Influence of synthesis parameters on the properties of LiFePO4/C cathode material☆
2016-06-01ZhengweiXiaoYingjieZhangGuorongHu
Zhengwei Xiao ,Yingjie Zhang ,*,Guorong Hu
1 Faculty of Metallurgical and Energy Engineering,Kunming University of Science and Technology,Kunming 650093,China
2 School of Metallurgy and Environment,Central South University,Changsha 410083,China
1.Introduction
The issues of energy crisis and pollution are formidable to mankind and a sustainable development needs green energy supply.The burning of fossil fuels for heat and electricity generation has long seriously contributed to the rise in CO2concentration in atmosphere,resulting in drastic climate changes worldwide.At present,electric vehicles are regarded as the solution to CO2emission reduction through higher energy efficiency by making use of regenerative braking[1].However,this reduction is limited by the present electrical energy supply nearly 70%of which is generated by burning fossil fuels[2].Thus,the adoption of electric vehicles in cities to a certain degree can only transfer urban pollution to places where electricity is generated[3].The introduction of a green grid is the ultimate solution,but the solar and wind power is unstable due to weather changes and as a result causes fluctuations on the grid.Therefore,the increase in percentage of renewable power on the grid depends on the successful large-scale stationary storage of electrical energy[4].
The lithium ion cell outperforms other battery systems,such as leadacid,Ni-Cd and Ni-MH,in many aspects,for example,cell voltage,gravimetric and volumetric energy density/power,cycle life and so on[5].Among the cathode materials for lithium ion cells,olivine-structured LiFePO4holds the desirable merits of abundant raw materials,nontoxicity,high thermal stability,suitable voltage of 3.45 V(vsLi+/Li)and theoretical capacity of 170 mA·h·g-1[6-8].It meets both demands of high energy density and environmental friendliness and is an adequate cathode for power battery and stationary storage of electrical energy generated by renewable power[9].The worst drawback of the cathode is its intrinsic low electronic conductivity and a viable solution is the application of LiFePO4/C composite[10].
The adoption of Fe2+source FeC2O4·2H2O for LiFePO4/C synthesisviasolid state reaction possesses advantageous attributes of simple procedure and product with good electrochemical performance,which is in particular suitable for mass production of the cathode material[11].But FeC2O4·2H2O,along with FePO4·2H2O and Fe3(PO4)2·8H2O,contains crystal water,which tends to be lost and brings about the need of chemical analysis for stoichiometric use in LiFePO4/C preparation.In addition,the oxidation of Fe2+in FeC2O4·2H2O and Fe3(PO4)2·8H2O results in the same issue.Fe2O3is chemically stable,consists of no crystallized water and is an ideal iron source for producing LiFePO4/C.The ferric iron in Fe2O3must be reduced in LiFePO4formation,which is generally realized by use of carbon or carbon-containing reductants[12].In the study,Fe2O3,NH4H2PO4,Li2CO3and glucose(C6H12O6·H2O)are applied to LiFePO4/C synthesis and effects of synthesis parameters on the properties of LiFePO4/C are investigated.The interesting results of low sintering temperature favoring carbon maintenance in LiFePO4/C and ball-milling dispersive agent affecting the properties of LiFePO4/C are for the first time reported by the work.
2.Experimental
Stoichiometric Fe2O3,NH4H2PO4and Li2CO3and certain amount of glucose were mixed and ball-milled for 4 h in a dispersive agent to ensure homogenous mixing.The pulp was dried at 80°C overnight to vaporize volatile components.The dried mixture was pressed in a crucible and sintered for15 h in an argon atmosphere.Changes in sintering temperature,carbon content and dispersive agent were carried out for LiFePO4/C synthesis.Carbon content of LiFePO4/C was determined by dissolving LiFePO4/C in hydrochloric acid to collect insoluble carbon for calculating the mass percentage of dried carbon in LiFePO4/C.
X-ray powder diffractions(XRD)of sintered products were performed on a Philips X-pert powder diffractometer using Cu Kαradiation.The morphology of samples was characterized using scanning electron microscopy(SEM)on a JSM-5600LV,JEOL.
