CO2/CH4 and CH4/N2 separation on isomeric metal organic frameworks☆
2016-06-12XiaoqingWangLiboLiJiangfengYangJinpingLi
Xiaoqing Wang,Libo Li,Jiangfeng Yang*,Jinping Li
Research Institute of Special Chemicals,Taiyuan University of Technology,Taiyuan 030024,China
1.Introduction
The Earth's climate is undergoing warming because of the excessive emission of greenhouse gases,such as carbon dioxide and methane[1–3].Simultaneously,naturalgas(CH4)is regarded as a good candidate due to the demand for alternative fuels that is greater now than ever with energy shortages.However,significant quantities of natural gas(biogas/land fill gas/coal bed methane)cannot be used efficiently because of the presence of CO2and N2in these gas sources,which lowers their energy content[4].Therefore,capturing and separating CO2and N2from CH4is important for the industrial application of natural gas.
Among the possible separation methods,adsorption(e.g.,pressure swing adsorption(PSA))is considered a very promising technology for commercial and industrial applications[5,6].However,the success of this approach is strongly dependent on the development of suitable adsorbents that possess a high selectivity for CO2or CH4from CO2/CH4/N2mixtures[7].Metal–organic frameworks(MOFs)have attracted a lot of attention over the last few decades since they appeared.One of the most important reasons for this is that MOFs have great potential in gas separation as an adsorbent[8–10],owing to their diverse structures.
In the investigation of MOFs,it was found that the structures of MOFs have a pronounced effect on the adsorption selectivity,whose different pore properties(e.g.,pore size,pore volume and surface area)and host–guest interactions have an influence on their adsorption abilities and kinetic selectivity towards a target gas[11].For example,Leeet al.synthesized a series of isostructural MOFs to probe the influence of their structures on molecular transport and kinetic selectivity[12].Li et al.studied the pore properties of MOF materials,to regulate their uptake capacity and selectivity,by modification of the ligands in the MOFs with functional groups[13].
In order to explore the relationship between MOF structures and their gas separation properties,we have synthesized two MOFs with different structures butidentical compositions(also named isomeric MOFs)[14]comprised of Cu(II)cations and OTf(trifluoromethanesulfonate[OSO2CF3-])counter ions,and studied their gas adsorption properties and mixed gas breakthrough.
The two isomeric MOFs,one with a two-dimensional(2D)structure and the other with a three-dimensional(3D)structure,were synthesized and reported by AtsushiKondoet al.[15–17].The 2Dand 3D frameworks have the same ‘square planar’units in which each metal is coordinated by four 4,4′-bipyridine(4,4′-bpy)ligands in the equatorial positions and two OTf anions in the axial positions with the only difference being the ways in which they are assembled,as shown in Fig.1.
The 2D structure formed square-grid sheets by connecting the‘square planar’units in the same plane and the resulting sheets stacked in a zigzag fashion to form uniform micropores[17,18].When adsorbing special guest molecules at a certain temperature and pressure,this material exhibited a unique step adsorption behavior,which was always accompanied with expansion and shrinkage of the structure.While the 3D structure comprised of two identical interwoven nets where all the metals were equivalent and played the role of square-planar nodes in the network,and gave two distincttypes of the latterchannels.The MOF expands the diameter of the hydrophilic and hydrophobic pores and elongates the length of both types of the pores in length upon cooling,andvice versa[19].In addition,the flexible MOFs showed that it can be slightly stretched or compressed in the direction of the c-axis when the solvent is removed[15].Although the two kinds of MOFs both have flexible frameworks,the 2D MOF showed significantly more flexibility than the 3D MOF,giving rise to different flexibility-associated gas adsorption behavior.

Fig.1.A view of the 2D and 3D structures of the Cu(4,4′-bpy)2(OTf)2 frameworks[15,16].
2.Experimental
2.1.Materials
All chemicals and solutions were purchased in their highest available commercial purity(>98%)from Sigma–Aldrich.Distilled water was made in our laboratory.
Synthesisofthe 2DMOF:This sample was synthesized following previously described methodology[20].An ethanol solution(10.0 ml)of 4,4′-bipyridine(4,4′-bpy)(60.0 mmol·L-1)was carefully layered onto an aqueous solution(10 ml)of Cu(OTf)2(30 mmol·L-1),after adding 3 ml of pure ethanolas a diffusion layer in a 100-mlbeaker at room temperature,then sealed with plastic wrap.A dark blue precipitate formed gradually on the beaker walls.The mixture was left sitting undisturbed for 2 weeks,then filtered and washed with water and ethanol.
Synthesis of the 3D MOF:this sample was synthesized using a new method,which is different from the method described in the literature[17].An aqueous solution(10 ml)consisting of 30 mmol of Cu(OTf)2and 15 mmol of KCF3BF3was prepared in a 100-mlbeaker,then carefully layered onto an alcohol solution(ethanol)of 4,4′-bipyridine(60.0 mmol·L-1,10.0 ml),after adding 3 ml of pure alcohol as the diffusion layer,then sealed with plastic wrap.It should be pointed out that KCF3BF3was used as a directing agent.The reaction was left at room temperature for 2 days and a dark blue precipitate formed on the beaker walls.The mixture was left sitting undisturbed for 1 week,then filtered and washed with water and ethanol.
2.2.Characterization
The crystallinity and phase purity of the samples were measured by powder X-ray diffraction(XRD)using a Rigaku MiniFlex IIX-ray diffractometer with Cu Kαradiation operated at 30 kV and 15 mA.The scanning range was 5°–40°(2 theta)at a rate of 1(°)·min-1.TGA was carried out in a flowing atmosphere(argon flow rate:100 ml·min-1)ata heating rate of5 K·min-1using a Netzsch STA-409-C balance.Scanning electron microscopy images were observed using JEOL JSM-6700F equipment at 10 kV.N2adsorption isotherms were measured on a QUADRASORB SI at 77 K for 15 min at each point along the isotherm.
