole was first reacted with an equal molar amount of γ-chloropropyl triethoxysilane overnight at 383 K,and then the primary product was washed with a copious amount of diethyl ether thrice to obtain a light yellow transparent liquid(I),1-butyl-3-(triethoxysilylpropyl)imidazolium chloride.Subsequently,the liquid(I)was dissolved in the anhydrous dichloromethane and an equal molar of concentrated H2SO4was added dropwise at room temperature.The mixture was re fluxed at 323 K for 12 h.At the end of reaction,the solvent was removed by rotary evaporation,and a viscous yellow liquid(II),1-butyl-3-(triethoxysilylpropyl)imidazolium hydrogen sulfate,was finally obtained.The above experiments were all carried out under nitrogen atmosphere.The resultant[BTPIm][HSO4]ionic liquid was ascertained by the1H NMR measurement in the DMSO solvent.1H NMR(400 MHz,DMSO)of[BTPIm][HSO4](δ):0.62(2H SiCH2),0.95(3H CH3),1.21(9H CH3),1.38(2H,CH3–CH2),1.91(2HCH2–CH2–CH2),2.02(2HCH2–CH2–CH2),3.82(6HOCH2),4.38(4H NCH2),7.35(1H NCH),7.42(1H NCH),10.68(1H NCHN);the characteristic peaks of FT-IR(KBr,cm-1):2965,2867,1560,1459,1165.

Fig.1.The synthesis route of ionic liquid[BTPIm][HSO4]and the immobilization of ionic liquid on MCM-36.
2.4.Covalent conjugation of[BTPIm][HSO4]on the MCM-36
The loading of ionic liquid onto the solid support could be accomplished by either simple impregnation or covalent conjugation[23,24].As showed in Fig.1,the covalent conjugation was performed by self assembly reaction in this study for better stability in the catalytic reaction.Typically,a 2.0 g sample of[BTPIm][HSO4]ionic liquid was dissolved in anhydrous ethanol and then mixed with 1.0 g of dried MCM-36 support.The mixtures were allowed to re flux at 353 K for 24 h.After the reaction,the solid was isolated by filtration and washed with copious amounts of ethanol thrice to remove the unreacted ionic liquid.The resultant[BTPIm][HSO4]-immobilized MCM-36 zeolite(defined as MCM-36-IL)was dried at333 K for 24 h prior to further characterization and adsorption experiment.All of the above experiments were carried out under nitrogen atmosphere.
2.5.Characterizations
FT-IR spectra of solid samples were carried out on a Fourier Transform Infrared Spectroscopy(FT-IR-850,Spectrum Two L1600300,Perkin Elmer)with the spectral range of 400–4000 cm-1.The as synthesized MCM-36 and MCM-36-IL were characterized by Powder X-ray diffraction(XRD)on a Dandong DX-2700 using Cu Kαradiation.The voltage and anode currentwas 40 kVand 30 mA,respectively.Thermal Gravity Analysis–Differential Thermal Gravity Analysis(TG–DTG)(Beijing Hengjiu HTG-2)of MCM-36-IL was performed in a nitrogen atmosphere from room temperature to about 700°C with a rate of 10 °C·min-1.X-ray Photoelectron Spectroscopy(XPS)was recorded on a XSAM800 spectrometer(XSAM800,Kratos,UK)with a monochromatized Al Ka X-ray source radiation at constant dwell time of 100 ms and a pass energy of 40 eV.The morphologies and crystal sizes of MCM-36 and MCM-36-IL were examined by Scanning Electron Microscopy(SEM,JSM-7500F,JEOL,Tokyo,Japan).Nitrogen adsorption measurements were carried out at 77 K with Tristar Π 3020(Micromeritics,USA).The amounts of the surface acid of solid samples were measured by a method ofn-butylamine titration using a similar procedure described previously[25].
2.6.Adsorption experiments
To determine the adsorption performance of the as-synthesized MCM-36 and MCM-36-IL,a home-made adsorption equipment was established,as schematically shown in Fig.2.The pressure was measured by an absolute pressure transducer(0–689.5kPa,±0.03%,Omega Industrial Measurements,USA).The temperature was controlled by a thermostat water bath and measured by a high precision thermometer(0–50 °C,±0.1 °C).The effective volume of the sample cellVscwas 10.9 ml.The effective volume of the gas reservoir Vr(the volume between valve 3,valve 5 and valve 7,including the gas reservoir and pipes)was determined as 89.8 ml with an uncertaintyu(V)of 0.1 ml.
The adsorption property of isobutane and 1-butene on MCM-36 and MCM-36-IL were measured with a previously-established static volumetric method[26,27].Typically,the sample was accurately weighed and added into the sample cell.At a predetermined temperature,adsorption system was degassed to circa 1 Pa by a vacuum pump,and then the adsorbate gas was filled into the gas reservoir.The equilibrium temperature and pressure of the gas reservoir were recorded to calculate the moles of gas by Peng–Robinson state equation.Subsequently,valve 5 was opened to let the gas flow into the sample cell.When the temperature and pressure kept at a constant value for at least 60 min,it was believed to reach adsorption equilibrium,and the equilibrium temperature and pressure were recorded.When Helium gas was used as adsorbate gas,the adsorption capacity of He on the sample was neglected.After filling the sample,the effective volume of the gas phase inVscwas denoted asVss.On the basis of the variation of pressure and the volume of gas reservoir,Vsswas calculated by the following formula:

When isobutane or 1-butene was used as adsorbate,adsorption capacity of the sample was calculated by the Eq.(2):

Finally,valve 5 was closed and the supplementary gas was filled into the gas reservoir by opening valve 2 and valve 3.The mass of the supplementary gas was calculated by the increment of pressure.Repeating the above procedures,the adsorption capacity at an increased equilibrium pressure was obtained by the following Eq.(3):

3.Results and Discussion
3.1.Characterization of the[BTPIm][HSO4]-immobilized MCM-36
3.1.1.FT-IR spectra
Fig.3 shows the respective FT-IR spectra of MCM-36,MCM-36-IL and[BTPIm][HSO4]in a wavelength range of4000–400 cm-1.Some differences of the FT-IR spectra between the MCM-36 and MCM-36-IL are distinguished.The successful immobilization of[BTPIm][HSO4]can be deduced from the appearance of two additional characteristic peaks at 1560 and 1459 cm-1on the FT-IR spectra of MMC-36-IL,which are attributable to the stretching vibration of C–N and C–C on the imidazole ring[20,28].The C–H bending stretching and deformation vibrations of the imidazole ring are also observed in the range of 2800–3200 and 1385 cm-1[20].Moreover,the specific peak at 1237 cm-1,assigned to the S=O stretching vibration[22],is also observed in the FT-IR spectra of MCM-36-IL and[BTPIm][HSO4].These above results are well consistent with the fact that[BTPIm][HSO4]has been successfully immobilized on the surface of MCM-36 support,and that the surface chemical compositions of the MCM-36 support are significantly changed after immobilization of ILs.