Button cells of 2025 type based on LiFePO4/C cathode were assembled in a glovebox.The charge-discharge process was realized galvanostatically on a LAND BTI-40 in 2.5-4.1 V.Electrolyte was 1 mol·L-1LiPF6dissolved in a mixed solvent of ethylene carbonate(EC),dimethyl carbonate(DMC)and ethylmethyl carbonate(EMC)with volume ratio of 1:1:1.Lithium disc was used as the anode electrode,and membrane was microporous polypropylene Celgard 2400.The cathode electrode consisted of LiFePO4/C,PVdF binder and acetylene black with mass ratio of 8:1:1.N-methyl pyrrolidinone was used as the organic solvent ground along with the three cathode ingredients to dissolve PVdF binder,and the obtained slurry was evenly spread on an Al foil current collector.The wet cathode electrode was dried under vacuum at 120°C overnight.Cathode discs with an area of 0.785 cm2and LiFePO4/C load of 2 mg each were punched for cell assembly.
3.Results and Discussion

Fig.1.XRD patterns of products sintered at different temperatures.Δ—Fe2O3,*—Li3PO4,□—Li3Fe2(PO4)3,○—Fe2P.
Fig.1 indicates that LiFePO4starts crystallizing even below 300°C.Fe2O3,NH4H2PO4,Li2CO3and glucose can be completely converted into LiFePO4/C at 500°C below which the main impurities in the sintered products are Fe2O3,Li3PO4and Li3Fe2(PO4)3.In particular at 300°C,Li3Fe2(PO4)3is detected,which is similar to the work by Ravet and co-workers.in which FePO4·2H2O instead of Fe2O3and NH4H2PO4was used as the Fe and PO4sources[13].For convenience,the samples obtained at 500 °C,600 °C,700 °C and 800 °C are designated as LiFePO4/C500,LiFePO4/C600,LiFePO4/C700and LiFePO4/C800,respectively.Both LiFePO4/C500and LiFePO4/C600exhibita diffraction pattern indexed to an orthorhombic crystal structure,space group Pnma.A high degree of crystallinity for LiFePO4synthesized at 600°C is proved by the sharp and perfect characteristic peaks in its XRD pattern.No impurity phases consisting of lithium,iron,and/or phosphorus are detected,suggesting a high purity for the samples prepared at both 500 °C and 600 °C.Thus,a temperature above 500°C is high enough for complete crystallization of LiFePO4.No characteristic diffraction peaks for crystalline carbon are revealed,indicating the amorphous form of the conductive reagent derived from the anaerobic pyrolysis of C6H12O6·H2O.
With the elevation of sintering temperature in the range of 300-1000°C,the degree of crystallinity of LiFePO4increases,which is reflected in the smaller full width at half maximum(FWHM)of higher(311)peak of XRD pattern for LiFePO4obtained at a higher temperature.Meanwhile,a smaller FWHM endows a bigger crystallite size in accordance with the Scherrer equation.Thus,the elevation of temperature gives rise to the growth ofLiFePO4crystallites which tend to agglomerate harder.The samples sintered at 700 °C and 800 °C consist of Fe2P phase,as shown in Fig.1,and a higher temperature leads to a higher Fe2P content.As there is no Fe2P present in the samples obtained at 500°C and 600 °C,the phosphide formed above 600 °C must have been from Fe2+in LiFePO4instead of Fe3+[14]in the presence of carbon and PO4

In this way,carbon is consumed on Fe2P formation.This can partially explain the carbon content reduction trend of 14.15%,11.03%and 6.95%for LiFePO4/C formed at 600 °C,700 °C and 800 °C,respectively.However,other reasons to be revealed should be responsible for the carbon content difference between LiFePO4/C500(17.33%)and LiFePO4/C600.The presence ofFe2P has been proved to contribute to the improvement on the electrochemical performance of LiFePO4due to its high electrical conductivity[15,16].In the case of LiFePO4/Fe2P composite,Fe2P forms conductive connections between LiFePO4particles,exhibiting a similar behavior to the conductive carbon in LiFePO4/C composite.
Fig.2 indicates that sintering temperature exerts great influence on the morphology of LiFePO4/C,and the change is reflected mainly in two aspects,existence state of carbon and LiFePO4particle size.As indicated in Fig.2,as the sintering temperature increases in the range of 600-800°C the residual carbon in LiFePO4/C decreases.Accumulative amorphous carbon is seen in the SEM image of LiFePO4/C600,but the conductive carbon is only observed to form connections between LiFePO4particles in the samples prepared at 700 °C and 800 °C.