2.3.Gas adsorption isotherm measurements
The sorption isotherms for CO2,CH4and N2were measured using an Intelligent Gravimetric Analyzer(IGA001 series,Hiden Analytical Ltd).The system was out gassed at 373 K for 12 h and no further mass losses were observed;the system temperature was then adjusted to the desired adsorption temperature and the sample cell was kept under vacuum for 30 min before the first adsorption measurement was taken.After a stable pressure was obtained,the adsorption equilibrium data were collected and mass had been maintained for 30 min at each point along the isotherm.All the isotherms for the samples were obtained using a single sample.
2.4.Breakthrough separation experiments
The breakthrough curves for the 2Dand 3D MOFs were measured on a homemade apparatus using the gas mixtures CO2/CH4(50%/50%,V/V)and CH4/N2(50%/50%,V/V).The experimental setup has been discussed in detail in our previous work[21,22]and performed in a stainless steel column with a length of 150 mm and an internal diameter of 9 mm.
Particles were obtained using an electric tablet press(SDY-20,Tian-Jin KeQi High and new technology company)by pressing the samples under a pressure of 3 MPa into a solid disk for 1–1.5 min,crushing and sieving to obtain 40–60 mesh particles(with a diameter of about 0.5–0.8 mm)and then drying for 1 h at 373 K under vacuum.The XRD of the particles has no difference with the as-synthesized powder.Then,the samples were introduced to the adsorption bed.A carrier gas(He≥99.999%)was used to purge the adsorption bed for more than 1 h to ensure that the adsorption bed was saturated with He.The raw mixed gases comprised of CO2/CH4(50%/50%)and CH4/N2(50%/50%)at a flow rate of 10 cm3·min-1(STP)were passed through the adsorption bed.The out let gas was analyzed using gas chromatography(Shimadzu,GC-2014C,Japan).The packing material in the gas chromatograph(GC)for CO2/CH4and CH4/N2mixture breakthrough was TDX-01(carbon molecular sieve)and zeolite 5A,respectively.The detector in the GC was a thermal conductivity detector and the temperature of the GC column was 80°C.In addition,the residence time of the gas in the column was about 5(CH4/N2)to 10 min(CO2/CH4).
3.Results and Discussion
3.1.Synthesis and characterization
The 2D MOF was obtained using an aqueous solution of Cu(OTf)2and various solvent-solution systems to regulate the materials structure.Which are listed in the Table S1(Supplementary Material)and their powder XRD patterns shown the Fig.S1(Supplementary Material).In addition,we also chose different solvents to dissolve 4,4′-bpy such as dichloromethane(CH2Cl2),tetrachloromethane(CCl4)and tetrahydrofuran(THF),and it is regrettable that we did not obtain the material what we expected.However,in the process of changing the solvent-solution system,we gained a 3D structure upon adding KCF3BF3to the aqueous solution of Cu(OTf)2as a directing agent.
Fig.2 shows the simulated and experimental XRD patterns of the MOFs.It was confirmed that we had successfully synthesized the 2D and 3D MOFs since the peak positions and relative diffraction intensities were consistent with the standard data[15–17].
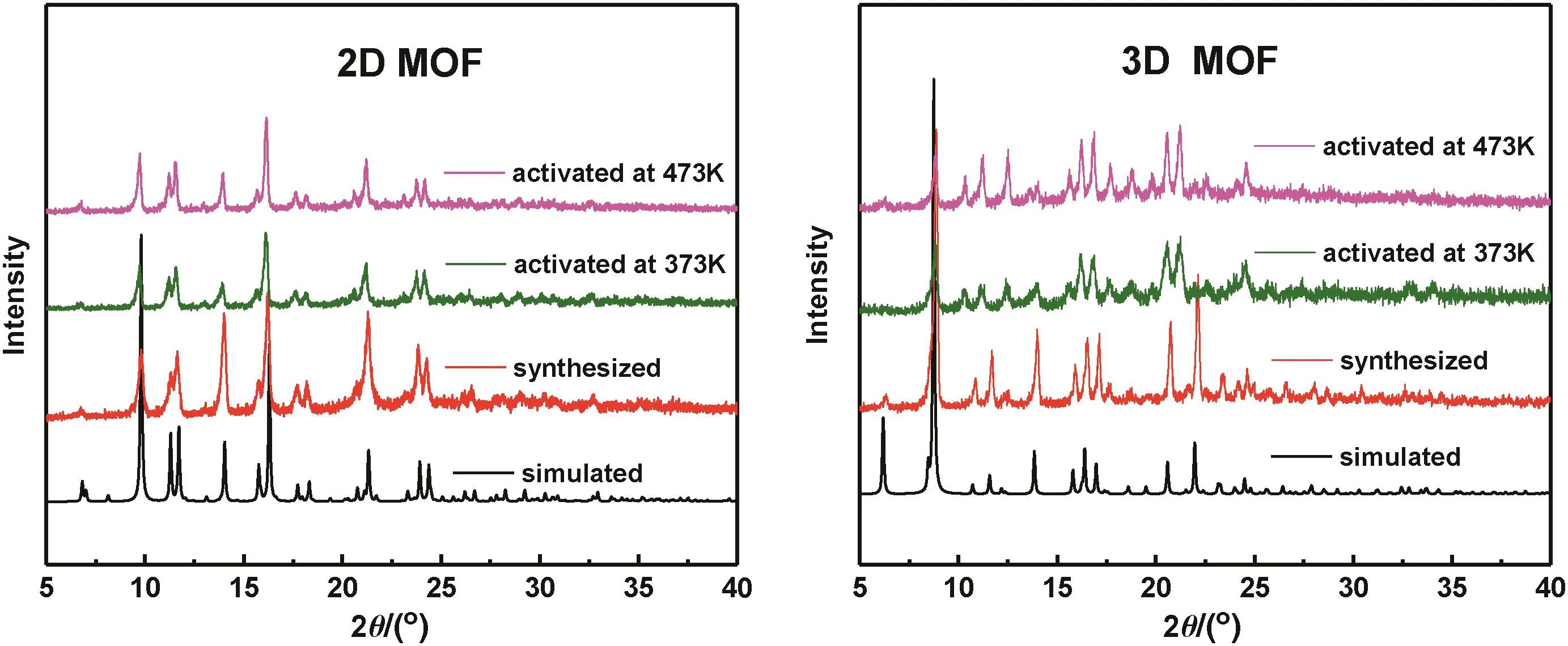
Fig.2.The XRD patterns of the 2D and 3D MOFs.