3.1.2.XPS spectra
Surface chemical compositions of the as-synthesized MCM-36 and MCM-36-IL were further determined by the XPS measurement.Fig.4 shows the wide scan,C 1s and S 2s core-level XPS spectra for the MCM-36 and MCM-36-IL samples.The appearance of three additional signals with binding energies(BEs)at 163,233 and 400 eV,attributable to the S 2p,S 2s and N 1s species respectively,in the wide scan XPS spectra of the MCM-36-IL as compared to that of the MCM-36 sample indicates the successful immobilization of[BTPIm][HSO4]ionic liquid on the MCM-36(Fig.4(a)).The core-level C 1s peak of the MCM-36-IL can be curve- fitted into four peak components with BEs at 283.1,284.6,285.7,and 287.6 eV,associated with the C–Si,C–C,C–N+and C–O–Si species,respectively[29].The area ratio of peak components of[C–N+]:[C–Si]:[C–O–Si]is approximately 2:1:1,comparable to the theoretical values of 2:1:3 for the[BTPIm][HSO4]molecular structure.The predominated core-level S 2s spectrum further confirms the presence of sulfonate groups(SO24-)on the MCM-36-IL surfaces(Fig.4(c)).These above XPS spectra further confirm the covalent conjugation of[BTPIm][HSO4]ionic liquids on the MCM-36 support.
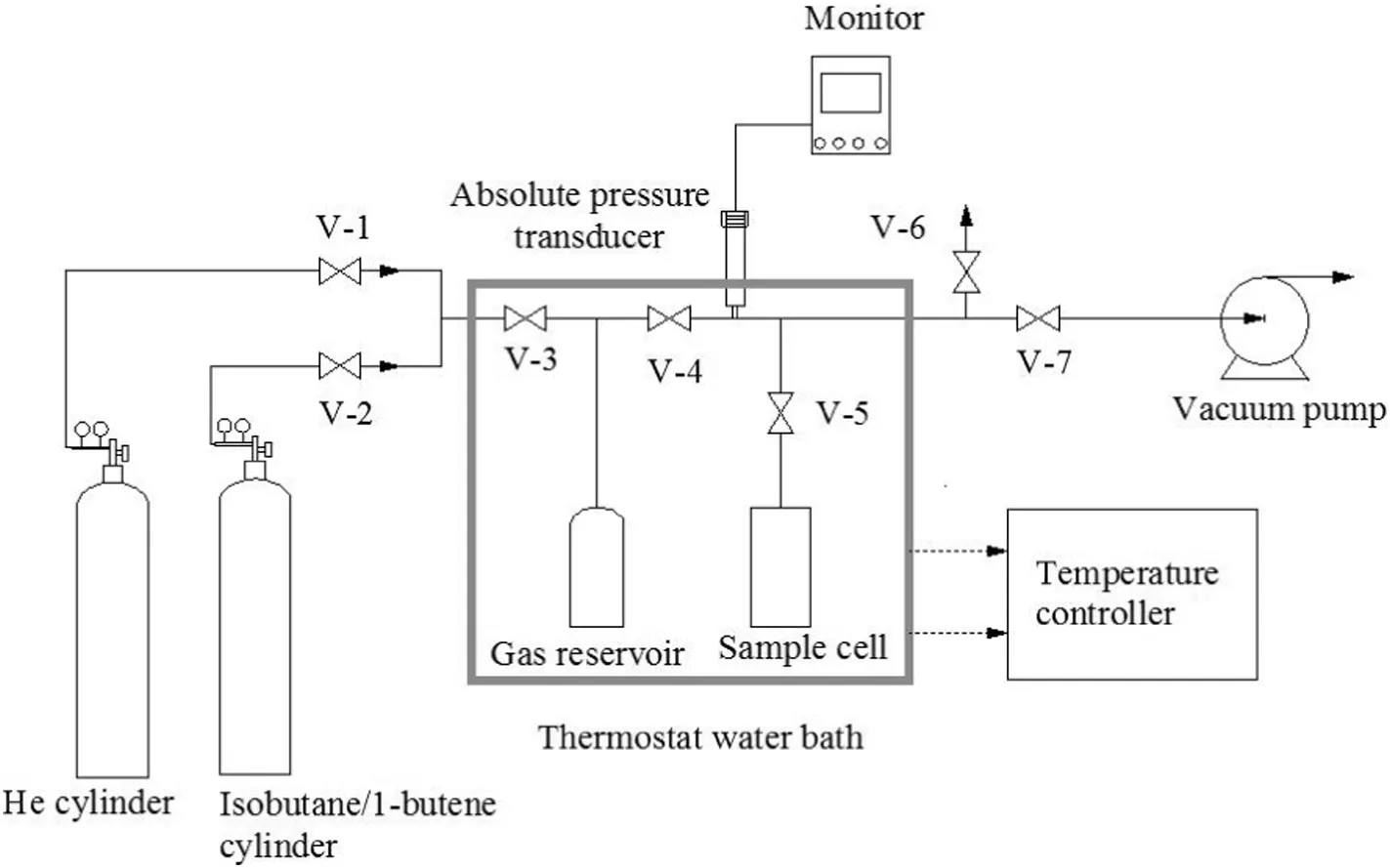
Fig.2.Schematic diagram of the experimental apparatus.
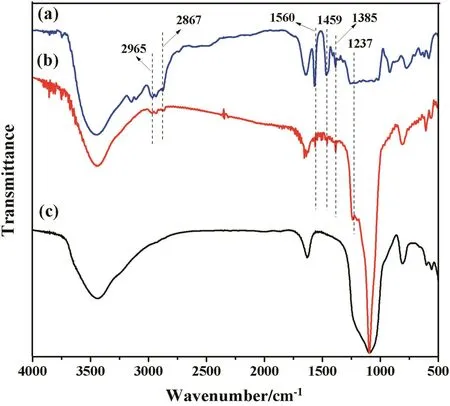
Fig.3.The FT-IR spectra of ionic liquid[BTPIm][HSO4](a),MCM-36-IL(b)and MCM-36(c).
3.1.3.XRD pattern and SEM images
To determine the change in the crystalline structures of MCM-36 before and after the[BTPIm][HSO4]immobilization,XRD patterns were recorded for the MCM-36 and MCM-36-IL samples,and the results are shown in Fig.5.Obviously,no change can be observed for the locations of XRD peaks of MCM-36 and MCM-36-IL samples,indicating that the immobilization of[BTPIm][HSO4]ionic liquid has no effect on the crystalline structure of MCM-36.
The change in surface morphology before and after the immobilization of[BTPIm][HSO4]ionic liquid was characterized by SEM imaging.Fig.6 shows the SEM images of the MCM-36 and MCM-36-IL samples,respectively.The MCM-36 shows a comparatively loose surface(Fig.6a),while the surface of the MCM-36-IL(Fig.6b)appears to be slightly flat and smooth.[BTPIm][HSO4]ionic liquid is covalently bonded on the MCM-36 through Si–O–Si and the channels of the MCM-36 are covered with a monolayer of ionic liquid,thus leading to the change in surface morphology of the MCM-36 support.