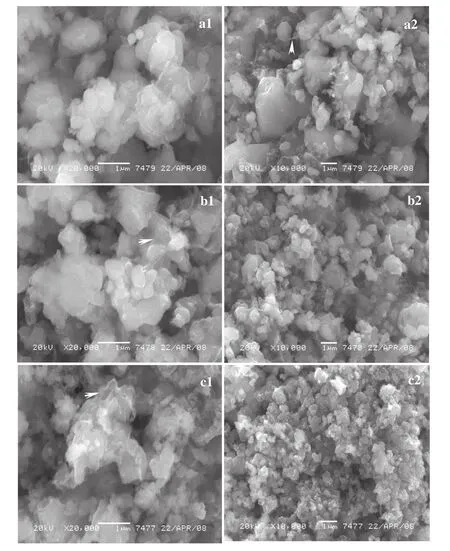
Fig.2.SEM images of LiFePO4/C synthesized at 800 °C(a1 and a2),700 °C(b1 and b2)and 600 °C(c1 and c2)(arrows showing amorphous carbon).
LiFePO4particle size increases significantly as the sintering temperature increases in the range of 600-800°C,which can be imputable to two principal reasons.Most primary particles in LiFePO4/C prepared at 600 °C are smaller than 1 μm which tend to fuse and agglomerate at elevated temperatures.The distinction between the smooth surface of LiFePO4in Fig.2(a2)and rough surface of LiFePO4in Fig.2(c2)gives the evidence for LiFePO4fusion.As discussed above,Fe2P formation consumes carbon whose loss makes the growth of LiFePO4particles more readily at higher temperatures since carbon is capable of inhibiting LiFePO4particle growth in LiFePO4/C preparation[17].
As 500°Cis high enough to obtain pure LiFePO4,the charge-discharge performance of the samples synthesized at 500 °C,600 °C,700 °C and 800°Cis examined,as shown in Fig.3.With the increase in sintering temperature,the discharge voltage plateau increases for LiFePO4/C prepared,which can be attributed to the higher degree of crystallinity of LiFePO4made at a higher temperature and formation of conductive Fe2P above 600°C.Though it possesses the highest carbon content,LiFePO4/C500displays inferior plateau voltages and biggest polarization in charge-discharge process.This can be ascribed to the low degree of crystallinity of LiFePO4made at the low temperature.LiFePO4/C800displays a relatively low discharge capacity.It can be attributed to the heavy loss of conductive carbon at this elevated temperature and deviation of molar ratio for Li:Fe:P from 1:1:1 in LiFePO4/C caused by formation of Fe2P.In addition,higher temperature favors the growth of LiFePO4crystallites/particles and more severe agglomeration of LiFePO4/C primary and secondary particles.Bigger crystallites/particles lead to longer paths for Li+migration in electrochemical process,which is unfavorable for its electrochemical kinetics[18].LiFePO4/C700exhibits the best charge-discharge performance among all the samples,although its carbon content is lower than that of LiFePO4/C600and its Fe2P content and degree of crystallinity are smaller than those of LiFePO4/C800.The principal reason accounting for the phenomenon is the compromised degree of crystallinity of LiFePO4and contents of conductive Fe2P and carbon.
An increased addition of glucose in preparation resulted in an increased carbon content in final product LiFePO4/C.Fig.4 shows the electrochemical performance of the samples with different carbon contents.It is demonstrated that when carbon content varies in the range of 6.02%-11.03%the discharge capacities and charge-discharge plateaus of the samples are similar,but the sample with 4.48%carbon content exhibits an obvious lower capacity and inferior plateau voltages.The samples with carbon contents of 11.03%,8.01%,6.02%and 4.48%deliver capacities of 150.2,152.1,146.7 and 137.6 mA·h·g-1,respectively,at 0.1C.The decrease in carbon content from 11.03%to 8.01%gives rise to an abnormal capacity increase of 2 mA·h·g-1.This can be attributed to too high a redox inactive carbon content reducing achievable capacity because the measured nominal capacity refers to that of LiFePO4/C but not that of pure LiFePO4in LiFePO4/C.A similar result[19]was observed by Tang and co-workers.