Thermal analyses showed that the two materials have higher stability(Fig.3).However,both samples showed steady mass at temperatures lower than 473 K and the 2D MOF was more stable than 3D MOF up to 523 K.In addition,both samples decomposed in two steps.The first collapse temperature range for the 2D MOF was 523–573 K,which was narrower than the 3D MOF range of 473–563 K,and then both samples began to undergo the second decomposition step until 700 K.
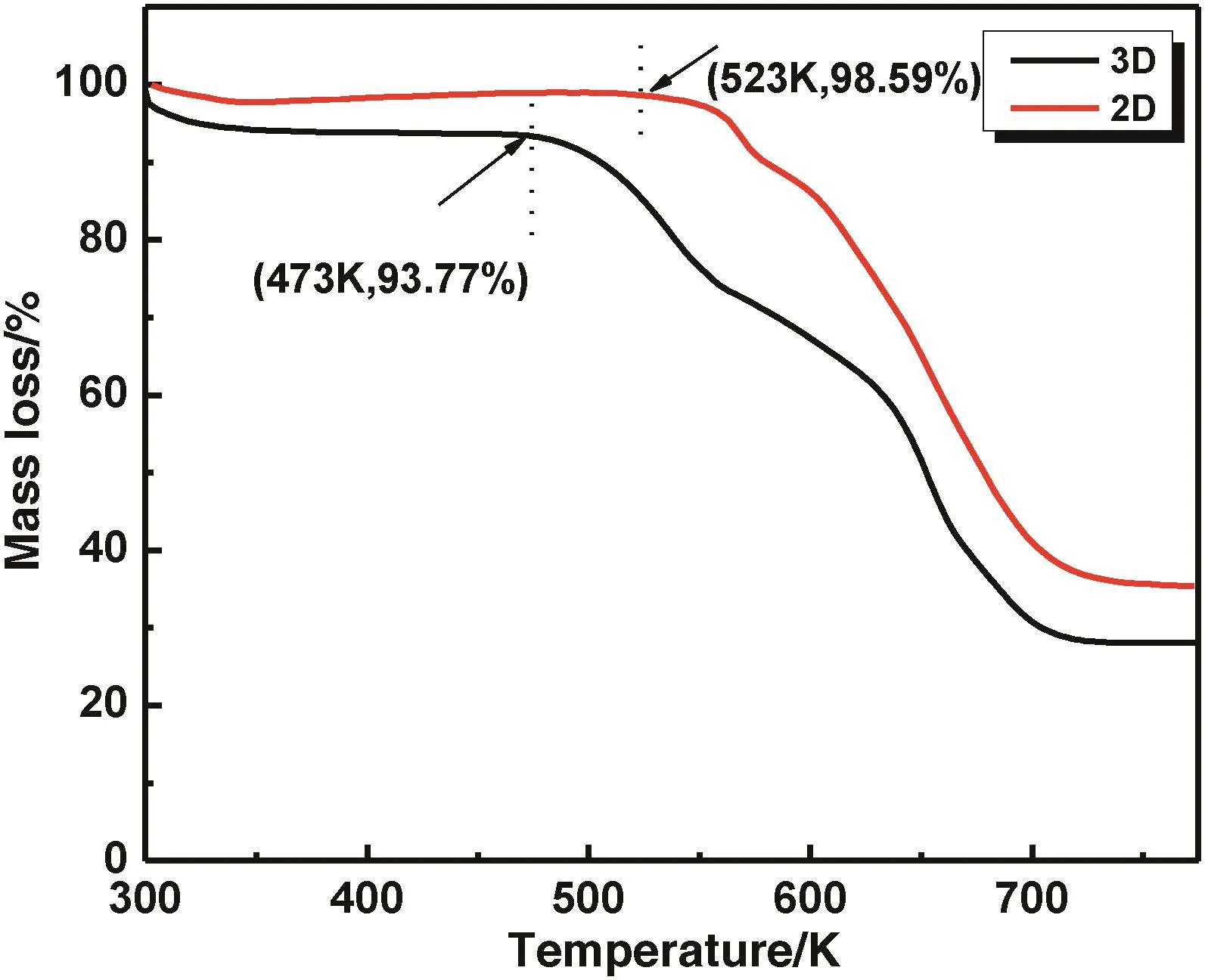
Fig.3.The TGA curves obtained for the 2D and 3D MOFs.
The morphologies of the 2D and 3D MOFs were observed using scanning electron microscopy(SEM)and are shown in Fig.4.As described above,the 2D and 3D frameworks have the same‘square planar’units in which each metal was coordinated by four 4,4′-bipyridine(4,4′-bpy)ligands in the equatorial positions and two OTf anions in the axial positions.When these ‘square planar’units were connected in the same plane,the 2DMOF was formed with a square-sheet and layered structure as shown in Fig.4.The side length was about 100 μm and the thickness of the layer was about 10 μm.While the 3D structure consisted of identical interwoven nets of these units,giving an octahedral shape and a size of about 10 μm.
The N2sorption isotherms obtained for the two MOFs at 77 K are shown in Fig.S2.For the 2D MOF,the adsorption branch was characterized by a definite double step and a marked hysteresis was present.The gate-opening pressure was 0.03 MPa and the total adsorbing capacity was about 190 cm3·g-1.While the isotherm obtained for the 3D MOF displayed Type Ι behavior and the total adsorption capacity was only about a half of that found for the 2D MOF.This was attributed to the 2D MOF having a structural transformation at 77 K,which resulted in a sudden increase in the adsorption quantity.Then,the Brunauer–Emmett–Teller(BET)surface areas were calculated according to the N2adsorption isotherms at 77 K.The 2D MOF had a similar BET surface area(205.6 m2·g-1)to the 3D MOF(225.4 m2·g-1)and the pore volume was 0.09 cm3·g-1(before gate opening)and 0.15 cm3·g-1(after gate opening);the 3D MOF had a pore volume 0.12 cm3·g-1.