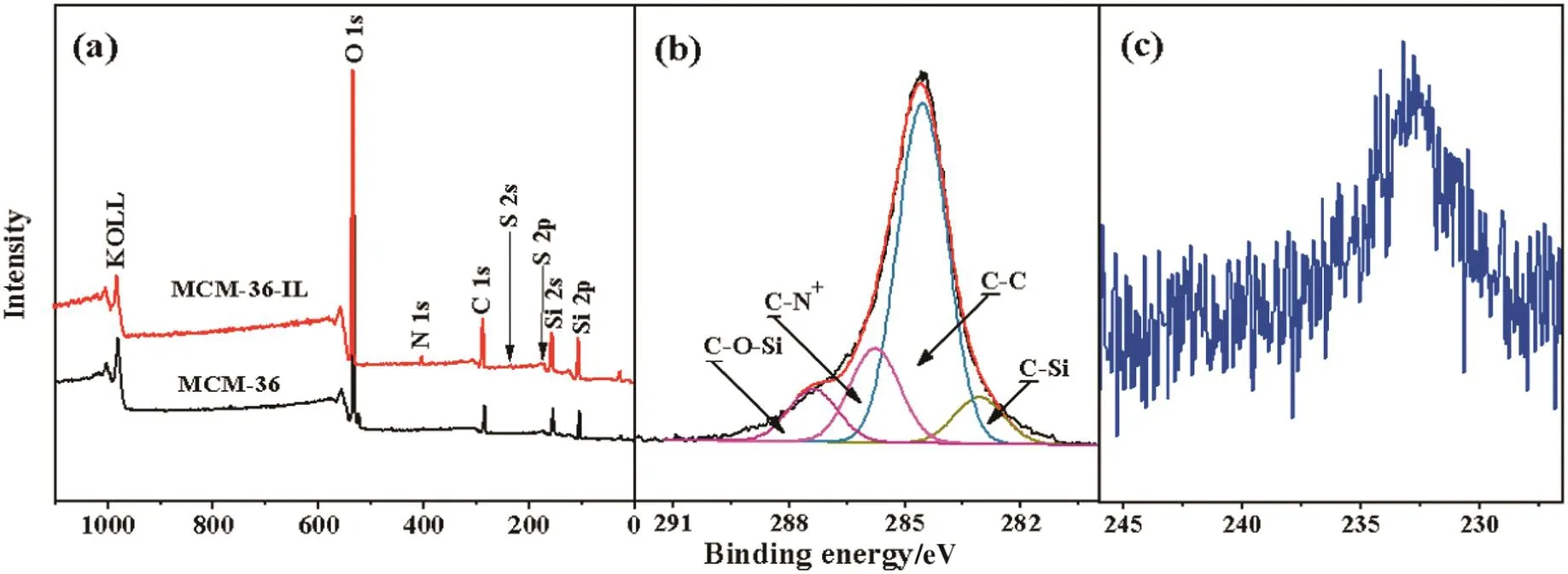
Fig.4.XPS spectra of MCM-36 and MCM-36-IL(a),deconvolution C 1s(b)and S 2s(c)spectra of MCM-36-IL

Fig.5.XRD patterns of MCM-36 and MCM-36-IL.
3.1.4.TG-DTG analysis of the MCM-36-IL
The loading amount of[BTPIm][HSO4]ionic liquid on the MCM-36-IL and the thermostability of MCM-36 and MCM-36-IL were characterized by TG–DTG analysis.The results are shown in Fig.7.Two mass loss stages can be observed in the TG curve of MCM-36-IL.The first one appears in a temperature range from 0 °C to 150 °C with a mass loss of about 5%,comparable to that of the MCM-36 sample with a mass loss of about 4%,which is mainly associated with the loss of physically adsorbed moisture.The other mass loss stage in the temperature region from 220 °C to 550 °C for the MCM-36-IL is mainly attributed to the loss of[BTPIm][HSO4]ionic liquid loaded onto the MCM-36.The observed 11.7%mass loss from the TG curve represents the amount of ionic liquid loaded on the MCM-36-IL.This result is consistent with the calculated loading amount of the immobilized ionic liquids,which is about 12.5 wt%,from the mass difference before and after immobilization.Taken together,the TG–DTG curve suggests that the[BTPIm][HSO4]ionic liquid immobilized on the MCM-36 is stable below about 220°C.
3.1.5.Nitrogen adsorption-desorption measurement
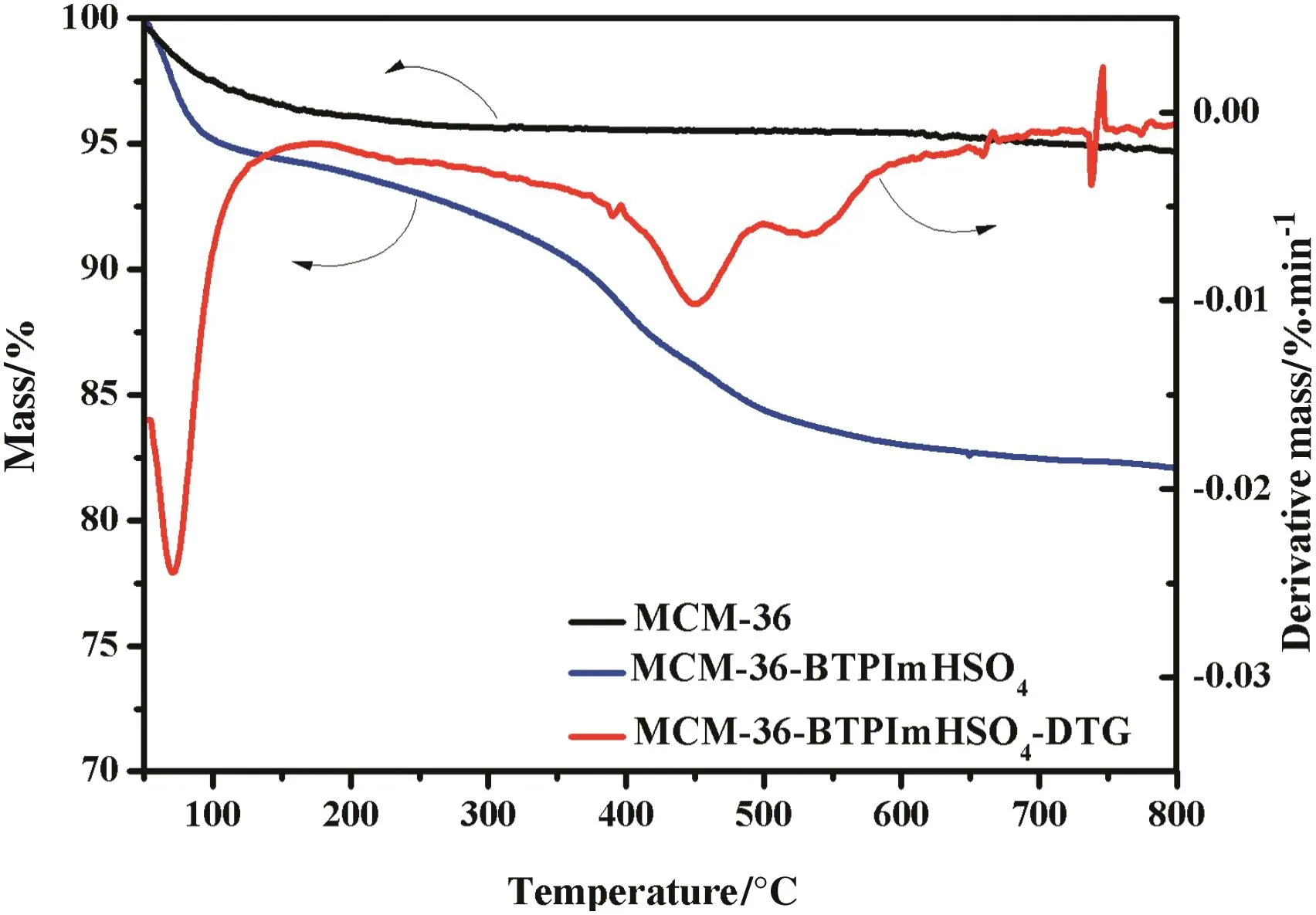
Fig.7.TG–DTG analysis of MCM-36 and MCM-36-IL.