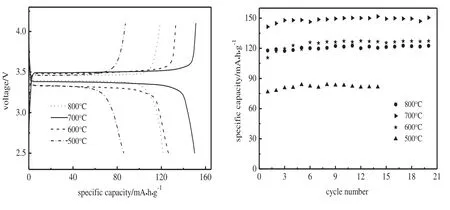
Fig.3.Charge-discharge profiles and rate capability of LiFePO4/C synthesized at different temperatures.

Fig.4.Charge-discharge profiles and rate capability of LiFePO4/C with different carbon contents.
At1C,the trend is apparent that higher carbon contents lead to higher discharge capacities.The two samples with carbon contents of 11.03%and 8.01%display similar capacities but the former is slightly higher.The unfavorable intrinsic low conductivity limits the practical use of LiFePO4,but the introduction of carbon has become the simplest and effective solution to the problem.Highercarbon contents facilitate efficient use of redox active LiFePO4in charge-discharge process.However,more incorporated carbon greatly reduces the tap density of LiFePO4/C composite,resulting in lower volumetric and gravimetric energy densities.By considering both capacity and tap density,the LiFePO4/C sample with 8.01%carbon is preferred to the one with 11.03%carbon.
Ethanol and acetone have been reported as grinding dispersive agents in ball-milling for LiFePO4/C synthesis[20,21].However,no work has compared their effect on the properties of the final products.In the study,ethanol,acetone and water were used respectively in ball-milling to mix and mechanically activate Fe2O3,NH4H2PO4,Li2CO3and glucose,and the as-prepared cathodes are designated as LiFePO4/Cethanol,LiFePO4/Cacetoneand LiFePO4/Cwater,respectively,for convenience.As indicated in Fig.5,the dispersive agent in milling is potent to affect the electrochemical performance of LiFePO4/C.First,the three samples exhibit distinct charge-discharge plateau voltages.The highest discharge voltage and lowest charge voltage are equivalent to the lowest polarization in charge-discharge process.Therefore,LiFePO4/Cacetoneshows superior plateau voltages to those obtained by using ethanol and water.Water is not suitable as a dispersive agent in milling,although it is the only one capable of dissolving glucose whose even distribution in ball-milled productfavors an even coating/mixing in LiFePO4/C.However,glucose melts at 130-150°C[22]and this property can ensure its good distribution to a certain extent in sintering.Both acetone and ethanol are qualified as dispersive agents in milling.The two samples synthesized by using the organic agents deliver a similar capacity at 0.1C,but at 1Cand 2C,the difference in discharge capacity for the two samples is distinct.LiFePO4/Cethanoland LiFePO4/Cacetoneexhibit an average of122.5 and 128.3 mA·h·g-1respectively at1Cin the first50 cycles,revealing a difference of 5.8 mA·h·g-1;at 2C,the corresponding data are 101.8 and 120.1 mA·h·g-1,revealing a difference of 18.3 mA·h·g-1which comprises 10%of the theoretical capacity of LiFePO4.Therefore,LiFePO4/Cacetonehas a superior rate capability to LiFePO4/Cethanol.
In SEM images of both LiFePO4/Cethanoland LiFePO4/Cacetone,conductive carbon is observed to be present between and on the LiFePO4particles and the LiFePO4particles are revealed to distribute between carbon connections,as shown in Fig.6.Thus,in the secondary particles of both samples,the carbon presents as a whole which is embedded with micrometer-sized LiFePO4particles,resulting in the formation of a continuous conductive connection between the LiFePO4particles.In this way,a mutual embedding state and an interconnected matrix for the conductive carbon and LiFePO4particles is achieved in the as-prepared LiFePO4/C,which may lead to fast electron transfer and small polarization for the low conductivity cathode material.Faster electron kinetics and smaller polarization bring about a better rate capability for the LiFePO4/C electrode.
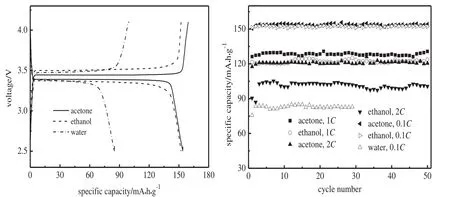
Fig.5.Charge-discharge profiles and rate capability of LiFePO4/C synthesized with different dispersive agents.