3.2.Single-component adsorption measurements
Fig.5 shows the adsorption equilibrium isotherms obtained for CO2,CH4and N2on the 2Dand 3DMOFs at temperatures of263,273,283 and 298 K and a pressure of 0.1 MPa.All the isotherms displayed Type Ι behavior on the 2D and 3D MOFs,which was attributed to micropore adsorption,with almost no change to the structure of the adsorbent.It should be pointed out that only the adsorption isotherms are given because the desorption isotherms showed no hysteresis.As with the previous description,the flexible MOFs showed a step-adsorption phenomenon at a certain temperatures and pressures[23,24],and the 2D MOF showed none of this behavior in our testing range.In addition,Fig.5 shows that the CO2,CH4and N2adsorption capacities on the 3D MOF were slightly higher than those found on the 2D MOF.This is because gate opening phenomena of 2D MOF will not happen at this pressure(<0.1 MPa),so 3DMOF has a largerpore volume(0.12 cm3·g-1)to accommodate guest molecules than 2D MOF(0.09 cm3·g-1).It can be seen that all the materials have a higher adsorption at low temperatures,decreasing with increasing temperature,due to the adsorption of CO2,CH4and N2viaphysical adsorption on both the samples.As shown in Fig.5,the 2D and 3D MOFs can take a moderate amount of CO2(17.8 and 24.2 cm3·g-1)but a negligible amount of CH4(5.7 and 7.9 cm3·g-1)at 0.1 MPa and 298 K,and with a similar adsorption ratio of CO2/CH4was 3.1 and 3.0,respectively.In this comparison,the two materials can be seen to be promising adsorption materials to separate CO2/CH4atroom temperature.Under the same conditions,though the volumes of CH4and N2adsorption(1.6 and 2.5 cm3·g-1)were lower than CO2,the adsorption ratios of CH4/N2was 3.6 for the 2D MOF and 3.2 for 3D MOF,which indicated that the 2D MOF has greater potential for selective adsorption than the 3D MOF,and that both MOFs could separate CH4/N2mixtures.

Fig.4.SEM of the 2D(a)and 3D(b)MOFs.
3.3.Adsorption heats
In order to estimate the temperature change during the adsorption process and design the experimental scheme,we often consider the isosteric heat of adsorption(Qst)in a gas adsorption process.TheQstis the adsorption isosteric enthalpy(-ΔH),which can be calculated as a function of loading using the adsorption data at different temperaturesviathe Clausius–Clapeyron equation[25]:
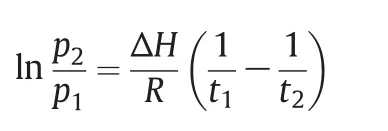
wherepis the pressure in MPa,tis the temperature in K andRis the gas constant(8.314 J·K-1·mol-1).When considering Fig.6,it was found that CO2has a higher adsorption heat than CH4and N2on both the 2D and 3D MOFs.This order was consistent with the gas polarization data(CO2,26.5×10-25cm3;CH4,26.0×10-25cm3;N2,17.6×10-25cm3).Combining the results of their single component isotherms,one can expect that the two samples will show preferential adsorption of CO2over CH4and CH4over N2in a binary mixture,making the 2D and 3D MOFs potentially useful materials in CO2/CH4and CH4/N2separation.The results also showed that the adsorption heat of CO2,CH4and N2on the 2D MOF was higher than that found on the 3D MOF,which indicated the binding force of the host-guest on the 2D MOF was stronger than that found for the 3D MOF.Combining these results with the adsorption ratios of CO2/CH4(3.1 and 3.0)and CH4/N2(3.6 and 3.2)for 2D and 3D MOF,it can be expected that the 2D MOF has a better separation effect than the 3D MOF.In addition,the difference of the interaction force results in the intensity of the structural transformation,which was observed as a clear difference of the gate-opening pressures on the 2D MOF with different gases[26].
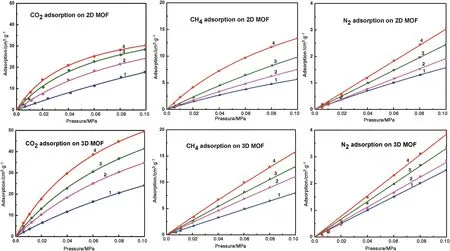
Fig.5.CO2,CH4 and N2 adsorption on the 2D and 3D MOFs at 263–298 K(solid,experimental point;line,Langmuir fitting curve.1—298 K;2—283 K;3—273 K;4—263 K).