Specific surface area and pore volume are important properties of catalysts.To determine the change in the specific surface area and the pore size of the MCM-36 after the immobilization of ionic liquid,the isothermal adsorption–desorption of nitrogen was recorded,and the isotherm curve is shown in Fig.8.A H3 type hysteresis loop was observed in aP/P0range of 0.4 to 1.0,indicating that a capillary condensation phenomenon had taken place.This result also suggests that mesoporous pores are formed in MCM-36 skeleton structure according to IUPAC classification[30].The calculated specific surface areas SBET,micropore volumeVmicroand mesopore volumeVmesoare listed in Table 1.The specific surface area of the MCM-36 undergoes an evident decrease from 672.3 to 248.6 m2·g-1owing to the immobilization of[BPTIm][HSO4]ionic liquids.However,the specific surface area of the MCM-36-IL is larger than that of MCM-22-IL as reported by Jin[20].The decrease in the specific surface area is consistent with the fact that a monolayer of the ionic liquids has been successfully immobilized on the surface of MCM-36.On the other hand,the immobilization of a monolayer ionic liquids results in a noticeable decrease in the pore volume,together with theVmicrovalue decreasing from 0.057 to 0.013 cm3·g-1and theVmesovalue from 0.367 to 0.214 cm3·g-1(Table 1).In fact,the ionic liquids can cause the block of a small fraction of the pores,and thus leading to the aggregation of adjacent plate-like particles as can be observed from SEM images.
3.1.6.Determination of the surface acidity
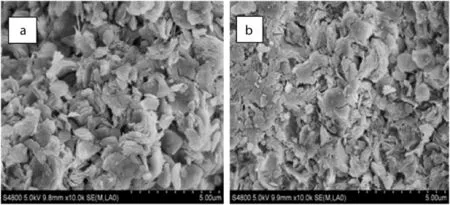
Fig.6.SEM images of MCM-36(a)and MCM-36-IL(b).
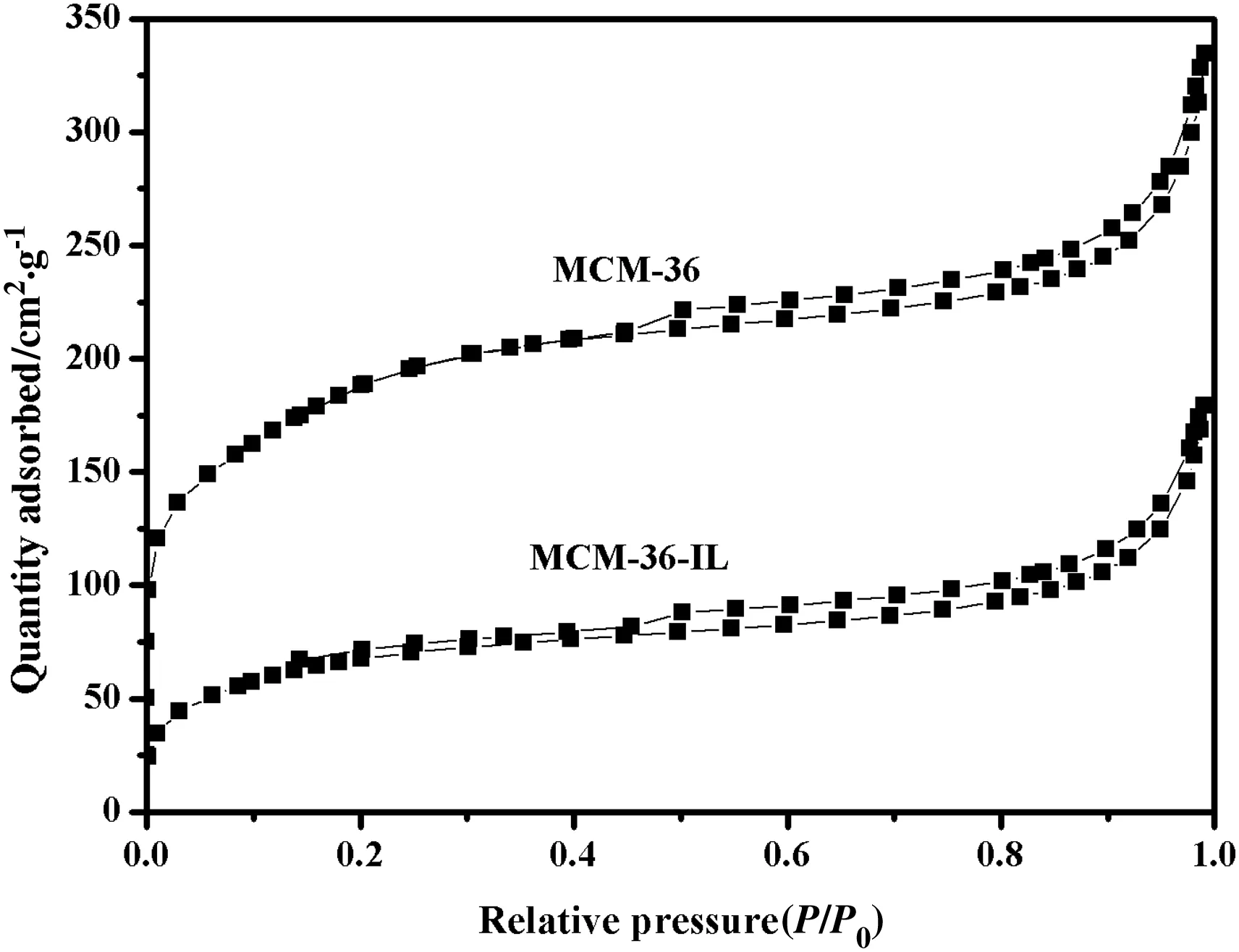
Fig.8.N2 adsorption–desorption analysis of MCM-36 and MCM-36-IL.
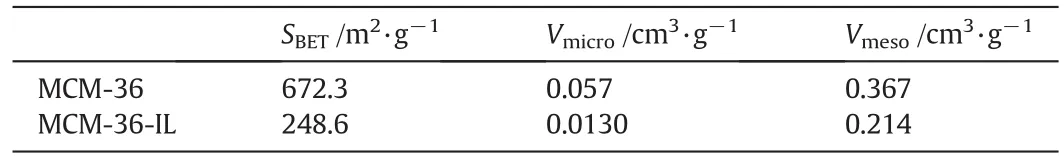
Table 1BET analysis results of MCM-36 and MCM-36-IL
Since acidity of a solid acid catalyst is one of the most important features in heterogeneous catalysis process,the acidity of the[BTPIm][HSO4]-immobilized MCM-36 zeolite was determined by a well-established n-butylamine titration method[25].The surface density of acid on the MCM-36-IL is circa 0.0035 mmol·m-2,much higher than that on the MCM-36 at circa 0.0014 mmol·m-2.The assynthesized[BTPIm][HSO4]ionic liquid is an acidic ionic liquid due to the presence of anionic[HSO4]moieties.The terminated[HSO4]groups are readily dissociated to provide proton H+.Therefore,the increase in the surface acidity on the MCM-36 can be accomplished by surface functionalization with acidic ionic liquids.