Micrometer-sized pores are seen in the secondary LiFePO4/C particles in Fig.6(a1)and(b1).These pores are capable of extending the available surface area and yield more sites for the electrochemical reaction which only proceeds at the points where active material,conductive carbon and electrolyte meet[23].LiFePO4/Cacetonediffers from LiFePO4/Cethanolin its more even carbon distribution than the latter,as shown in Fig.6(b1)and(b2).In LiFePO4/Cacetone,most LiFePO4particles are observed to be coated and well-connected by carbon,which is favorable for fast electron transfer incurred at high charge-discharge rates.Thus,at 1Cand 2C,LiFePO4/Cacetoneshows a much better charge-discharge performance than LiFePO4/Cethanol.
The difference in electrochemical performance for the three samples synthesized with ethanol,acetone and water may originate from the different states of dispersion,segregation and agglomeration of particles in dried ball-milled products,which affects the following solid state reaction and the physical properties of finalproducts.The three dispersive agents possess different functional groups and distinct physical chemical properties.First,acetone has the lowest boiling point of 56.2°C and the wet ball-milled product can be dried up fastest to avoid severe segregation of ingredients in drying.Second,to micro/nano-sized particles,their aggregation and dispersion states in ball-milled pulp are directly correlated with their surface charge which is the measure of inter-particle electrostatic repulsive force and whose value is influenced greatly by pH[24].Owing to the difference in hydrogen bond forming abilities of different functional groups,water,ethanol and acetone can differently influence the surface charge of mechanically activated raw material particles in ball-milled pulp,and subsequently determine the agglomeration of particles during the drying process and finally the electrochemical performance of LiFePO4.Third,the pH value dependent on the dispersive agent determines the Zeta potential[25]of ball-milled particles.This potential then dictates the segregation and agglomeration of the wet ball-milled particles in drying.Different dispersive agents bring about different Zeta potentials but which cannot be precisely measured in non-aqueous solutions/suspensions.
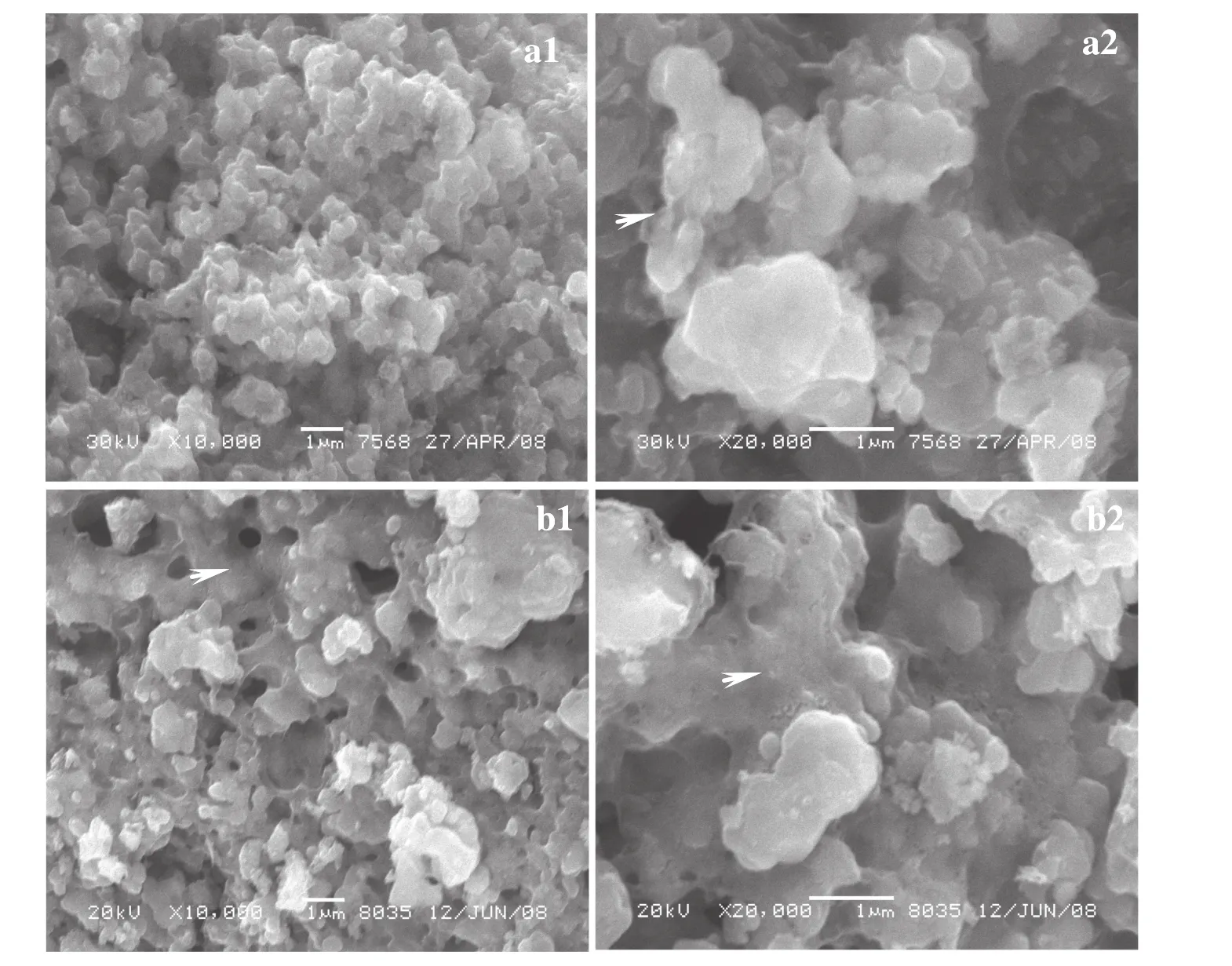
Fig.6.SEM images of LiFePO4/C synthesized with different dispersive agents:ethanol(a1 and a2)and acetone(b1 and b2)(arrows indicating amorphous carbon).