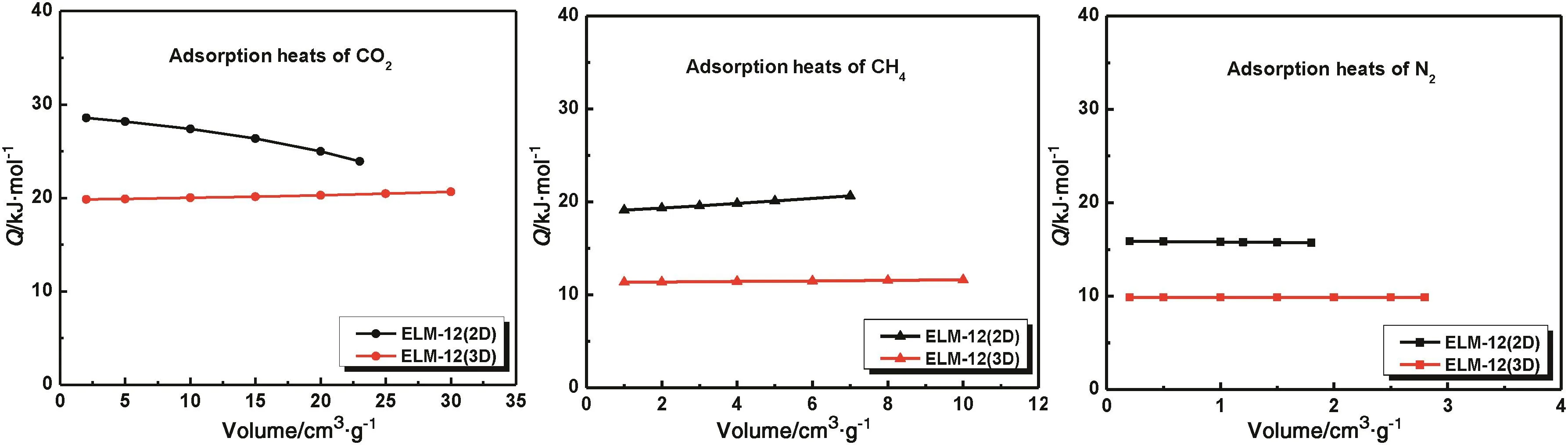
Fig.6.Adsorption heats for CO2,CH4 and N2 on the 2D and 3D MOFs.
3.4.Adsorption selectivity
Adsorbents must have a sufficient adsorption capacity or good adsorption separation performance,but high selectivity plays a decisive role.Generally,the most accurate method for testing the separation of a gas mixture is to use a binary adsorption equilibrium system,giving the separation selectivities for binary mixtures of CO2/CH4(50%/50%)and CH4/N2(50%/50%)at different pressures according to the ideal adsorbed solution theory(IAST)model[27,28].Analytical expressions of the adsorption isotherm are needed to apply the method and in the present work a Langmuir equation was used[29].The two-equation systemwas then solved simultaneously for the phase equilibrium to determine the component molar fractions in the gas(xg)and adsorbed phases(xa),as follows:

The selectivity values were calculated using the following relationship:
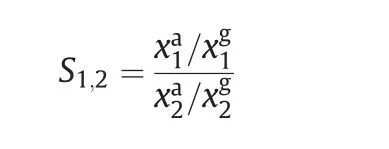
The adsorption selectivity values for equimolar mixtures of CO2/CH4and CH4/N2on the 2D and 3D MOFs at 273–298 K and 0.1 MPa are shown in Table 1(detailed data are shown in Table S2.).It can be seen that the values ofSCO2/CH4on the two samples are close at 298 K,which means their separation ability was similar.However,the SCO2/CH4value on the 2D MOF has a large difference with the 3D MOF,when we calculated the results at 283 and 273 K.In other words,the 2D MOF showed a greater potential for the separation of CO2and CH4compared to the 3D MOF at lower temperatures.
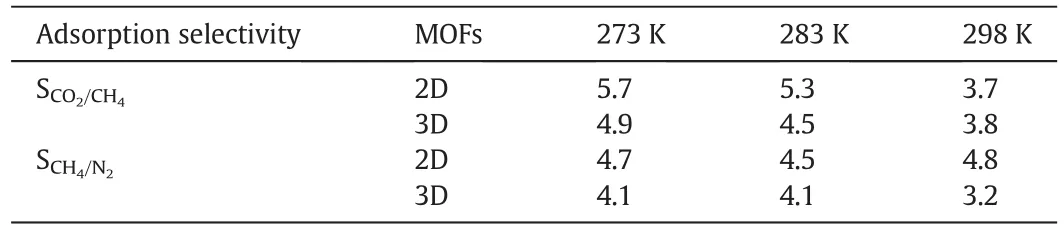
Table 1Adsorption selectivity for equimolar mixtures of CO2/CH4(50%/50%)and CH4/N2(50%/50%)on the 2D and 3D MOFs
Unlike the selectivity values for CO2/CH4,however,SCH4/N2showed better separation selectivity at 298 K,the value for the 2D MOF was 4.8 while the 3D MOF was 3.2,which exhibited greater potential for the separation of CH4and N2.At lower temperatures,the 2D MOF showed greater potential for the separation ability than the 3D MOF,though the difference is small.
Based on these calculations,we speculated that the separation effect of the 2D MOF was better than the 3D MOF.To further analyze the gas separation behavior of the two samples,we measured the breakthrough separation experiments for CO2/CH4and CH4/N2mixtures on our homemade apparatus.
3.5.Breakthrough experiments:2D versus 3D MOF
We measured the breakthrough curves using a column with a length of 150 mm and an internal diameter of 9 mm,packed with 4.6 g of 2D MOF and 4.3 g of 3D MOF pellets.To further analyze this behavior in other porous materials,we compared our data with the three kinds of commercial adsorbents and two MOFs,respectively:zeolite-4A(5.1 g,Aladdin,China),zeolite-5A(5.4 g,Aladdin,China),activated carbon,MIL-100(Cr)and Cu3(BTC)2.It is worth mentioning that the breakthrough curves for MIL-100(Cr)and Cu3(BTC)2were obtained in our previous work[21,30].The CO2,CH4,and N2breakthrough curves were measured for these zeolites and the results were shown in Figs.S3 and S4.We activated the samples by flushing the adsorption bed with heat 373 K for 5 h before cooling it to room temperature.