3.2.Adsorption behaviors of isobutane/1-butene on MCM-36 and MCM-36-IL
The microstructures of MCM-36 have a significant effect on their adsorption capacity due to different pore sizes and diffusion channels for adsorbates[31].The Sip isotherm was commonly used to simulate the adsorption property of a porous zeolite,and the expression is shown as follows Eq.(4)[32,33]:

Figs.9–11 show the adsorption isotherms of isobutane and 1-butene on the MCM-36 and MCM-36-IL at the adsorption temperature of 293.15,298.15 and 303.15 K,respectively.The corresponding isoparaffin/olefin(I/O)ratios on MCM-36 and MCM-36-IL at different temperatures are calculated,and are also shown in Figs.9–11.The fitted parameters of Sip isotherm are summarized in Table 2.Obviously,Sip model shows a good fitting to the adsorption isotherms of isobutane/1-butene on MCM-36 and MCM-36-IL with a high correlation coefficient R2of 0.994.In Sip model,the parameter n is used to represent system heterogeneity.As shown in Table 2,the parameternis larger than unity,indicating a heterogeneous surface of the MCM-36 and MCM-36-IL samples.
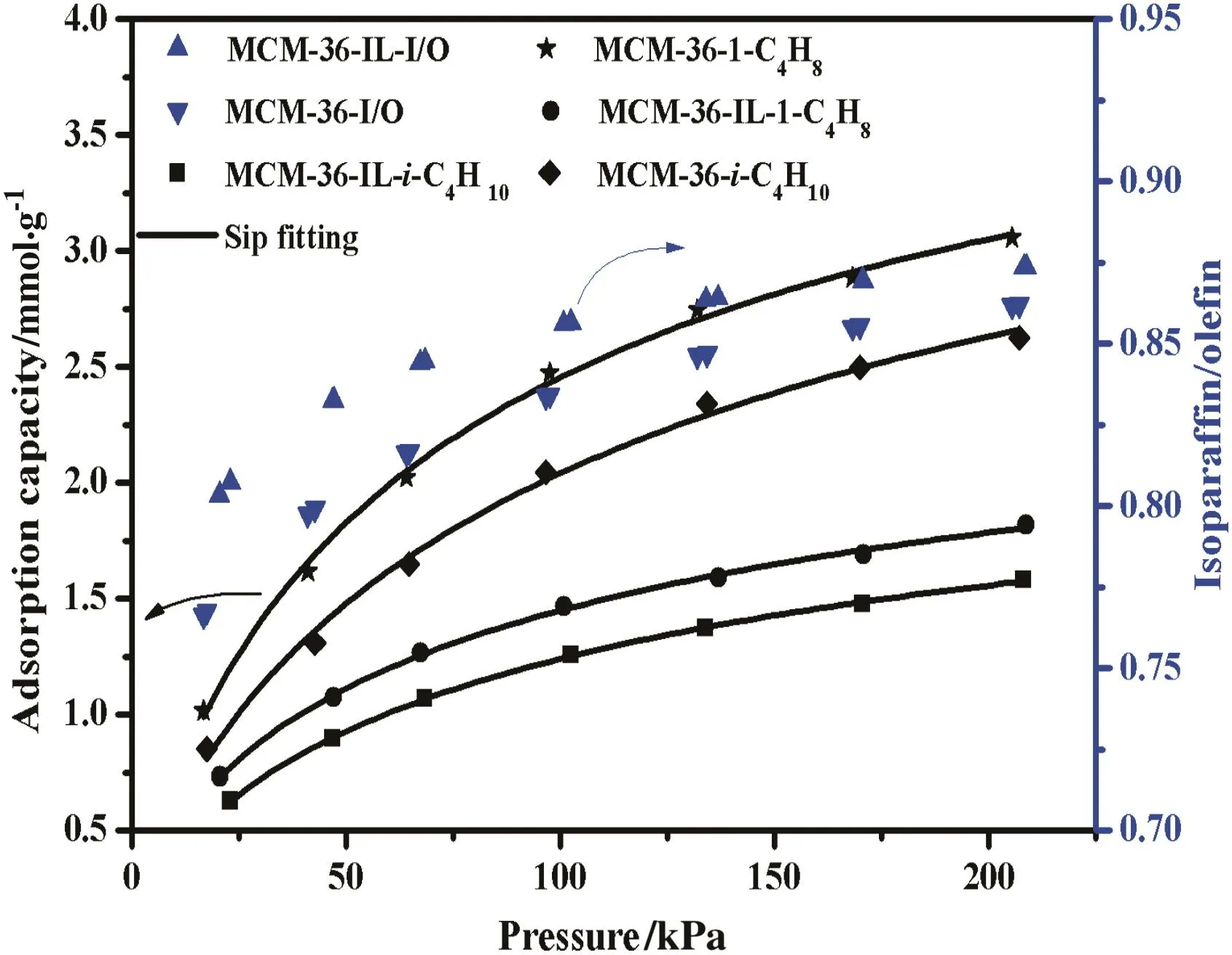
Fig.9.Adsorption I/O ratio and adsorption capacities of isobutane/1-butene on MCM-36 and MCM-36-IL at 293.15 K.Adsorption capacities of isobutane(■)and 1-butene(●)on MCM-36-IL,adsorption capacities of isobutane(♦)and 1-butene(★)on MCM-36,I/O ratio on MCM-36-IL()and MCM-36().
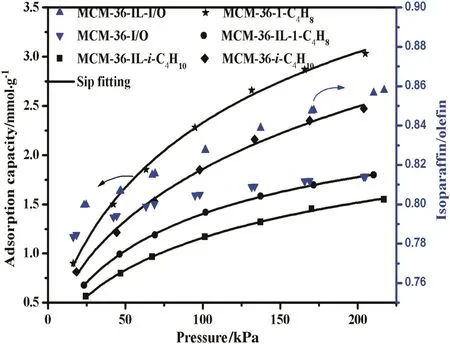
Fig.10.Adsorption I/O ratio and adsorption capacities of isobutane/1-butene on MCM-36 and MCM-36-IL at 298.15 K.Adsorption capacities of isobutane(■)and 1-butene(●)on MCM-36-IL,adsorption capacities of isobutane(◆)and 1-butene(★)on MCM-36,I/Oratio on MCM-36-IL()and MCM-36().