4.Conclusions
From above,it can be concluded that sintering temperature and carbon content must be carefully optimized for synthesizing LiFePO4/C with superior electrochemical performance and tap density.The increase in sintering temperature leads to a higher degree of crystallinity and bigger crystallite/particle size for LiFePO4,more Fe2P formation and lower carbon content in LiFePO4/C.700°C is the optimum sintering temperature.Higher carbon content in the range of 4.48%-11.03%leads to better rate capability for LiFePO4/C but impacts its tap density deleteriously.Acetone is a better dispersive agent in ball-milling than ethanol and water and may better alleviate segregation and agglomeration in the following drying, finally resulting in an idealcarbon existence state in LiFePO4/C.That is,an even distribution in the form of coating and connecting LiFePO4particles.LiFePO4/C synthesized at 700°C by using acetone as the dispersive agent exhibits an average of 153.8,128.3 and 121.0 mA·h·g-1at 0.1C,1Cand 2C,respectively,in the first 50 cycles.These synthesis parameters are worth further and more deeply studying for synthesis of LiFePO4/C with high quality.
[1]J.Apt,S.B.Peterson,J.F.Whitacre,Battery vehicles reduce CO2emissions,Science333(6044)(2011)823.
[2]S.Chu,A.Majumdar,Opportunities and challenges for a sustainable energy future,Nature488(7411)(2012)294-303.
[3]R.F.Service,Battery FAQs,Science332(6037)(2011)1495.
[4]B.Dunn,H.Kamath,J.M.Tarascon,Electrical energy storage for the grid:A battery of choices,Science334(6085)(2011)928-935.
[5]Z.W.Xiao,G.R.Hu,K.Du,Z.D.Peng,A facile route for synthesis of LiFePO4/C cathode material with nano-sized primary particles,Chin.J.Chem.Eng.22(5)(2014)590-595.
[6]K.D.Yang,F.X.Tan,F.Wang,Y.F.Long,Y.X.Wen,Response surface optimization for process parameters of LiFePO4/C preparation by carbothermal reduction technology,Chin.J.Chem.Eng.20(4)(2012)793-802.
[7]W.L.Yu,Y.P.Zhao,Q.L.Rao,Rapid and continuous production of LiFePO4/C nanoparticles in super heated water,Chin.J.Chem.Eng.17(1)(2009)171-174.
[8]Z.W.Xiao,G.R.Hu,K.Du,Z.D.Peng,X.R.Deng,High density LiFePO4/C composite cathode material for lithium ion batteries,Chin.J.Non-Ferrous Met.17(12)(2007)2040-2045(in Chinese).
[9]Z.Yang,J.Zhang,M.C.W.Kintner-Meyer,X.Lu,D.Choi,J.P.Lemmon,J.Liu,Electrochemical energy storage for green grid,Chem.Rev.111(5)(2011)3577-3613.