Fig.7 shows the breakthrough curves for the separation of CO2/CH4(50%/50%)at 298 and 273 K at 0.1 MPa with a flow gas velocity of 10 ml·min-1.According to the single gas adsorption curve,the adsorption capacity ofCO2was higher than CH4on both samples,which shows high CO2adsorption selectivity and causes longer elution times.So CH4first breaks through from the gas mixture and CO2breaks through after a further period of time.In addition,good materials for gas separation require not only a longer retention time but also a shorter adsorption equilibrium time[21].Table 2 shows the breakthrough times obtained for CO2and CH4on the MOF samples at 298 K and 0.1 MPa.From Table 2,when the flow gas velocity was 10 ml·min-1,the retention time on the 2D MOF was slightly longer than that found on the 3D MOF,and far away from the zeolites.Considering the pore sizes of the 2D and 3D MOFs both were 0.8–1.0 nm,and zeolite 4A and 5A were about 0.4 nm and 0.5 nm,respectively.So CH4,CO2and N2can be adsorbed in the pores of all the samples(MOFs and zeolites),which indicates that the pore size was not a key factor for CO2/CH4/N2separation.The metal sites of the MOFs have stronger interactions with small molecular gases than the cations in the zeolites,leading to the separation of CO2/CH4/N2mixtures on the zeolite being less good than that found on the MOFs used in this research.When comparing the 2D and 3D MOFs with MIL-100(Cr)and Cu3(BTC)2,due to the difference of flow gas velocity,we added the breakthrough curves obtained for the 2D and 3D MOFs at 298 K and 0.1 MPa with a flow gas velocity of 20 ml·min-1in Fig.S5.It shows the elution time both are 5.5 min for the 2D MOF at 20 ml·min-1and Cu3(BTC)2at 16.6 ml·min-1and because the elution time will be longer with a reduced flow gas velocity,the 2DMOF has a better separation of CO2/CH4than Cu3(BTC)2.For MIL-100(Cr),the elution time cannot be compared in detaildue to the difference in the conditions,but it can be inferred that it has a similar separation ability as the 2D and 3D MOFs for CO2/CH4separation.In addition,it is obvious the CO2/CH4separation abilities of both 2D and 3D were better than activated carbon.When comparing 2D and 3D MOFs,though they are both flexible frameworks,the 2D MOF has a dramatic structural transformation compared to the 3D MOF,which indicates that the 2D has stronger host–guest interactions than the 3D MOF.The adsorption heat for CO2,CH4and N2on the 2D MOF was higher than those found on the 3D MOF,therefore,the 2D MOF has a stronger adsorbing ability and longer retention time.In addition,the 2D MOF quickly reached equilibrium,which is favorable for separation from the viewpoint of the technology.
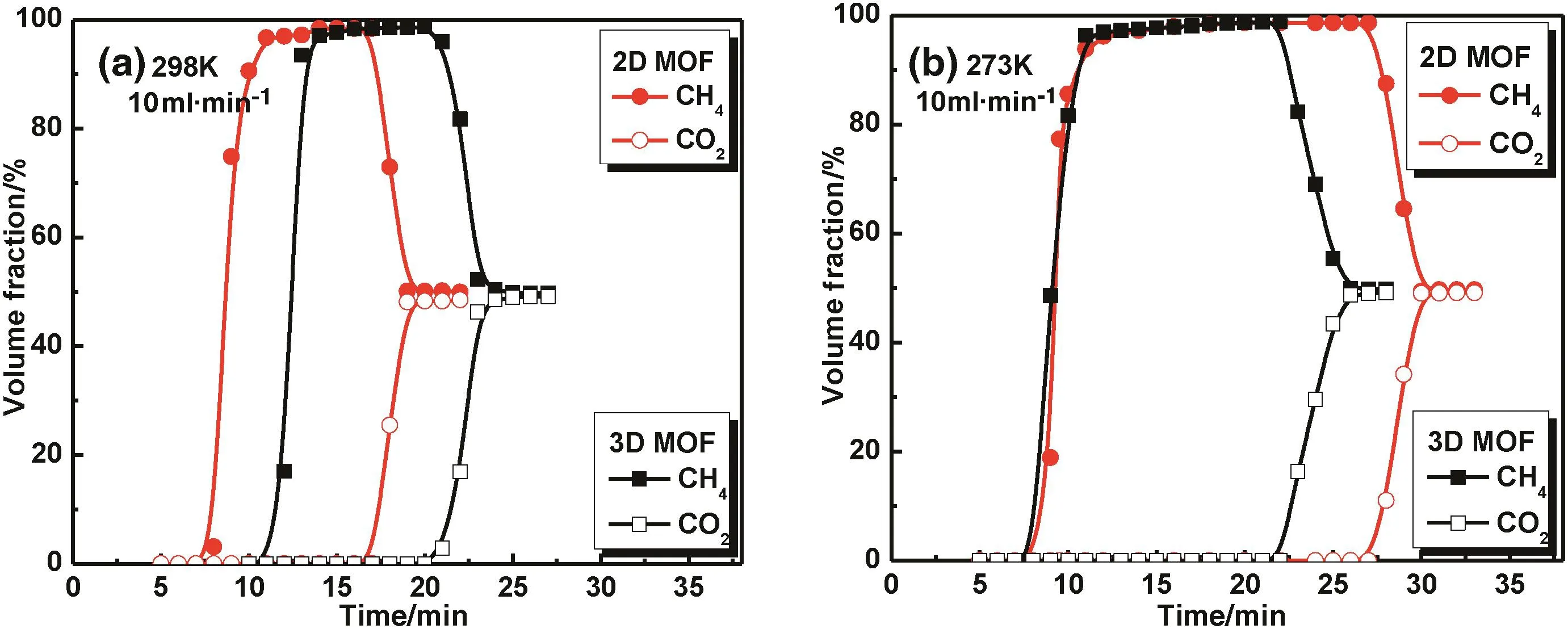
Fig.7.Breakthrough curves for the gas separation experiments at 10 ml·min-1 using CO2/CH4(50%/50%)mixtures at 298 K(a)and 273 K(b).

Table 2Breakthrough times for gas separation experiments using CO2/CH4(50%/50%)mixtures at 298 K and 0.1 MPa.
Fig.7 shows that the 2D MOF has an obviously longer retention time at273 K,which can be attributed to CO2having an advantage during the competitive adsorption between CH4and CO2at lower temperature,so the 2D sample has a higher CO2/CH4adsorption selectivity than that found at298 K.It was found that the gas separation ability of the flexible MOFs was significantly affected by temperature because the competitive adsorption increases with decreasing temperature.