The adsorption capacities of isobutane and 1-butene on the MCM-36 and MCM-36-IL all increase with increasing equilibrium pressure,while undergoing a decrease with the equilibrium temperature.These results are in good agreement with the general adsorption behavior of most adsorbents.As a consequence,the adsorption I/O ratios gradually increase with increasing operation pressure,while they undergo a decrease with increasing adsorption temperature.These above results suggest that a high adsorption I/O ratio can be regulated by increasing pressure or decreasing temperature for the MCM-36 and MCM-36-IL samples,whereas,the adsorption capacities of isobutane and 1-butene on the MCM-36-IL are much lower than those of the corresponding MCM-36.These results are ascribed to the decrease of specific surface area and mean pore volume upon the grafting of[BTPIm][HSO4]ionic liquid on the MCM-36 support.The decrease in specific surface area essentially results in the decrease in adsorption sites,while the smaller pore sizes cause the increase in diffusion resistance of adsorbate gases.Thus,the immobilization of an ionic liquid monolayer is virtually unbeneficial to the adsorption capacity of the MCM-36 support.
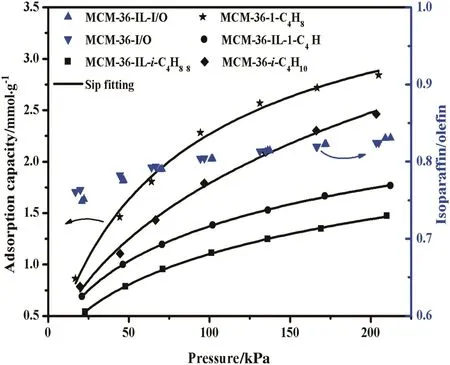
Fig.11.Adsorption I/O ratio and adsorption capacities of isobutane/1-butene on MCM-36 and MCM-36-IL at 303.15 K.Adsorption capacities of isobutane(■)and 1-butene(●)on MCM-36-IL,adsorption capacities of isobutane(◆)and 1-butene(★)on MCM-36,I/O ratio on MCM-36-IL()and MCM-36().
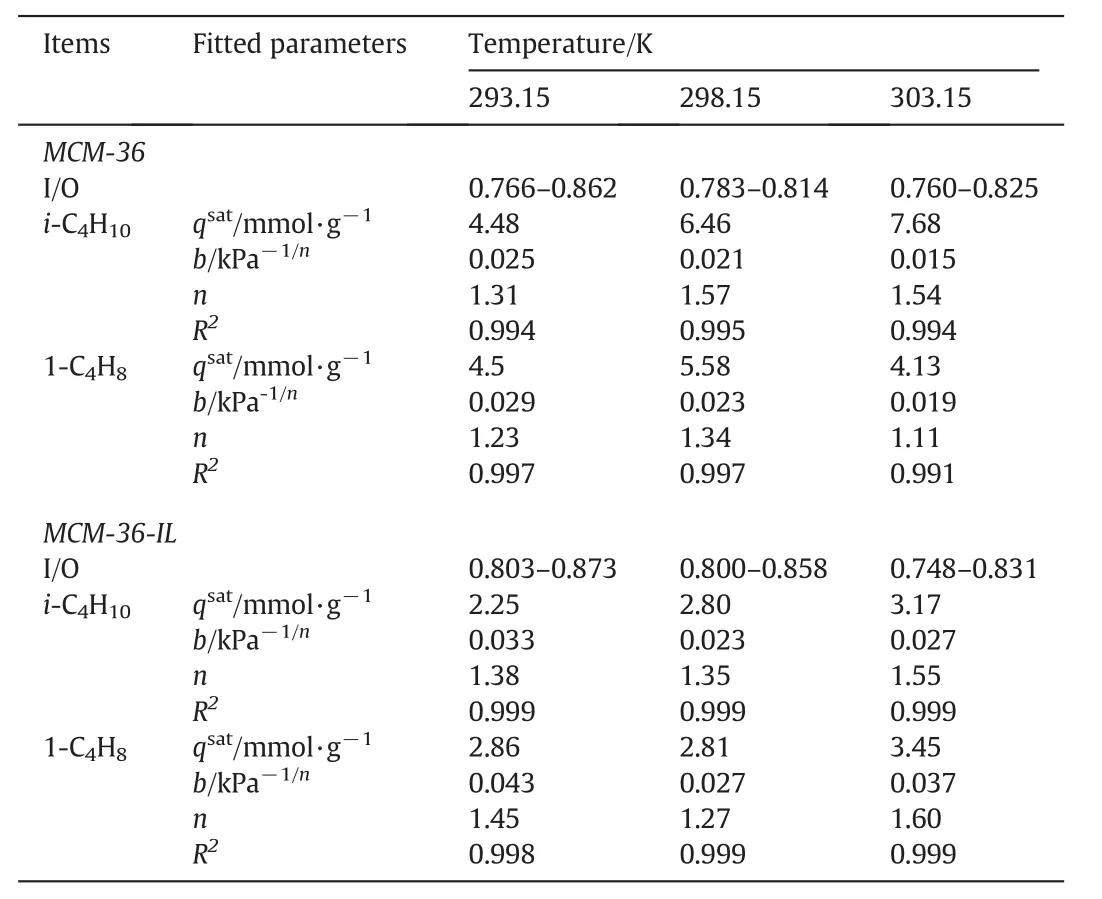
Table 2The isoparaffin/olefin ratios(I/O)and fitted parameters of Sip isotherm model
However,the grafting of acid[BTPIm][HSO4]ionic liquid is beneficial to increase the adsorption I/O ratio on the MCM-36 support.The surface-grafted ionic liquids monolayer can cause changes in the microstructure,surface features and wettability of the MCM-36,especially for surface hydrophobicity,which is adjusted due to interactions between surface hydroxyl groups on the MCM-36 and acidic ionic liquids,as well as the presence of non-polar butyl groups of[BTPIm][HSO4].The increase in surface hydrophobicity can substantially enhance the interaction between isobutane and adsorbent,but repel the 1-butene adsorption.On the contrary,the polargroups have a negative effect on the surface hydrophobicity.Thus,only slight increase in the I/O adsorption ratio was observed.In fact,the presence of abundantgroups provides enough acidity and acid amount for the solid acid catalysts,Therefore,the grafting of[BTPIm][HSO4]on the MCM-36 zeolite can not only substantially enhance the surface acidity and acid amount,but also regulate the I/O adsorption ratio for a slight increase in non-polar isobutane.These are all key factors to intensify the reaction catalyzed by solid acid catalyst.
3.3.Adsorption thermodynamics
The isosteric heat of adsorptionQSTand its variation provides useful information about the strength of adsorbate–adsorbent interaction and surface characteristics.It can be calculated according to the following equations[34,35]:

TheQSTvalues of isobutane or 1-butene on MCM-36 and MCM-36-IL at different adsorption amount are shown in Fig.12.TheQSTvalues of isobutane on the MCM-36 shows a slight increase with increasing adsorption quantity(Fig.12(a)).The results are agreed with the results of Nabil Lamia[36].The affinity of isobutane on MCM-36 is gradually enhanced with the accumulation of the adsorbed isobutane molecules.However,the QSTvalues of isobutane on MCM-36-IL decrease with increasing adsorption amount.In general,a stronger interaction between adsorption site and adsorbate brings a larger QSTvalue and the adsorbate is preferentially adsorbed on the more active adsorption sites.With the increasing adsorption quantity,the strong adsorption sites are occupied by adsorbate gradually and the interactions between the adsorbent and adsorbate decrease gradually.So theQSTvalues of isobutane on MCM-36-IL decrease slightly with increasing adsorption quantity.According to the variation tendency,we can speculate that theQSTvalues of isobutane on MCM-36-IL are higher than theQSTvalues of isobutane on MCM-36 at the range of adsorption quantity of isobutane less than 1.0 mmol·g-1.These results indicate that the interaction between isobutane and the adsorbent is enhanced by the immobilization of ionic liquid,which brings a largerQSTvalue.