[10]J.Wang,X.Sun,Understanding and recent development of carbon coating on LiFePO4cathode materials for lithium-ion batteries,Energy Environ.Sci.5(1)(2012)5163-5185.
[11]J.Zhang,J.Xie,C.Wu,G.Cao,X.Zhao,In-situ one-pot preparation of LiFePO4/carbonnano fibers composites and their electrochemical performance,J.Mater.Sci.Technol.27(11)(2011)1001-1005.
[12]Z.W.Xiao,G.R.Hu,A novel synthesis of LiFePO4/C from Fe2O3without extra carbon or carbon-containing reductant,J.Cent.South Univ.21(6)(2014)2143-2149.
[13]N.Ravet,M.Gauthier,K.Zaghib,J.B.Goodenough,A.Mauger,F.Gendron,C.M.Julien,Mechanism of the Fe3+reduction at low temperature for LiFePO4synthesis from a polymeric additive,Chem.Mater.19(10)(2007)2595-2602.
[14]C.W.Kim,J.S.Park,K.S.Lee,Effect of Fe2P on the electron conductivity and electrochemical performance of LiFePO4synthesized by mechanical alloying using Fe3+raw material,J.Power Sources163(1)(2006)144-150.
[15]S.H.Wu,J.J.Shi,J.Y.Lin,Effects of Fe2P and Li3PO4additives on the cycling performance of LiFePO4/C composite cathode materials,J.Power Sources196(16)(2011)6676-6681.
[16]M.M.Rahman,J.Z.Wang,R.Zeng,D.Wexler,H.K.Liu,LiFePO4-Fe2P-C composite cathode:an environmentally friendly promising electrode material for lithium-ion battery,J.Power Sources206(5)(2012)259-266.
[17]J.K.Kim,J.W.Choi,G.Cheruvally,J.U.Kim,J.H.Ahn,G.B.Cho,K.W.Kim,H.J.Ahn,A modified mechanical activation synthesis for carbon-coated LiFePO4cathode in lithium batteries,Mater.Lett.61(18)(2007)3822-3825.
[18]A.Singhal,G.Skandan,G.Amatucci,F.Badway,N.Ye,A.Manthiram,H.Ye,J.J.Xu,Nanostructured electrodes for next generation rechargeable electrochemical devices,J.Power Sources129(1)(2004)38-44.
[19]Y.L.Ruan,Z.Y.Tang,B.M.Huang,Effect of carbon content on electrochemical properties of LiFePO4/C composite cathode,Chin.J.Chem.Eng.13(5)(2005)686-690.
[20]Z.G.Xie,Electrochemical performance of cathode material LiFePO4of lithium ion batteries,Chin.J.Appl.Chem.24(2)(2007)238-240(in Chinese).
[21]Y.L.Ruan,Z.Y.Tang,Effects on the structure and electrochemical performance of LiFePO4by Zr4+doping,Electrochemistry12(3)(2006)315-318(in Chinese).
[22]M.Hurtta,I.Pitkanen,J.Knuutinen,Melting behavior of D-sucrose,D-glucose and D-fructose,Carbohydr.Res.339(13)(2004)2267-2273.
[23]M.S.Whittingham,Lithium batteries and cathode materials,Chem.Rev.104(10)(2004)4271-4301.
[24]S.Sakthivel,V.Venkatesan,B.Krishnan,B.Pitchumani,Influence of suspension stability on wet grinding for production of mineral nanoparticle,Particuology6(2)(2008)120-124.
[25]A.Martin,F.Martinez,J.Malfeito,L.Palacio,P.Pradanos,A.Hernandez,Zeta potential of membranes as a function of pH:optimization of isoelectric point evaluation,J.Membr.Sci.213(1-2)(2003)225-230.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Controlled release and enhanced antibacterial activity of salicylic acid by hydrogen bonding with chitosan☆
- The structure,tensile properties and water resistance of hydrolyzed feather keratin-based bioplastics☆
- Preparation of dendritic bismuth film electrodes and their application for detection of trace Pb(II)and Cd(II)☆
- Wheat straw pretreatment with KOH for enhancing biomethane production and fertilizer value in anaerobic digestion☆
- Performance evaluation of a modified step-feed anaerobic/anoxic/oxic process for organic and nutrient removal
- Biodiesel synthesis via metal oxides and metal chlorides catalysis from marine alga Melanothamnus afaqhusainii