CH4and N2are usually difficult to separate because they have very similar physical properties,however,we found a better separation of CH4/N2on the two MOFs.Fig.8 shows the breakthrough curves for CH4and N2upon separation of a CH4/N2(50%/50%)mixture at 298 and 273 Kat0.1 MPa.As we predicted from on the single gas adsorption curves,the adsorption capacity for CH4was higher than N2on both samples,which exhibit high CH4adsorption selectivity and causes longer elution times.So,N2breaks through first from the adsorption,followed by CH4after a period of time.Table 3 shows the breakthrough times of CH4and N2on the MOF samples at 298 K and 0.1 MPa.With a flow gas velocity of 10 ml·min-1,the elution times for the zeolites were very short and the elution time observed for the 2D MOF was slightly longer than those for MIL-100(Cr)and the 3D MOF.When compared with Cu3(BTC)2,the 3D MOF has the same elution time but smaller flow gas velocity,so the separation effect is not better than Cu3(BTC)2,and the 2D MOF cannot be compared in detail due to the difference in conditions.It should be noted that activated carbon has the best separation effect for CH4/N2.In conclusion,the 2D MOF has a longer elution time and is potentially useful for separating methane from a CH4/N2mixture.
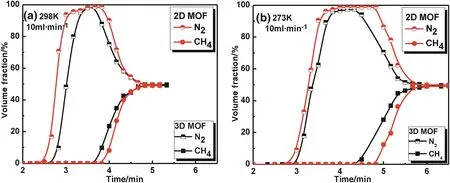
Fig.8.Breakthrough curves for the gas separation experiments at 10 ml·min-1 using CH4/N2(50%/50%)mixtures at 298 K(a)and 273 K(b).

Table 3Breakthrough times for gas separation experiments using CH4/N2(50%/50%)mixtures at 298 K and 0.1 MPa.
3.6.Cycling measurements
The regenerability of an adsorption material is one of its most important properties.To ensure the regenerability of the flexible 2D and 3D MOFs,we performed CO2/CH4separation cycling experiments on the two MOFs at 298 K at 0.1 MPa with a gas velocity of 10 ml·min-1(Fig.9).In the above CO2/CH4separation experiments,we found that CO2/CH4can be efficiently separated by the two flexible MOFs for 1 cycle.Upon further investigation,after five gas mixture separation cycles,the breakthrough profiles for the two samples were clearly well retained(Fig.9).In order to make the results more accurate,after every experiment,we activated all the samples by flushing the adsorption bed at 323 K for 1 h purging it with the carrier gas(He≥99.999%)at the same time.The results showed the 2D and 3D MOFs have good regenerability,making the 2Dand 3DMOFs potentially useful materials in gas separation.
4.Conclusions
The relationship between the sorbent structures and separation properties is very important for adsorption separation technology.In this report,we have synthesized two isomeric MOFs(2D and 3D structures)with identical composition,which were characterized by XRD,TGA and SEM.We studied their CO2,CH4and N2adsorption properties and calculated the adsorption heats and adsorption selectivity based on the adsorption isotherms.The results showed that the 2D MOF was potentially useful for separating CO2/CH4and CH4/N2even with its lower surface area and pore volume.Further analysis of the gas separation behavior using the two samples via dynamic separation experiments indicated that the 2DMOF possessed a significantly higher separation selectivity for CO2/CH4when compared to the 3DMOF at273 K.The separation experiment using CH4/N2showed that the elution time of the 2D MOF was longer than the 3D MOF.As a consequence,the 2D structure appears to be the better candidate as an adsorbent for CH4/CO2and CH4/N2separation when compared to the 3D structure of these two isomeric MOFs.

Fig.9.CO2/CH4(50%/50%)separation cycling experiments on the 2D(left)and 3D(right)MOFs at 298 K and 0.1 MPa.
Supplementary Material
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.cjche.2016.05.013.
[1]S.Kenji,D.L.Rogow,J.A.Mason,T.M.McDonald,E.D.Bloch,Z.R.Herm,T.H.Bae,J.R.Long,Carbon dioxide capture in metal–organic frameworks,Chem.Rev.112(2012)724–781.
[2]Z.J.Zhang,Y.Q.Zhao,Q.H.Gong,Z.Li,J.Li,MOFs for CO2capture and separation from flue gas mixtures:The effect of multifunctional sites on their adsorption capacity and selectivity,Chem.Commun.49(2013)653–661.
[3]P.L.Llewellyn,S.Bourrelly,C.Serre,Y.Filinchuk,G.Férey,How hydration drastically improves adsorption selectivity for CO2over CH4in the flexible chromium terephthalate MIL-53†,Angew.Chem.Int.Ed.118(2006)7915–7918.
[4]J.R.Li,J.L.Sculley,H.C.Zhou,Metal–organic frameworks for separations,Chem.Rev.112(2012)869–932.
[5]S.Sircar,Publications on adsorption science and technology,Adsorption6(2000)359–365.
[6]J.F.Yang,J.M.Li,W.Wang,L.B.Li,J.P.Li,Adsorption of CO2,CH4,and N2on 8-,10-,and 12-membered ring hydrophobic microporous high-silica zeolites:DDR,silicalite-1,and beta,Ind.Eng.Chem.Res.52(2013)17856–17864.
[7]S.Sircar,Application of gas separation by adsorption for the future,Adsorpt.Sci.Technol.19(2001)347–366.
[8]T.Fukushima,S.Horike,Y.Inubushi,K.Nakagawa,Y.Kubota,M.Takata,S.Kitagawa,Solid solutions of soft porous coordination polymers:Fine-tuning of gas adsorption properties,Angew.Chem.Int.Ed.49(2010)4820–4824.
[9]H.Sato,W.Kosaka,R.Matsuda,A.Hori,Y.Hijikata,R.V.Belosludov,S.Sakaki,M.Takata,S.Kitagawa,Self-accelerating CO sorption in a soft nanoporous crystal,Science343(2014)167–170.