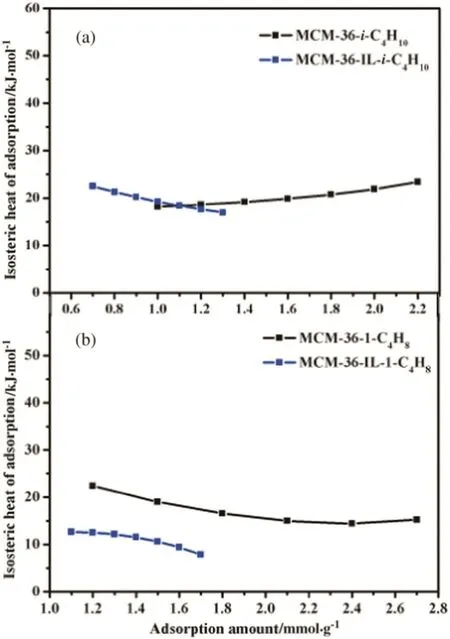
Fig.12.The isosteric heat of adsorption of isobutane and 1-butene on MCM-36 and MCM-36-IL.(a):The Q ST of isobutane on MCM-36 and MCM-36-IL;(b):The Q ST of 1-buene on MCM-36 and MCM-36-IL.
TheQSTvalues of 1-butene on the MCM-36 and MCM-36-IL both decrease with increasing adsorption amount(Fig.12(b)).It is easy to understand this tendency by the preferential adsorption of the strong adsorption sites.TheQSTvalues of 1-butene on the MCM-36-IL are smaller than that on the MCM-36 indicating that the interaction between adsorbent and 1-butene becomes weak after the immobilization of ionic liquid.
Taken together,the immobilization of[BTPIm][HSO4]ionic liquid on the MCM-36 support has an effect on the interaction between adsorbent and adsorbates.The interaction between the MCM-36 and isobutane is substantially enhanced by the immobilized ionic liquid[BTPIm][HSO4],while the interaction between the MCM-36 and 1-butene is reduced.Consequently,the adsorption properties of isobutane and 1-butene on the MCM-36,as well as the adsorption I/O ratio,can be regulated by the grafting of ionic liquids for the enhancement of the solid acidcatalyzed reaction.
4.Conclusions
Anovelacidic ionic liquid,1-butyl-3-(triethoxysilylpropyl)imidazolium hydrogen sulfate(i.e.[BTPIm][HSO4],was synthesized and subsequently immobilized on the MCM-36 zeolite via self-assembly reaction.Success in the[BTPIm][HSO4]synthesis and subsequent functionalization of MCM-36 were ascertained by the FTIR,XPS,TGA,XRD and BET measurements.The immobilization of[BTPIm][HSO4]ionic liquids resulted in a decrease in specific surface area and the pore volume,while it led to an increase in surface acidity and surface acid amount.The adsorption experimental results revealed that the interaction between MCM-36 and isobutane was substantially enhanced by the ionic liquid immobilization,while the interaction between 1-butene and the ionic liquid-immobilized MCM-36 was weakened.The noticeable increase in the I/O adsorption ratio suggested that the adsorption properties of isobutane and 1-butene on the MCM-36 could be regulated by grafting ionic liquid.In a whole,the improvement in the I/Oadsorption ratio and surface acid amount has a positive effect on the catalyst life and reaction yield for the acid catalytic reactions,such as the alkylation process,acylation and isomerization.
Nomenclature
ΔHThe differential enthalpy of adsorption,J·mol-1
k The coefficient of Sip isotherm model
mThe mass of catalyst,g
nThe solid heterogeneity parameter(>1)
niThe adsorption capacity of adsorbate on adsorbent,mmol·g-1
pThe adsorption equilibrium pressure,Pa
QSTThe isosteric heat of adsorption,J·mol-1
qThe adsorption capacity of adsorbate on adsorbent,mol·g-1
qsatThe saturation adsorption capacity of adsorbate on adsorbent,mol·g-1
TThe equilibrium temperature,K
VrThe effective volume of the gas reservoir(=89.8 ml),ml
VscThe effective volume of the sample cell(=10.9 ml),ml
VssThe effective volume of gas phase in the sample cell after filling sample,ml
vHe-rThe molar volume of He calculated by PR equation according to the initial equilibrium pressure and temperature in gas reservoir,ml·mmol-1
vHe-rssThe molar volume of He calculated by PR equation according to the equilibrium pressure and temperature in gas reservoir and sample cell,ml·mmol-1
vr,iThe molar volume of isobutane or 1-butene calculated by PR equation according to the equilibrium pressure and temperature in gas reservoir,ml·mmol-1
vrss,iThe molar volume of isobutane or 1-butene calculated by PR equation according to the equilibrium pressure and temperature in gas reservoir and sample cell,ml·mmol-1
[1]Z.Zhao,B.Yuan,W.Qiao,Z.Li,G.Wang,L.Chang,The metal ion modified ionic liquids promoted free-solvent alkylation of α-methylnaphthalene with long-chain olefins,J.Mol.Catal.A Chem.235(2005)74–80.
[2]J.F.Haw,Zeolite acid strength and reaction mechanisms in catalysis,Phys.Chem.Chem.Phys.4(2002)5431–5441.
[3]M.A.Granato,N.Lamia,T.J.H.Vlugt,A.E.Rodrigues,Adsorption equilibrium of isobutane and 1-butene in zeolite 13X by molecular simulation,Ind.Eng.Chem.Res.47(2008)6166–6174.
[4]C.A.Grande,J.D.Araujo,S.Cavenati,N.Firpo,E.Basaldella,A.E.Rodrigues,New πcomplexation adsorbents for propane-propylene separation,Langmuir20(2004)5291–5297.
[5]B.L.Newalkar,N.V.Choudary,U.T.Turaga,R.P.Vijayalakshmi,P.Kumar,S.Komarneni,T.S.G.Bhat,Adsorption of light hydrocarbons on HMS type mesoporous silica,Microporous Mesoporous Mater.65(2003)267–276.
[6]W.Zhu,F.Kapteijn,J.A.Moulijn,M.C.den Exter,J.C.Jansen,Shape selectivity in adsorption on the all-silica DD3R,Langmuir16(2000)3322–3329.
[7]W.Shen,Y.Gu,H.Xu,D.Dubé,S.Kaliaguine,Alkylation of isobutane/1-butene on methyl-modified Nafion/SBA-15 materials,Appl.Catal.A377(2010)1–8.
[8]I.Ogino,Y.Suzuki,S.R.Mukai,Tuning the pore structure and surface properties of carbon-based acid catalysts for liquid-phase reactions,ACS Catal.5(2015)4951–4958.
[9]C.Guücüyener,J.van den Bergh,J.Gascon,F.Kapteijn,Ethane/ethene separation turned on its head:Selective ethane adsorption on the metal–organic framework ZIF-7 through a gate-opening mechanism,J.Am.Chem.Soc.132(2010)17704–17706.