[10]L.B.Li,J.F.Yang,Q.Zhao,J.P.Li,One-dimensional inter penetrated coordination polymers showing step gas sorption properties,Cryst Eng Comm15(2013)1689–1692.
[11]A.Kondo,N.Kojima,H.Kajiro,H.Noguchi,Y.Hattori,F.Okino,K.Maeda,T.Ohba,K.Kaneko,H.Kanoh,Gas adsorption mechanism and kinetics of an elastic layer structured metal–organic framework,J.Phys.Chem.C116(2012)4157–4162.
[12]C.Y.Lee,Y.S.Bae,N.C.Jeong,O.K.Farha,A.A.Sarjeant,C.L.Stern,P.Nickias,R.Q.Snurr,J.T.Hupp,S.T.Nguyen,Kinetic separation of propene and propane in metal organic frameworks:Controlling diffusion rates in plate-shaped crystals via tuning of pore apertures and crystallite aspect ratios,J.Am.Chem.Soc.133(2011)5228–5231.
[13]Y.Zhao,H.Wu,T.J.Emge,Q.Gong,N.Nijem,Y.J.Chabal,L.Kong,D.C.Langreth,H.Liu,H.Zeng,J.Li,Enhancing gas adsorption and separation capacity through ligand functionalization of microporous metal–organic framework structures,Chem.Eur.J.17(2011)5101–5109.
[14]T.A.Makal,A.A.Yakovenko,H.C.Zhou,Isomerism in metal–organic frameworks:Framework isomers,J.Phys.Chem.Lett.2(14)(2011)1682–1689.
[15]L.Carlucci,N.Cozzi,G.Ciani,M.Moret,D.M.Proserpio,S.Rizzato,A threedimensional nanoporous flexible network of ‘square-planar’copper(II)centres with an unusual topology,Chem.Commun.13(2002)1354–1355.
[16]A.Kondo,H.Noguchi,L.Carlucci,D.M.Proserpio,G.Ciani,H.Kajiro,T.Ohba,H.Kanoh,K.Kaneko,Double-step gas sorption of a two-dimensional metal–organic framework,J.Am.Chem.Soc.129(2007)12362–12363.
[17]A.Kondo,H.Kajiro,H.Noguchi,L.Carlucci,D.M.Proserpio,G.Ciani,K.Kato,M.Takata,H.Seki,M.Sakamoto,Y.Hattori,F.Okino,K.Maeda,T.Ohba,K.Kaneko,H.Kanoh,Super flexibility of a 2D Cu-based porous coordination framework on gas adsorption in comparison with a 3D framework of identical composition:Framework dimensionality-dependent gas adsorptivities,J.Am.Chem.Soc.133(2011)10512–10522.
[18]H.Kajiro,A.Kondo,K.Kaneko,H.Kanoh,Flexible two-dimensional square-grid coordination polymers:Structures and functions,Int.J.Mol.Sci.11(2010)3803–3845.
[19]A.Kondo,K.Maeda,Anisotropic thermal expansion of a 3D metal–organic framework with hydrophilic and hydrophobic pores,J.Solid State Chem.221(2015)126–131.
[20]T.D.Tran,Molecular simulation of carbon dioxide capture on elastic layered metal–organic framework adsorbents,University of Michigan,2012.
[21]L.B.Li,J.F.Yang,J.M.Li,Y.Chen,J.P.Li,Separation of CO2/CH4and CH4/N2mixtures by M/DOBDC:A detailed dynamic comparison with MIL-100(Cr)and activated carbon,Microporous Mesoporous Mater.198(2014)236–246.
[22]J.F.Yang,R.Krishna,J.M.Li,J.P.Li,Experiments and simulations on separating a CO2/CH4mixture using K-KFI at low and high pressures,Microporous Mesoporous Mater.184(2014)21–27.
[23]J.F.Yang,Q.H.Yu,Q.Zhao,J.M.Liang,J.X.Dong,J.P.Li,Adsorption CO2,CH4and N2on two different spacing flexible layer MOFs,Microporous Mesoporous Mater.161(2012)154–159.
[24]T.K.Maji,R.Matsuda,S.Kitagawa,A flexible interpenetrating coordination framework with a bimodal porous functionality,Nat.Mater.6(2007)142–148.
[25]T.L.Hill,Statistical mechanics of adsorption.V.Thermodynamics and heat of adsorption,J.Chem.Phys.17(1949)520–535.
[26]R.Kitaura,K.Seki,G.Akiyama,S.Kitagawa,Porous coordination-polymer crystals with gated channels specific for supercritical gases,Angew.Chem.Int.Ed.42(2003)428–431.
[27]A.L.Myers,Equation of state for adsorption of gases and their mixtures in porous materials,Adsorption9(2003)9–16.
[28]Y.He,R.Krishna,B.Chen,Metal–organic frameworks with potential for energy efficient adsorptive separation of light hydrocarbons,Energy Environ.Sci.5(2012)9107.
[29]J.K.Syers,M.G.Browman,G.W.Smillie,Phosphate sorption by soils evaluated by the Langmuir adsorption equation,Soil Sci.Soc.Am.J.37(1973)358–363.
[30]J.M.Li,J.F.Yang,L.B.Li,J.P.Li,Separation of CO2/CH4and CH4/N2mixtures using MOF-5 and Cu3(BTC)2,J.Energy Chem.23(4)(2014)453–460.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Hemicellulose in corn straw:Extracted fromalkali solution and produced 5-hydroxymethyl furfural in HCOOH/HCOONa buffer solution☆
- Molar volume of eutectic solvents as a function of molar composition and temperature☆
- Investigation on molar heat capacity,standard molar enthalpy of combustion for guaiacol and acetyl guaiacol ester
- Statistical mechanics and artificial intelligence to model the thermodynamic properties of pure and mixture of ionic liquids☆
- A comprehensive fractal char combustion model☆
- Development of a bifurcation analysis approach based on gPROMS platform☆