[10]M.Rufete-Beneite,M.C.Román-Martínez,A.Linares-Solano,Insight into the immobilization of ionic liquids on porous carbons,Carbon77(2014)947–957.
[11]M.I.Onishchenko,I.A.Tyablikov,E.E.Knyazeva,V.V.Chernyshev,A.V.Yatsenko,B.V.Romanovsky,Modification of MCM-41 and SBA-15 mesoporous silicas by imidazolium ionic liquids,Russ.J.Phys.Chem.A87(2013)108–113.
[12]H.Li,P.S.Bhadury,B.Song,S.Yang,Immobilized functional ionic liquids:Efficient,green,and reusable catalysts,RSC Adv.2(2012)12525.
[13]F.Wang,Z.Zhang,J.Yang,L.Wang,Y.Lin,Y.Wei,Immobilization of room temperature ionic liquid(RTIL)on silica gel for adsorption removal of thiophenic sulfur compounds from fuel,Fuel107(2013)394–399.
[14]X.Gao,R.Li,G.Zhu,J.Fan,Effect of alkyl chain length on adsorption properties of alkyl imidazolium ionic liquids surface imprinting polymers,Monatsh.Chem.146(2014)475–484.
[15]G.Severa,K.Bethune,R.Rocheleau,S.Higgins,SO2sorption by activated carbon supported ionic liquids under simulated atmospheric conditions,Chem.Eng.J.265(2015)249–258.
[16]Y.Zhao,J.Wang,H.Jiang,Y.Hu,Desulfurization performance ofether-functionalized imidazolium-based ionic liquids supported on porous silica gel,Energy Fuel29(2015)1941–1945.
[17]A.Erto,A.Silvestre-Albero,J.Silvestre-Albero,F.Rodríguez-Reinoso,M.Balsamo,A.Lancia,F.Montagnaro,Carbon-supported ionic liquids as innovative adsorbents for CO2separation from synthetic flue-gas,J.Colloid Interface Sci.448(2015)41–50.
[18]Y.Lin,F.Wang,Z.Zhang,J.Yang,Y.Wei,Polymer-supported ionic liquids:Synthesis,characterization and application in fuel desulfurization,Fuel116(2014)273–280.
[19]A.Zukal,I.Dominguez,J.Mayerova,J.Cejka,Functionalization of delaminated zeolite ITQ-6 for the adsorption of carbon dioxide,Langmuir25(2009)10314–10321.
[20]K.Jin,T.Zhang,J.Ji,M.Zhang,Y.Zhang,S.Tang,Functionalization of MCM-22 by dual acidic ionic liquid and its paraffin absorption modulation properties,Ind.Eng.Chem.Res.54(2015)164–170.
[21]M.Zhang,K.Jin,Y.Zhang,T.Zhang,S.Tang,Static synthesis of MCM-22 and MCM-36 zeolites using piperidine as an organic template(in Chinese),J.Chem.Eng.Chin.Univ.4(2015)1–9.
[22]S.Rostamnia,A.Hassankhani,H.G.Hossieni,B.Gholipour,H.Xin,Brønsted acidic hydrogensulfate ionic liquid immobilized SBA-15:[MPIm][HSO4]@SBA-15 as an environmentally friendly,metal-and halogen-free recyclable catalyst for Knoevenagel–Michael-cyclization processes,J.Mol.Catal.A395(2014)463–469.
[23]S.X.Heng,Y.Zhou,Y.Yang,Y.Zhang,Z.Zhang,S.Zhou,X.Fu,S.Zhao,Synthesis of immobilized heteropolyanion-basedionic liquids on mesoporous silica SBA-15 as aheterogeneous catalyst for alkylation,RSC Adv.4(2014)30697–30703.
[24]X.Ma,X.Wang,C.Song,“Molecular Basket”sorbents for separation of CO2and H2S,J.Am.Chem.Soc.131(2009)5777–5783.
[25]J.Papp,S.Soled,K.Dwight,A.Wold,Surface acidity and photocatalytic activity of TiO2,WO3/TiO2,and MoO3/TiO2photocatalysts,Chem.Mater.6(1994)496–500.
[26]Y.K.Ryu,J.W.Chang,S.Y.Jung,C.H.Lee,Adsorption isotherms of toluene and gasoline vapors on DAY zeolite,J.Chem.Eng.Data47(2002)363–366.
[27]Y.Zhang,T.Zhang,P.Gan,H.Li,M.Zhang,K.Jin,S.Tang,Solubility of isobutane in ionic liquids[BMIm][PF6],[BMIm][BF4],and[BMIm][Tf2N],J.Chem.Eng.Data60(2015)1706–1714.
[28]Q.Zhang,J.Luo,Y.Wei,A silica gel supported dual acidic ionic liquid:An efficient and recyclable heterogeneous catalyst for the one-pot synthesis of amidoalkyl naphthols,Green Chem.12(2010)2246.
[29]H.Jiang,X.B.Wang,C.Y.Li,J.S.Li,F.J.Xu,C.Mao,W.T.Yang,J.Shen,Improvement of hemocompatibility of polycaprolactone film surfaces with zwitterionic polymer brushes,Langmuir27(2011)11575–11581.
[30]J.Wang,S.Jaenicke,G.K.Chuah,W.Hua,Y.Yue,Z.Gao,Acidity and porosity modulation of MWW type zeolites for Nopol production by Prins condensation,Catal.Commun.12(2011)1131–1135.
[31]S.Maheshwari,C.Martínez,M.T.Portilla,F.J.Llopis,A.Corma,M.Tsapatsis,Influence of layer structure presservation on the catalytic properties of the pillared zeolite MCM-36,J.Catal.272(2010)298–308.
[32]B.U.Choi,D.K.Choi,Y.W.Lee,B.K.Lee,Adsorption equilibria of methane,ethane,ethylene,nitrogen,and hydrogen onto activated carbon,J.Chem.Eng.Data48(2003)603–607.
[33]G.M.Nam,B.M.Jeong,S.H.Kang,B.K.Lee,D.K.Choi,Equilibrium isotherms of CH4,C2H6,C2H4,N2,and H2on zeolite 5A,J.Chem.Eng.Data50(2005)72–76.
[34]M.Bülow,D.Shen,S.Jale,Measurement of sorption equilibria under isosteric conditions:the principles,advantages and limitations,Appl.Surf.Sci.196(2002)157–172.
[35]Y.Y.Huang,The temperature dependence of isosteric heat of adsorptionon the heterogeneous surface,J.Catal.25(1972)131–138.
[36]N.Lamia,M.Jorge,M.A.Granato,F.A.Almeida Paz,H.Chevreau,A.E.Rodrigues,Adsorption of propane,propylene and isobutane on a metal–organic framework:Molecular simulation and experiment,Chem.Eng.Sci.64(2009)3246–3259.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Hemicellulose in corn straw:Extracted fromalkali solution and produced 5-hydroxymethyl furfural in HCOOH/HCOONa buffer solution☆
- Molar volume of eutectic solvents as a function of molar composition and temperature☆
- Investigation on molar heat capacity,standard molar enthalpy of combustion for guaiacol and acetyl guaiacol ester
- Statistical mechanics and artificial intelligence to model the thermodynamic properties of pure and mixture of ionic liquids☆
- A comprehensive fractal char combustion model☆
- Development of a bifurcation analysis approach based on gPROMS platform☆
